ABSTRACT
Patients with chronic obstructive pulmonary disease (COPD) show impairments in the autonomic nervous systems (ANS) function, which is responsible for cardiac autonomic regulation. This study assessed the autonomic function and cardio-vagal reactivity in conveniently sampled subjects with COPD participating in a pulmonary rehabilitation (PR) program. Twenty-six subjects with COPD and 22 age and gender matched control subjects were evaluated. R-R intervals were collected at rest in supine position. Thereafter, resting autonomic function parameters comprising linear and nonlinear analyses of heart rate variability (HRV) and baroreceptor sensitivity (BRS) were calculated. Autonomic reactivity tests comprising deep breathing (DB), Valsalva maneuver (VM), and head up tilt (HUT) were also performed. The results of this study indicated that resting autonomic function variables were generally reduced in COPD compared to controls. However, this difference was only statistically significant for a few HRV parameters: mean RR intervals, low frequency (LF), standard deviation of dispersion of points perpendicular to the line-of-identity (SD1), and approximate entropy (ApEn) (p < 0.05). The results also indicated that all cardio-vagal indices following the autonomic reactivity tests were comparable between COPD and controls (p > 0.05). It was concluded that subtle autonomic impairments exists in physically active COPD patients, and these autonomic function deficits were mainly recognized by resting HRV indices and not autonomic reactivity tests.
Introduction
Chronic obstructive pulmonary disease (COPD) is a pulmonary disease mainly characterized by airflow limitation in the lungs and also multi-systemic manifestations on a number of extra-pulmonary systems especially the cardiovascular and autonomic nervous systems (ANS) (Citation1–3). These manifestations are associated with COPD comorbidities, most of which contribute to poor prognosis, risks of arrhythmias, and COPD-related mortality rates (Citation1, Citation4). Specifically, patients with COPD present with functional alterations and dysfunction of the cardiac autonomic modulation, which is primarily reflected in the form of an elevation in the resting heart rate and/or reduction in heart rate variability (HRV) indices (Citation2, Citation3, Citation5).
Therefore, the assessment of various ANS parameters has been the focal point in understanding the autonomic function, and its clinical implications in COPD Citation(3). Current reports have also shown that ANS parameters (physiological biomarkers) such as heart rate variability (HRV) (Citation1, Citation6, Citation7), heart rate response (HRR) to exercise Citation(1), muscle sympathetic nerve activity (MSNA), and baroreceptor sensitivity (BRS) Citation(8) are significantly impaired in patients with COPD. The HRV in particular is an autonomic function parameter that represents a powerful research tool for quantifying the state of the ANS, with reliable clinical applications Citation(9). Moreover, autonomic function parameters are utilized for calculating the sympathetic tone, vagal modulation of the sinus node, and the sympatho-vagal balance with a view to optimizing treatment Citation(10). A number of limitations have been observed in the existing studies over the years. For example, in our recent systematic review, we found that most of the available studies often do not take into account variables such as differences in disease stage (global obstructive lung disease [GOLD] criteria) or presence of variable COPD comorbidities and no evidence existed for a number of autonomic function variables Citation(11). Additionally, autonomic function was also found to be influenced by several factors such as circadian rhythm Citation(12), clinical and even demographic variables, and physical activity status Citation(11).
Furthermore, several important autonomic function parameters like the nonlinear HRV analyses (including entropy changes), which has recently become more useful in assessing the ANS function remain under-reported in patients with COPD (Citation2, Citation13–15). Specifically, the nonlinear HRV values have the capacity to reflect important nonlinear heart dynamics or patterns in the ECG data. Moreover, no systematic evidence is presently available to support severely impaired nonlinear HRV indices in COPD Citation(11). Additionally, conflicting results have been reported among different subgroups of COPD. Cardiac autonomic control is a complex mechanism that requires careful interpretation and application in clinical settings Citation(4). Nevertheless, since autonomic function is widely reported in COPD, it is largely assumed to be impaired in these patients. We hypothesize that there will be a diminished or impaired ANS function (e.g., lower LF or impaired baroreflex activation) among a subgroup of individuals with COPD who are physically active and fairly homogenous. Furthermore, the use of the widely used autonomic reactivity tests (comprising validated test battery for assessing autonomic failure) of cardio-vagal autonomic function indices has been inadequately reported for patients with COPD Citation(16).
