Abstract
COPD and obesity often coexist and there is a complex interaction between them. Our aim was to evaluate the prevalence of obesity in a secondary care COPD population. Furthermore, the presence of comorbidities in obese (COPDOB) and non-obese COPD (COPDNO) individuals was studied. In 1654 COPD patients (aged ≥18 years) who visited a pulmonologist between January 2015 and December 2015, patient characteristics, pulmonary function tests and comorbidities were obtained from the medical records. Subjects were categorized according their BMI as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2) or obese (BMI ≥30.0 kg/m2). The Charlson comorbidity index and COTE index were used to quantify comorbidities. The prevalence of obesity was 21.8% in our COPD population. Obesity was significantly less common in GOLD stage IV (10.1%) compared to GOLD I (20.5%), II (27.8%) and III (18.9%). COPDOB had different comorbidities compared with COPDNO. Hypertension, diabetes mellitus, atrial fibrillation and congestive heart failure were significantly more prevalent in COPDOB compared with COPDNO. Osteoporosis and lung cancer were significantly more common in COPDNO compared with COPDOB. Obesity is common in patients with COPD and is most prevalent in COPD GOLD I-II and least prevalent in COPD GOLD IV. Obese COPD patients have different comorbidities than non-obese COPD patients. Cardiovascular and metabolic comorbidities, especially hypertension and diabetes mellitus, are highly prevalent in obese COPD patients. Active screening for these conditions should be a priority for physicians treating obese COPD patients.
Introduction
Obesity and Chronic Obstructive Pulmonary Disease (COPD) frequently coexist (Citation1–4). In 2014, 13% of the global adult population met the criteria for obesity (Body Mass Index (BMI) > 30 kg/m2) (Citation5). Also, in the Netherlands the prevalence of obesity was 13.7% in 2015 (Citation6). Existing data show a great variation in prevalence rates of COPD due to differences in demography, diagnostic criteria and survey methods (Citation7). Estimations vary from 210 to 600 million people having COPD worldwide (Citation8). In a systematic review the global prevalence of COPD was estimated to be 9–10% (Citation9). The prevalence of both conditions is projected to increase in the future (Citation7,Citation10).
The prevalence of obesity in COPD has been studied in several countries but the results are inconsistent and ranges between 18 and 54% (Citation2,Citation11–17). Most studies suggest a higher prevalence of obesity in COPD patients compared to non-COPD individuals (Citation11,Citation12,Citation14). In these studies the prevalence of obesity in the COPD cohorts exceeds the prevalence of obesity in the studied general populations which varies between 10 and 24%. However, epidemiological data from a Latin American study demonstrated a lower prevalence of obesity in COPD compared with non-COPD subjects (23% vs 31% respectively) (Citation13).
Factors that may explain the variability in data regarding the prevalence of obesity in COPD are the study method, degree of airflow limitation, sex, genetic and socio-demographic differences. Most of these studies relied on self-reported data and could not include objective information like: pulmonary function, anthropometric measurements and/or physician made diagnosis (Citation2,Citation12,Citation13,Citation15). Other limitations are small number of subjects (n = 213–355) or having an age limit for inclusion or excluding severe comorbidities (Citation11,Citation14,Citation17).
There is increasing evidence indicating different patterns of comorbidities in COPD patients compared to non-COPD individuals (Citation18,Citation19). It is known that cardiovascular, metabolic and cognitive comorbidities are relatively common in COPD (Citation20–28). Most of these comorbidities lead to higher hospitalization rates and in-hospital mortality (Citation29). However, it is relatively unknown which role the co-existence of obesity plays on the occurrence of comorbidities in COPD.
The primary aim of this study was to evaluate the prevalence of obesity in a well-defined, large COPD population from a secondary care clinic. Furthermore, the presence of comorbidities in obese (COPDOB) and non-obese COPD (COPDNO) groups was studied. Additionally, we compared the prevalence of obesity and the coexistence of comorbidities between GOLD stages.