To address these gaps, this study was conducted to evaluate both parameters of resting autonomic function and cardio-vagal responses to validate autonomic reactivity tests among non-sedentary individuals with COPD.
Methods
Subjects
The population of this study comprised 26 patients with severe COPD (23 males and 3 females; %predictedFEV1 of 39.5%) aged between 50 and 80 years. All COPD subjects were non-sedentary, and this was defined as participating in a pulmonary rehabilitation (PR) program continuously for at least 2 months. COPD subjects fulfilling the following inclusion criteria were eligible for participation in the study: (i) a stable condition (no acute exacerbation or change in therapeutic treatment plan in the past 3 months), (ii) no severe co-morbidity such as chronic heart diseases, uncontrolled diabetes mellitus, neurological problems, cancer, mental disorder, and complex cardiac arrhythmias, and (iii) no recent surgery (<6 months).
A control group comprising 22 age and sex-matched healthy individuals (19 males and 3 females) without COPD or any other chronic disease was recruited from the general population. The participants in the control group were screen using the Baecke physical activity questionnaire Citation(17), and only those with a moderate or hard physical activity level participated in the study.
All participants signed an informed consent form prior to commencement of study measurements. The research protocol was approved by the ethical committee of Ghent University Hospital.
Procedure
The measurements were conducted in a room with constant temperature between 21 and 23°C. All measurements were carried out in the morning hours between 8 and 11 am to avoid the influence of circadian rhythm. The participants were also instructed to refrain from coffee and nicotine use prior to the test moment on the scheduled day. Prior to the measurements, the participants were questioned regrading age, gender, and smoking status, alcohol use was measured, and their body mass index (BMI) was also calculated. Information regarding medical history, and medication use of subjects with COPD was retrieved from their hospital based medical records. The ECG and beat-to-beat blood pressure recordings were conducted via 3-lead electrodes (placed on the chest) and bicep/finger (plethysmography) cuffs attached to a Finometer PRO® ECG machine (Finapres Medical Systems, The Netherlands). The finger volume pulse waveform measured by Finometer device has been recently confirmed to be a reliable source for analysis of HRV Citation(18).
Autonomic function tests
Data acquisition and analysis
The data files were retrieved from the Finameter Pro machine. The files were then transformed into a readable (excel) format in order to extract stable signals corresponding to the middle 5 minutes of resting autonomic function recordings. The pulse interval (RRi) in TXT format was then imported into Kubios (software package version 2.2, University of Eastern Finland, Kuopio) Citation(19) for further analyses. Artifact correction was set at ‘‘very strong’’ in all cases.
Resting heart rate variability analysis
The resting HRV was assessed by linear as well as by nonlinear analyses. For linear HRV analyses, this was done for both time and frequency domain parameters. The time domain HRV analyses included; mean of RR (mean RR) intervals, the standard deviation of the mean of all RR intervals (SDRR or SDNN), the square root of the mean squared differences of successive RR intervals (RMSSD), mean heart rate (HR), number of pairs of adjacent NN intervals differing by more than 50 milliseconds in the entire recording (NN50), NN50 count divided by the total number of all NN intervals (pNN50), the triangular index (RRi tri), and triangular interpolation of NN interval histogram (TINN). In general, higher values in the time domain HRV analyses reflect vagal influence over the heart control.
The frequency domain HRV analyses, which were based on the Fast Fourier Transformation (Welsh periodogram estimates) included the very low frequency (VLF), low frequency (LF), and high frequency (HF), were expressed in milliseconds squared (ms2). Higher values of the HF index represent parasympathetic activity, while the LF characterizes both sympathetic and parasympathetic outflows. The normalized units (nu) of the LF and HF, as well as the LF/HF ratio, which represents the sympathovagal balance were also calculated.