Methods
Study design and patients
This study was performed in the outpatient clinic of Rijnstate hospital, a large teaching hospital in the Netherlands. The study was approved by Rijnstate Hospital institutional review board and the local ethics committee (LTC number: 2015-0686). Medical records of individuals (aged ≥18 years) registered with the ICD-10 diagnose of COPD between January 2015 and December 2015 were evaluated retrospectively. Data was extracted from the electronic database which is used as part of usual medical care in our hospital. The electronic records contain documentation of in- and outpatient contacts from all specialties within the hospital, data on all examinations (lab, pulmonary function, radiology etc.), medication use and referral letters from the general practitioners.
Patients were only selected when a pulmonologist had diagnosed them with COPD. The indicated diagnosis of COPD was checked and verified by evaluating the most recent Pulmonary Function Test (PFT) using the post bronchodilator values. The European Community for Coal and Steel reference equations were used to calculate predicted values (Citation30). Obstructive disease was confirmed if the ratio of Forced Expiratory Volume in one second (FEV1) to Vital Capacity (VC) was below the 5th percentile of the predicted value (Lower Limit of Normal (LLN)) (Citation31).
Patients with a history of asthma, asthma-COPD overlap syndrome (ACOS), solely restrictive lung disease and those with FEV1/VC ≥ LLN were excluded. Furthermore, patients with diagnose of COPD but without available PFT were also excluded from analysis.
When all the above mentioned criteria of inclusion were met, medical records were reviewed thoroughly, including documentation of all in- and outpatient contacts from other medical specialist in order to gain information on comorbidities.
Variables of interest
Patient characteristics like sex, age, height, weight, BMI, diagnosis of COPD by a pulmonologist, comorbidities, use of inhalers and smoking status as well as pulmonary function tests parameters were obtained from the medical records
Height, weight and BMI were obtained from the most recent PFT reports. Patients were categorized to weight classes according to WHO criteria (Citation5): underweight (UW) defined as BMI <18.5 kg/m2, normal-weight (NW) defined as BMI 18.5–24.99 kg/m2, overweight (OW) defined as BMI 25.0–29.99 kg/m2 and obese (OB) defined as BMI ≥30.0 kg/m2. The severity of COPD was classified according to GOLD guidelines (Citation7).
We chose to review the medical records on comorbidities used in the Charlson comorbidity index, COTE index and three most common comorbidities in COPD not listed in the mentioned indexes (hypertension, depression and osteoporosis) (Citation17,Citation32–34). Comorbidities were scored on physician based diagnoses which were retrieved from the documentation of specialists and general practitioners.
Statistics
Descriptive statistics were used to characterize the study population at baseline. Continuous variables are expressed as mean ± SD while discrete variables are shown as percentages. A two-tailed p value <0.05 was considered statistically significant. Between-group comparisons were made using the independent t-test (for 2 groups) or the ANOVA (for >2 groups). Post hoc analysis using Bonferroni or Games-Howell (depending on homogeneity of variance according to Levene’s test) were used to correct for multiple comparisons. The Chi-squared test was used to assess the differences in prevalence rates between GOLD stages. Post hoc analysis using Bonferroni were used to correct for multiple comparisons. For comparisons of comorbidities between obese and non-obese, binary logistic regression was used and the models were adjusted for age, sex, smoking status and FEV1% predicted. Analyses were performed with SPSS 21.0 for Windows (SPSS, IBM, USA).
Results
Description of the cohort
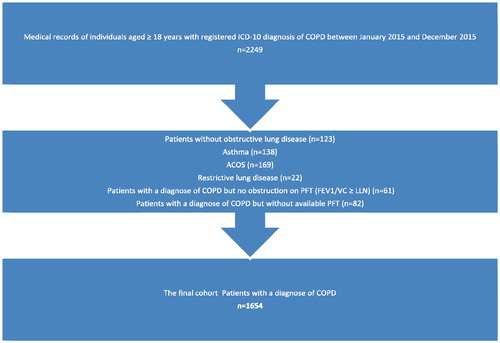
A total of 2,249 medical records were reviewed retrospectively. 595 cases did not meet the inclusion criteria. Most patients were excluded because they appeared to have a diagnosis of Asthma or ACOS (n = 307). For the final analysis we included 1,654 cases, who had met the inclusion criteria ().
Baseline characteristics
Baseline characteristics of the 1654 included subjects with a diagnosis of COPD are presented in . The proportion of male and female subjects was in balance (52 and 48% respectively). The mean age of our COPD population was 68.5 ± 10.5 years. According to the Gold classification, 7.4% of the study population was classified as GOLD I, 43.7% as GOLD II, 36.9% as GOLD III and 12.0% as GOLD IV.
Table 1. Patient’s characteristics categorized by GOLD stage (I–IV).
Subjects in GOLD II did have a higher BMI compared to GOLD III/IV. DLCO decreased with the GOLD stage. When looking at smoking status, 36% of the study population was currently smoking. There were relatively more current smokers in COPD GOLD I, while the prevalence of ex-smokers was highest in GOLD III.
Prevalence of obesity
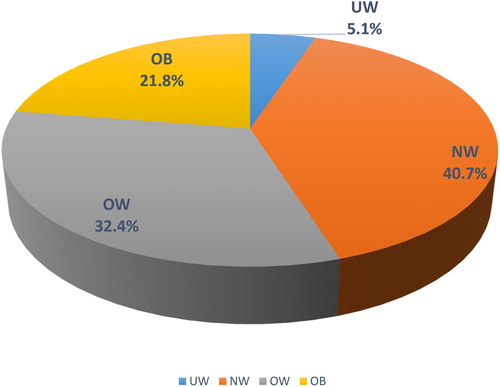
In the entire COPD population, the prevalence of obesity was 21.8%. Only 5.1% of the population was underweight (). Most of the COPD patients were normal weight (40.7%) or overweight (32.4%).
Figure 2. Distribution of weight classes in the COPD population. The prevalence (in percentages) of each weight class is provided in the pie-chart. Abbreviations: UW, under-weight; NW, normal weight; OW, overweight; OB, obese.
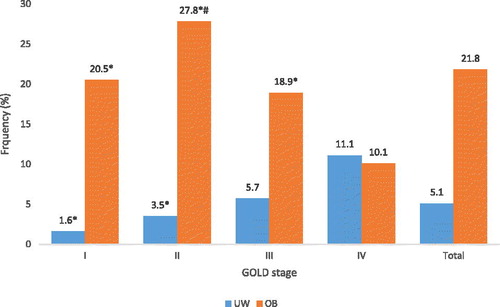
In the prevalence of both extremes in weight classes (obesity and underweight) are presented for each GOLD stage. Obesity was significantly more prevalent in GOLD stages I (20.5%), II (27.8%) and III (18.9%) than in GOLD IV (10.1%). When looking at UW, the prevalence was significantly higher in patients with COPD GOLD IV (11.1%) compared with GOLD I (1.6%) and II (3.5%) (p < 0.05).
Figure 3. Prevalence (%) of obese (OB) and under-weight (UW) patients in different GOLD stages (I–IV). *p < 0.05 compared to GOLD class IV of the same weight class, # p < 0.05 compared to GOLD class III of the same weight class.
There was no significant difference in the prevalence of OB between males and females (19.9% vs 23.9% respectively; p = 0.06). OW was more prevalent in male subjects in comparison to females (38.4% vs 26.0%; p < 0.01). Overall there was no significant difference in mean BMI between females and males (mean BMI females: 26.3 ± 6.4; males: 26.3 ± 5.1 respectively; p = 0.84). Both UW and NW were more common in females in comparison with males, but only with UW the difference was significant (UW: 7.3% vs 3.0% p < 0.01; NW: 42.8% vs 38.7% p = 0.09).
Comorbidities
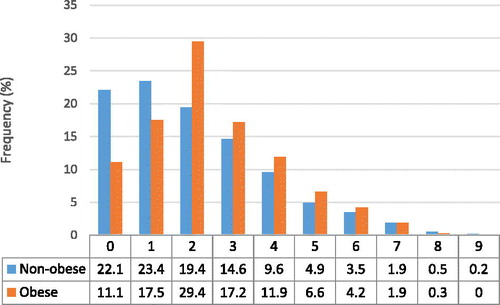
The prevalence of comorbidities within our study population is listed in . Medical records were screened for 29 comorbidities and all of these were present in our COPD population. The prevalence of the described comorbidities ranged from 0.1 to 46%. Hypertension (45.5%), cancer (23.4%), coronary artery disease (CAD) (18.5%) and diabetes mellitus (17.5%) were the most prevalent comorbidities. shows the proportion of COPDOB and COPDNO individuals with a certain number of comorbidities. The number of comorbidities for each patient ranged from 0 to 9. Within the COPDOB group 72% had two or more comorbidities, in comparison 54% of COPDNO patients had two or more comorbidities (p < 0.01).