The indices of the nonlinear HRV analysis, which provide a more robust information relating to the behavior of the cardiac control were also retrieved. These included the standard deviation measuring the dispersion of points in the plot perpendicular to the line of identity (SD1) and the standard deviation measuring the dispersion of points along the line of identity (SD2). The SD1 measures short-term HRV in ms, while the SD2 represents both the short and long term HRV in milliseconds (ms). Other nonlinear parameters included the (i) approximate entropy (ApEn), which measures the complexity or irregularity or randomness within the HRV data (series). Small ApEn values is indicative of a regular and predictable signal Citation(20) (ii) Shannon entropy (ShannEn), is also a measure of short-term HRV that reflects the degree of complexity of the signal similar to ApEn, and (iii) mean line length (Lmean) and max line length (Lmax) of the data series. Furthermore, we also reported on the sample entropy (SampEn), which is a measure of signal regularity and complexity, and small values also represent regular signals. The detrended fluctuation analysis (DFA) measures the rate of fluctuation in the data series, and it is described in terms of both brief (α1) and long-term (α2) fluctuations that represents baroreceptor reflex and regulatory mechanisms that limit fluctuation of the beat cycle was also assessed (Citation20, Citation21). DFA is decreased in cardiovascular disorders such as myocardial infarction. And lastly, we calculated the correlation dimension (D2) which estimates the minimum number of variables required to construct a model of system dynamics. Here, higher values indicate greater complexity Citation(20).
Resting baroreceptor reflex sensitivity
The resting BRS reported in this study was the time domain cross-correlation BRS (xBRS), which was computed according to the method of Westerhof Citation(22). Other xBRS parameters such as interval (in milliseconds), delay time (in seconds), and the regression coefficient of determination (R2) were also extracted.
Autonomic reactivity tests
The autonomic reactivity tests of cardio-vagal indices were conducted by means of continuous ECG and cardiovascular monitoring during three test moments based on a validated and standardized protocol Citation(23). They include heart rate response to deep breathing (HRDB), Valsalva maneuver (VM), and head up tilt (HUT).
Heart rate response to deep breathing
For the HRDB, the participants were asked to perform eight cycles of deep inspirations (via the nose) and expirations (via the mouth) at a breathing rate corresponding to 6 breaths/minute. They were provided with a visual biofeedback on a computer screen. The maneuver was repeated twice with a resting time interval of 5 minutes. The best trial comprising at least five cycles good of breaths was retrieved. The average of the range of maximum HR achieved during inspiration and the minimum HR during expiration for each cycle of breath was calculated. This parameter assesses the vagal control of the heart, a measure of parasympathetic reactivity (normal: >15 beat/minute, borderline: 11–14 beats/minute, and abnormal: <11 beats/minute) Citation(24).
Valsalva maneuver
The VM was evoked by asking the subjects to continuously blow for 15 seconds, through a mouthpiece attached onto a sphygmomanometer at pressure between 40 and 50 mmHg. A visual feedback was provided on a computer screen to aid the maneuver. This maneuver was repeated three times with a resting interval time of 3 minutes between the maneuvers. For each subject, only the best of the three trials showing adequate pressure, duration, and normal mean arterial pressure response that reflects the four phases of VM (Citation23, Citation25) were selected for analyses. This decision was taken after a careful inspection of the maneuver (excel graph plot) was made.
Several parameters were calculated from the data of subjects who completed at least one adequate VM trial. They include: Valsalva ratio (VR), which represents vagal activity, was calculated as the ratio of the maximum HR (HRmax) during Valsalva maneuver and the maximum HR within 30 seconds from the HRmax. The Valsalva index (VI), which is also an indirect measure of the ANS integrity, was calculated as the ratio between the longest RRi during VM recovery (phase 4) and the shortest RRi during the peak of VM. A VI of less than 1.4 is considered abnormal. Three BRS parameters comprising adrenergic baroreceptor sensitivity (BRS_a), vagal baroreceptor sensitivity (BRS_v), and global baroreceptor sensitivity (BRS_g) indices were also calculated using an existing protocol Citation(26).
Head up tilting
After a 5-minute period of continuous recording in a supine position, the table was slowly tilted up to 60° angle and maintained for 10 minutes. The participants were asked to report any symptoms of light headedness, feelings of dizziness, discomfort, or pain throughout the duration of the tilt. The HUT test was terminated only if severe orthostatic intolerance complaints or signs of vasovagal syncope were suspected Citation(27).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences vaersion_22 (SPSS Inc. Chicago IL, USA). Demographic and other clinical characteristics were mainly summarized using descriptive statistics. All the variables were subjected to the Shapiro–Wilk's test and manual inspection of both QQ plots and histograms to ascertain whether or not they were normality distributed. To evaluate the significance of differences of variables between COPD and controls, we used independent samples t-test or Mann–Whitney U-test, where appropriate. For non-parametric variable comparison, we used Chi-square test. Lastly, to assess the cardiovascular responses during the HUT at different categorical time points, a two-way repeated measures ANOVA was utilized. Alpha probability value was considered significant at < 0.05 level in all cases.
Results
Demographic characteristics
The demographic and baseline clinical characteristics are presented in . These results show that the majority of the study variables such as age, body weight, BMI, systolic and diastolic blood pressures as well as mean arterial pressure (MAP) variables were all comparable between patients with COPD and healthy control subjects (p > 0.05). However, the control subjects were significantly taller (p = 0.008). The table further shows that subjects with COPD had a significantly higher resting HR, and they reported significantly higher use of two vasoactive medications (beta blockers and ACE inhibitors) compared to controls (p < 0.05).
Table 1. Baseline characteristics.
Resting autonomic function
shows the results for resting autonomic function indices between subjects with COPD and the healthy controls. Subjects with COPD were found to consequently have lower values for almost all the time domain HRV analyses compared to controls. However, only the differences for mean RR was statistically significant (p = 0.011). This is suggestive of a decreased vagal activity of subjects with COPD compared to controls. Similarly, frequency domain indices with the exception of HF (nu) were decreased in COPD compared to controls, however, only the LF(ms2) power was of significance (p = 0.035).
Table 2. Comparison of resting autonomic function parameters between COPD and controls.
For the nonlinear HRV analyses, all reported variables were comparable between subjects with COPD and control subjects except for SD1, which was significantly decreased in COPD (p = 0.041). The ApEn was also found to be significantly increased in COPD compared to the controls (p = 0.02), suggesting a higher complexity and lower signal predictability. Lastly, the results of this study revealed that subjects with COPD had lower resting BRS parameters compared to the control group (5.5 vs. 8.0, ms.mmHg). However, these differences did not reach statistical significance (p > 0.05).
Cardio vagal autonomic reactivity
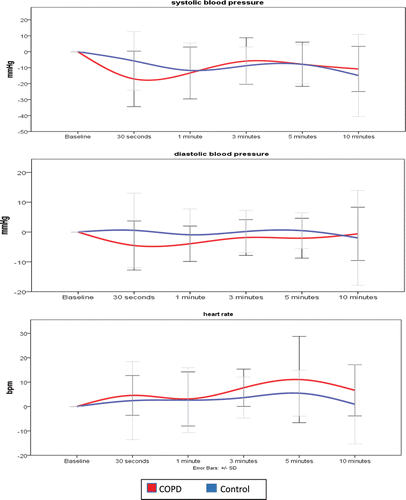
The results from the autonomic reactivity tests are presented in . The HRDB were comparable between subjects with COPD and controls (p = 0.869). All parameters derived from the VM including the VR, VI, BRS_a, BRS_v, BRS_g, PRT, and PP drop were comparable between subjects with COPD and controls (p > 0.05). For HUT, even though subjects with COPD reported more orthostatic symptoms compared with controls, these were not statistically significant (p > 0.05). Further analyses of the HUT results revealed that the subjects with COPD also recorded a slightly higher drop in both systolic and diastolic blood pressures, as well as a slightly increased heart rate during the HUT. The results from the two-way repeated measures ANOVA analyses indicated that these variations were no statistically significant interaction (between subjects effects) in the systolic blood pressure (F, 2.302; p = 137), diastolic blood pressure (F, 0.034; p = 0.855), and heart rate (F, 0.975; p = 0.330) of both COPD and control groups. These results are illustrated in a graphical manner in .
Table 3. Results of cardio-vagal indices for COPD and control subjects following autonomic reactivity tests.
Discussion
This study was aimed at providing additional extensive assessment of the ANS or autonomic function in COPD. We limited our study population to a specific cohort of COPD-patients that were non-sedentary or were ‘‘physically active’’ due to the heterogenous and complex nature of COPD, and also because of the feasibility of testing in this specific population. Consequently, it is important that our results should be understood in this context. We also included a control group so that our results can be viewed in perspective of the prevailing autonomic function health status of their counterparts in the general population. More importantly, our study reported several autonomic function parameters some of which are not frequently reported in COPD in existing studies. Also, including autonomic reactivity tests represents a first attempt in the area of autonomic function research in COPD.