Table 2. Prevalence of comorbidities (%) in the COPD population.
In , comorbidities with a prevalence of ≥5% are presented and compared between COPDOB and COPDNO. In , the adjusted odds ratio of comorbidities which were significantly more prevalent in obese vs. non obese and vice versa are presented. Hypertension (63.4%) and diabetes mellitus (34.1%) were the most prevalent comorbidities in COPDOB. The prevalence of hypertension was significantly higher in COPDOB compared to COPDNO (63.4% versus 40.5% respectively; p < 0.01). Also the prevalence of diabetes mellitus was significantly higher in COPDOB compared to COPDNO (34.1% versus 12.9% respectively; p < 0.01).
Figure 5. Prevalence of comorbidities (%) in obese and non-obese COPD patients. Abbreviations: HT, hypertension; CA, carcinoma; CAD, coronary artery disease; DM, diabetes mellitus; AF, atrial fibrillation or flutter; CHF, congestive heart failure; DP, depression; PVD, peripheral vascular disease; OS, osteoporosis; CKD, moderate to severe chronic kidney disease; MI, myocardial infarction; AN, anxiety; CVD, cerebrovascular disease; LUC, lung cancer. * Comorbidity is significantly more prevalent comparing to the other group (p < 0.05).
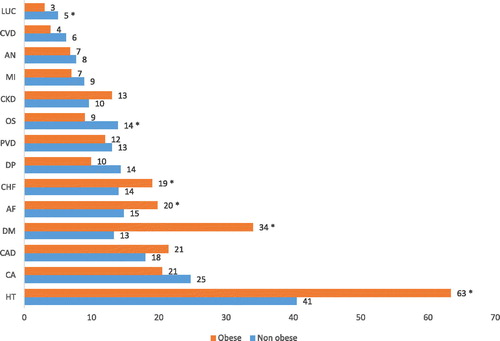
Table 3. Comorbidities which are significantly more prevalent in obese or non-obese COPD patients.
When looking at other cardiovascular diseases, congestive heart failure (COPDOB 19.4%; COPDNO 14.0%; p = 0.01) and atrial fibrillation/flutter (COPDOB 19.9%; COPDNO 14.8%; p = 0.02) were more prevalent in obese patients as well. There were no statistically significant differences in the prevalence of myocardial infarction, CAD and peripheral vascular disease between COPDOB and COPDNO. We also found significant differences between COPDOB and COPDNO when comparing the prevalence rates of osteoporosis (8.9% vs 13.8%) and lung cancer (2.8% vs 5.4%). These conditions were significantly more common in COPDNO compared with COPDOB (p < 0.05).
Discussion
Prevalence of obesity in COPD
The prevalence of obesity in our COPD population was 21.8%. This exceeded the prevalence of underweight (5.1%) by more than four times. Obesity was most prevalent in patients with COPD GOLD I and II (20.5% and 27.8% respectively) and least prevalent in patients with COPD GOLD IV (10.1%).
The prevalence of obesity in our study is comparable with results from Vanfleteren et al. (Citation17) (213 COPD patients with a mean FEV1% predicted of 51%; from a rehabilitation clinic; prevalence of COPDOB 23%), the PLATINO study (Citation13) (759 COPD patients recruited from a population-based epidemiological study; prevalence of COPDOB 23%) and a Canadian study (Citation12) (self-reported data of 3470 COPD patients from the Canadian National Health Survey; prevalence of COPDOB 24.6%). However it is not consistent with data from an earlier Dutch study in a primary care population of 317 COPD patients (Citation14) (prevalence of COPDOB 18%), a study with 355 COPD patients from Northern California recruited from a database of primary-to-tertiary care (prevalence of COPDOB 54%) (Citation11), a survey from Middle East and North Africa where 996 self-reported COPD patients from the general population were recruited (prevalence of COPDOB 30%) (Citation2) and a cohort of 2265 individuals referred to a tertiary care pulmonary function laboratory (prevalence of COPDOB 30%) (Citation16). In our study, obesity was most prevalent in patients with COPD GOLD I and II (20.5% and 27.8% respectively) and least prevalent in patients with COPD GOLD IV (10.1%). This later finding is in line with the study of Steuten et al. (Citation14).