The major results of our study are as follows: (i) subjects with COPD have a significantly lowered RR intervals compared to controls, (ii) most HRV and BRS indices were lower in subjects with COPD compared to the control group, however only a few of these indices were of statistical significance, (iii) the autonomic responses to the three autonomic reactivity tests of (deep breathing, valsalva maneuver, and tilting) were largely normal and similar between COPD and controls, and (iv) further analyses of tilting revealed that subjects with COPD have a slightly poorer cardiovascular response to tilting in comparison to controls. The significant decrease in LF among the COPD and marginal reduced the baroreflex control (measured by resting BRS) in COPD are important results that is in line with past trend. There is evidence—at least in cardiovascular diseases—that the baroreflex control and LF power in HRV are associated Citation(28). Additionally, we found that the ApEn, which is a measure of signal regularity and complexity was significantly increased in COPD. The ApEn has been reported to be a very useful tool for predicting individual risk of cardiovascular disease Citation(29). All of these results put together are suggestive of residual autonomic function deficits in COPD, thereby lending credence to earlier reports which stated that ANS is impaired in COPD (Citation3, Citation30). Nevertheless, our results for resting HRV parameters including mean RR, SDNN, RMSSD, LF(ms2) LF(n.u.), HF(ms2) HF (n.u), and LF/HF ratio, were largely comparable with the normative value reported among heathy adults during short-term HRV measurements in a systematic review by Nunan et al. Citation(31).
We understand that several factors may have influenced the results of our study. The use of COPD subjects participating in a rehabilitation program may have explained the similarity in the results in most of the autonomic function variables that were assessed, and also the non-statistical difference for the cardio-vagal indices following the autonomic reactivity tests. A number of studies including our recent systematic review has demonstrated that participating in an aerobic exercise training program, which forms the core of pulmonary rehabilitation or a maintenance program affects the autonomic function in COPD (Citation2, Citation7, Citation32–34). For example, a 4-week rehabilitation has been shown to significantly increase HRV indices, which were also associated with health-related quality of life in COPD Citation(35). Furthermore, pulmonary rehabilitation programs that may comprise interventions have been proven to influence the autonomic function in COPD such as controlled breathing techniques and noninvasive mechanical ventilation Citation(36). The LF/HF ratio, which is an index of sympathovagal balance was found to be higher among the controls, albeit not statistically significant. Nevertheless, this result is indicative of a generally higher sympathetic hyperactivity among the controls over the COPD group Citation(37). In addition, the results could mean that autonomic function, represented by both sympathetic and parasympathetic branches in COPD is deficient in COPD. Comparing the results in this study with existing studies demonstrate that the subjects in our study had a higher average resting HRV (Citation2, Citation38–40) and BRS Citation(41) parameters. However, the resting BRS was found to be similar to the values reported in COPD patients who were much younger (51 years) Citation(42). Again, the use of beta blockers and ACE inhibitors by subjects with COPD may have also contributed to the largely comparable results, since these medications are known to affect HRV in the direction of normalization.
Even though the cardio-vagal response indices (HRDB, BRS_a, BRS_v, BRS_g, VI, and VR) and the HUT parameters were comparable between COPD and controls, further analyses may provide extra information. For example, both groups had an HRDB value that has been classified to be a borderline parasympathetic response Citation(24), suggesting a possible influence of ageing. We also discovered that the average VI and VR in COPD recorded in our study was just above the normal reference cut-offs reported for healthy adults (Citation4, Citation24, Citation26), which may indicate that both groups have normal parasympathetic responses. Moreover, the HRV during deep breathing in the context of autonomic function testing has been successfully used for detecting parasympathetic disorders or rates of parasympathetic activity Citation(43). Nevertheless, more studies are needed for other COPD populations.
We used a number of VM parameters to describe the autonomic reaction to the maneuver all of which showed a normal trend, and even higher values for COPD. The higher VR results of subjects with COPD in our study can be explained by a more rapid HR increase during second phase of VM, which is as a result of vagal activity withdrawal over the sinus node that is only reactivated after the Valsalva strain is removed Citation(44).The average value of the adrenergic, vagal and global BRS indices and even the VR of the participants (COPD and control) of this study were comparable to the values obtained for subjects with orthostatic hypotension, but lower than those of the healthy controls in the study of Schrezenmaier et al. Citation(26). However, the participants of our study were much older compared to those in the latter study (65 vs. 51 years).
After further analyzing the cardiovascular responses to tilting (), a few differences were observed. Even though these differences were not statistically significant, they still represent signs of residual autonomic function impairments in subjects with COPD. During HUT, the ensuing orthostatic change triggers an increased sympathetic nervous system outflow Citation(45), which produces a shift in spectral power from HF bands to LF bands (or increased LF/HF ratio). This is reflected in the cardiovascular system in the form of peripheral vasoconstriction in order to maintain hemodynamic functioning. The marginal lowering of the systolic and diastolic blood pressures as well as the increased baseline HR we found in our results could be suggestive of a diminishing sympathovagal response to orthostatic stimuli, as is already known (Citation6, Citation8, Citation46).
As mentioned earlier, the non-sedentary nature of the subjects with COPD in this study may be an important factor that influenced the non-significant differences between COPD patients and healthy controls in the majority of the autonomic function indices. We also must take into account that the COPD patients used more vasoactive medications (beta blocker and ACE inhibitors) that are known to influence autonomic test results (Citation47, Citation48). The COPD patients in this study were not asked to discontinue their usual medication for several reasons among which was to assess the subjects autonomic function under their current conditions and for ethical concerns related to withdrawal of prescribed medications. Therefore, the extent by which any of these factors in addition to certain lifestyle circumstances may have affected the results is presently not clear. This study has highlighted the need for more focus on reporting tests of physiological biomarkers in various COPD phenotypes. Our study has provided new and noteworthy results that could form the basis for further autonomic function analyses in COPD. Notably, our study has shown that the cardiovascular response to tilting offers a more discriminatory ability of autonomic reactivity tests in COPD even when other cardio-vagal variables appear to be within normal range. All these findings can assist clinicians in defining the presence of autonomic failure and even their response to treatment Citation(49).
The results of this study must be viewed within the limitations of the study. A significant proportion of the participants was males and we did not carry out any gender-based analyses. However, this was taken into account by the gender/age matching (maximum 5 year interval) that was used during subject recruitment. Cardio-vagal index represents only 2 of 3 aspects of the composite autonomic scoring scale (CASS) Citation(50), which is a validated test protocol for assessing autonomic failure. Future studies assessing these tests in addition to sudomotor function which can be evaluated with the quantitative sudomotor axon reflex test and the thermoregulatory sweat test, also impaired in COPD (Citation51, Citation52), may be necessary. Lastly, the study utilized a cross-sectional case–control design. We recommend study designs with either larger number of participants and /or follow-up analyses in order to accurately evaluate the course of these indices (autonomic function and cardio-vagal) over time.
Conclusion
To summarize, our results in this study suggest that autonomic impairment exists in COPD. However, COPD patients who are non-sedentary or physically active may show several comparable autonomic function indices with their healthy counterparts. Autonomic reactivity tests are not of any discriminatory value over resting autonomic function indices in the COPD cohort in this study.
Conflicts of interest declaration
All the authors of this manuscript report no conflicts of interest.
References
- Andreas S, Anker SD, Scanlon PD, Somers VK. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest. 2005;128(5):3618–3624.
- Borghi-Silva A, Mendes RG, Trimer R, Oliveira CR, Fregonezi GA, Resqueti VR, et al. Potential effect of 6 versus 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary disease. Eur J Phys Rehabil Med. 2015;51(2):211–221.
- van Gestel AJ, Kohler M, Clarenbach CF. Sympathetic overactivity and cardiovascular disease in patients with chronic obstructive pulmonary disease (COPD). Discov Med. 2012;14(79):359–368.
- da Luz Goulart C, Cabiddu R, de Borba Schneiders P, San Martin EA, Trimer R, Borghi-Silva A, et al. Is cardiac autonomic modulation during upper limb isometric contraction and Valsalva maneuver impaired in COPD patients? Int J Chron Obstruct Pulmon Dis. 2017;12:849–857.