Some factors may explain the variability in prevalence between the studies. First, it is important to use a correct definition for ‘having COPD’ or ‘being obese’. Studies with a relatively large sample size have used self-reported diagnosis, weight and length to define COPD and obesity (Citation2,Citation12,Citation15). Utilization of self-reported data is a popular methodology in studies, however it is proved to be less reliable (Citation35–39). Our study population was diagnosed with COPD by a pulmonologist. Furthermore, we used data from the most recently performed PFT’s to confirm the diagnosis. Anthropometric measurements like weight and height are measured during each PFT in our hospital, so these data were extracted from the PFT reports to calculate the BMI. This method reduced the chance of incorrect information regarding the diagnosis and other used variables (BMI, COPD Gold stage etc.).
Second, use of FEV1/FVC ratio to diagnose obstructive disease is very common. This parameter was also used in most of the studies which confirmed the diagnosis of COPD with PFT (Citation11,Citation13,Citation14,Citation17). Measuring FVC is a practical way to estimate the ‘actual’ VC. However FEV1/FVC ratio seems to be less reliable comparing with FEV1/(slow) VC ratio to diagnose obstructive disease (Citation40–42). FVC is dependent on flow and for example, in case of air-trapping, FVC is smaller than VC.
Third, the prevalence of obesity is correlated with the severity of airflow obstruction. An explanation for this might be that COPD subjects with obesity die in earlier stage of COPD and therefore not reach severe stages of COPD. Nevertheless, the higher prevalence of obesity in milder COPD makes it assumable that selection of a study population strongly influences the prevalence rates. In general, COPD patients from a primary care population have milder disease stage (GOLD I and II) compared to patients at secondary care clinics. Hence one would expect that the prevalence of obesity would be higher in primary care than in secondary care. Contrary to our expectation, the prevalence of obesity was lower in a Dutch primary care population (Citation14). Despite having relatively more subjects with COPD GOLD I and II (78%), obesity was less common in this population comparing to ours (18% vs 21.8% respectively). Although it must be mentioned that this primary care study included a relatively small number of COPD patients (n = 317). Also surprisingly our secondary care population, consisting mostly of patients with COPD GOLD II and III, did not differ in the prevalence of obesity with patients from a rehabilitation clinic in the Netherlands (Citation17). When evaluating the data in more detail, the mean FEV1% predicted of our population matched with the rehabilitation clinic population (52 ± 18% predicted vs 51 ± 17% predicted respectively). This might explain similarity in our prevalence rates. Furthermore, patients with severe comorbidities (like active malignancy) were not included in the study performed at the rehabilitation clinic. It is assumable that inclusion of such patients would lead to lower prevalence rates of obesity.
Finally, other factors like genetic, socio-demographic differences and sex may also play a role in prevalence rates of obesity in COPD. Several studies indicate that obesity is more prevalent in women (Citation12,Citation13,Citation43). However, our data did not show any difference between mean BMI of males and females.
An interesting finding is the significantly higher prevalence of obesity in our COPD population compared to the prevalence of obesity in general (Dutch) population; 21.8% and 13.7% respectively (p < 0.01) (Citation6). We did not have a control group of non-COPD individuals to compare the rates, nevertheless our finding confirms the results of some earlier studies (Citation11–14,Citation17). When evaluating prevalence rates it is possible that we relatively find more obese individuals in a COPD population, simply because they live longer than non-obese COPD patients. Whether COPD is a risk factor for developing obesity or that obese patients are more prone for developing COPD remains unclear and needs to be studied prospectively.
Comorbidities in obese and non-obese COPD
Hypertension, cancer, diabetes mellitus and CAD were the most common comorbidities in our COPD cohort. This is in line with previous data (Citation17,Citation24,Citation44–46). COPDOB had significantly more comorbidities compared to COPDNO. Also the pattern of comorbidities differed between obese and non-obese COPD patients.