- Jensen MT, Marott JL, Lange P, Vestbo J, Schnohr P, Nielsen OW, et al. Resting heart rate is a predictor of mortality in COPD. Eur Respir J. 2013;42(2):341–349.
- Volterrani M, Scalvini S, Mazzuero G, Lanfranchi P, Colombo R, Clark AL, et al. Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest. 1994;106(5):1432–1437.
- Camillo CA, Laburu Vde M, Goncalves NS, Cavalheri V, Tomasi FP, Hernandes NA, et al. Improvement of heart rate variability after exercise training and its predictors in COPD. Respir Med. 2011;105(7):1054–1062.
- Costes F, Roche F, Pichot V, Vergnon JM, Garet M, Barthelemy JC. Influence of exercise training on cardiac baroreflex sensitivity in patients with COPD. Eur Respir J. 2004;23(3):396–401.
- Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, et al. Heart rate variability reflects severity of COPD in PiZ α 1-antitrypsin deficiency. Chest. 1998;113(2):327–333.
- Lombardi F. Clinical implications of present physiological understanding of HRV components. Cardiac Electrophysiol Rev. 2002;6(3):245–249.
- Mohammed J, Meeus M, Derom E, Da Silva H, Calders P. Evidence for autonomic function and its influencing factors in subjects with COPD: A systematic review. Respir Care. 2015;60(12):1841–1851.
- Massin MM, Maeyns K, Withofs N, Ravet F, Gérard P. Circadian rhythm of heart rate and heart rate variability. Arch of Dis Childhood. 2000;83(2):179–182.
- Zivanovic I, Zupanic E, Lainscak M, Avbelj V, Kalisnik JM. Nonlinear dynamics of heart rate variability in patients with chronic obstructive pulmonary disease and changes after 4-week rehabilitation. Eur Respir J. 2014;44(suppl. 58) P3541.
- Goulart Cda L, Simon JC, Schneiders Pde B, San Martin EA, Cabiddu R, Borghi-Silva A, et al. Respiratory muscle strength effect on linear and nonlinear heart rate variability parameters in COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;11(6):1671–1677.
- Mazzuco A, Medeiros WM, Sperling MP, de Souza AS, Alencar MC, Arbex FF, et al. Relationship between linear and nonlinear dynamics of heart rate and impairment of lung function in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1651–1661.
- Volterrani M, Scalvini S, Mazzuero G, Lanfranchi P, Colombo R, Clark AL, et al. Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest. 1994;106(5):1432–1437.
- Baecke JA, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942.
- Linder JR, Stauss HM, Gindes H, Pierce GL, Von Bergen NH, Haynes WG, et al. Finger volume pulse waveforms facilitate reliable assessment of heart rate variability, but not blood pressure variability or baroreflex function. BMC Cardiovascular Disorders. 2014;14(1):180–187.
- Langewouters G, Settels J, Roelandt R, Wesseling K. Why use Finapres or Portapres rather than intraarterial or intermittent non-invasive techniques of blood pressure measurement? J Med Eng Tech. 1998;22(1):37–43.
- Shaffer F, Ginsberg J. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258(1–17).
- Francesco B, Maria Grazia B, Emanuele G, Valentina F, Sara C, Chiara F, et al. Linear and nonlinear heart rate variability indexes in clinical practice. Comput Math Methods Med. 2012:1–6.
- Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM. Time-domain cross-correlation baroreflex sensitivity: Performance on the EUROBAVAR data set. J Hypertens. 2004;22(7):1371–1380.
- Novak P. Quantitative autonomic testing. J Visualized Exp. 2011; (53):e2502 (1–24).
- O'Brien I, O'Hare P, Corrall R. Heart rate variability in healthy subjects: Effect of age and the derivation of normal ranges for tests of autonomic function. Heart. 1986;55(4):348–354.
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65(10):1533–1537.
- Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J, Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007;64(3):381–386.
- De Wandele I, Rombaut L, Leybaert L, Van de Borne P, De Backer T, Malfait F, et al., eds. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers–Danlos syndrome. Sem Arthr and Rheum. 2014;44(1):93–100.
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low‐frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96(12):1255–1261.
- Wessel N, Schumann A, Schirdewan A, Voss A, Kurths J. Entropy measures in heart rate variability data. Lect Notes in Comp Sci. 2000;(1933):78–87.
- van Gestel AJ, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2010;2(4):215–222.
- Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short‐term heart rate variability in healthy adults. Pacing and Clin Electrophysiol. 2010;33(11):1407–1417.
- Mohammed J, Derom E, Van Oosterwijck J, Da Silva H, Calders P. Evidence for aerobic exercise training on the autonomic function in patients with chronic obstructive pulmonary disease (COPD): A systematic review. Physiotherapy. 2017; doi: 10.1016/j.physio.2017.07.004.
- Borghi-Silva A, Arena R, Castello V, Simoes RP, Martins LEB, Catai AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. 2009;103(10):1503–1510.
- Cheng ST, Wu YK, Yang MC, Huang CY, Huang HC, Chu WH, et al. Pulmonary rehabilitation improves heart rate variability at peak exercise, exercise capacity and health-related quality of life in chronic obstructive pulmonary disease. Heart Lung. 2014;43(3):249–255.
- Zupanic E, Zivanovic I, Kalisnik JM, Avbelj V, Lainscak M. The effect of 4-week rehabilitation on heart rate variability and QTc interval in patients with chronic obstructive pulmonary disease. COPD. 2014;11(6):659–669.
- Mohammed J, Da Silva H, Van Oosterwijck J, Calders P. Effect of respiratory rehabilitation techniques on the autonomic function in patients with chronic obstructive pulmonary disease: A systematic review. Chron Respir Dis. 2016;14(3):217–230.
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381.
- Carvalho TD, Pastre CM, de Godoy MF, Fereira C, Pitta FO, de Abreu LC, et al. Fractal correlation property of heart rate variability in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:23–28.
- Borghi-Silva A, Reis MS, Mendes RG, Pantoni CBF, Simoes RP, Martins LEB, et al. Noninvasive ventilation acutely modifies heart rate variability in chronic obstructive pulmonary disease patients. Respir Med. 2008;102(8):1117–1123.
- Borghi-Silva A, Arena R, Castello V, Simoes RP, Martins LEB, Cataii AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. 2009;103(10):1503–1510.
- Bartels MN, Gates GJ, Downey JA, Armstrong HF, De Meersman RE. Baroreceptor sensitivity after Valsalva maneuver in women with chronic obstructive pulmonary disease. Clin Auton Res. 2012;22(4):185–189.
- Haider T, Casucci G, Linser T, Faulhaber M, Gatterer H, Ott G, et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J Hypertens. 2009;27(8):1648–1654.
- Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed). 1982;285(6346):916–918.
- Minatel V, Karsten M, Neves LM, Beltrame T, Borghi-Silva A, Catai AM. Heart rate assessment during maximal static expiratory pressure and Valsalva maneuver in healthy young men. Rev Bras Fisioter. 2012;16(5):406–413.
- Burke D, Sundlof G, Wallin G. Postural effects on muscle nerve sympathetic activity in man. J Physiol. 1977;272(2):399–414.
- Pagani M, Lucini D, Pizzinelli P, Sergi M, Bosisio E, Mela GS, et al. Effects of aging and of chronic obstructive pulmonary disease on RR interval variability. J Autonom Nervous Sys. 1996;59(3):125–132.
- bv E-WN. COPD – samenvatting. 2015.
- Sharif R, Cuevas CR, Wang Y, Arora M, Sharma G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir Med. 2013;107(7):1046–1052.
- Low PA, Tomalia VA, Park K-J. Autonomic function tests: Some clinical applications. J Clin Neurol. 2013;9(1):1–8.
- Suarez GA, Opfer-Gehrking T, Offord K, Atkinson E, O'brien P, Low PA. The autonomic symptom profile a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–528.
- Bir LS, Özkurt S, Daloglu G, Kurt T. Impaired sympathetic skin response in chronic obstructive pulmonary disease. Tohoku J Exp Med. 2005;207(4):243–248.
- Stewart A, Marsh F, Waterhouse J, Howard P. Autonomic nerve dysfunction in COPD as assessed by the acetylcholine sweat-spot test. Eur Respir J. 1994;7(6):1090–1095.