In COPDOB hypertension and diabetes mellitus were the most prevalent comorbidities. The prevalence of both diseases was significantly higher in COPDOB compared with COPDNO. Also other cardiovascular comorbidities like atrial fibrillation/flutter and congestive heart failure were significantly more prevalent in COPDOB. Surprisingly, CAD was not more common in COPDOB compared to COPDNO (21.3% vs 17.7% respectively; p = 0.13). However in further analyses we found that CAD was significantly more prevalent in patients with BMI ≥25.0 kg/m2 compared with patients with BMI <25.0 kg/m2 (21.1% vs 15.5% respectively; p < 0.01). Various studies have demonstrated that cardiovascular comorbidities, including hypertension, often co-exist with COPD and are associated with increasing BMI, age and smoking (Citation44,Citation47–50). Inflammation might play a role in the development of cardiovascular diseases amongst COPD patients, however the exact mechanism remains unclear. Factors which might contribute in the development of diabetes mellitus in COPD patients are systemic inflammation and use of corticosteroids, especially systemic corticosteroids (Citation51,Citation52). However in this study we found no differences in the use of inhaled corticosteroids (ICS) between COPDOB and COPDNO (% of patients using ICS: COPDOB 63.4%; COPDNO 65.6; p = 0.5). Osteoporosis and lung cancer were significantly less prevalent in COPDOB compared to COPDNO.
In daily practice, underweight is one of the main focus points in COPD care. In part, this is due to the clear negative effects on important endpoints like mortality. Our study indicates that obesity is much more common in COPD than underweight. Therefore, physicians should be more aware of the common coexistence of COPD and obesity. Furthermore obese COPD patients seem to have more and different comorbidities compared to non-obese COPD. Hypertension and diabetes mellitus are the most common comorbidities in obese COPD patients. These treatable conditions can be easily diagnosed. Thus diagnosing these conditions should be a point of focus. Early referral for diagnosing the most common comorbidities may benefit the quality of life and reduce mortality. Whether, and if so, how to treat obesity in COPD patients remains a question that needs to be evaluated in the future.
Limitations and future studies
Some limitations of our study need to be addressed. We evaluated data from a single center. In order to generalize these prevalence rates, a multicenter study is more appropriate. Due to the cross-sectional nature of this study, potential causal links cannot be established. Future prospective studies with follow up of patients are needed to investigate causal links. Furthermore, the prevalence rate of obesity and comorbidities seems to be strongly depending on the demographic characteristics of the population. These figures might differ in other parts of the world and cannot be generalized to all COPD patients. Also it must be kept in mind that our COPD population represented a sample of patients being treated at a secondary care hospital. We did not have a control group of non-COPD individuals to compare the rates, nevertheless our finding confirms the results of some earlier studies. Obesity was only assessed by BMI in this study. Other adiposity measures may have added value, however BMI seems to be a measure which is as clinically important and accurate compared to other measures (Citation53,Citation54). The severity of COPD was based on the latest PFT. Although it is the policy within our department to only perform a PFT when patients are stable (have no exacerbation), we cannot rule out that some PFT’s were performed during an exacerbation, thus overestimating the severity of COPD.
Conclusion
Obesity is common in patients with COPD and is most prevalent in COPD GOLD I–II and least prevalent in COPD GOLD IV. Obese COPD patients have different comorbidities than non-obese COPD patients. Cardiovascular and metabolic comorbidities, especially hypertension and diabetes mellitus, are more prevalent in obese COPD patients. Recognition and active screening of these comorbidities, should be a priority for clinicians treating obese COPD patients.
Disclosure statement
This work was supported by an unrestricted grant from GlaxoSmithKline. The funding agency had no involvement in study design, data collection, data analysis, interpretation of data, or writing of the report.
References
- Franssen FME, O’Donnell DE, Goossens GH, Blaak EE, Schols AMWJ. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63(12):1110–7.
- Koniski ML, Salhi H, Lahlou A, Rashid N, El Hasnaoui A. Distribution of body mass index among subjects with COPD in the Middle East and North Africa region: data from the BREATHE study. Int J Chron Obstruct Pulmon Dis. 2015;10(1):1685–94.
- Liu Y, Pleasants RA, Croft JB, Lugigo N, Ohar J, Heidari K, et al. Body mass index, respiratory conditions, asthma, and chronic obstructive pulmonary disease. Respir Med. 2015;109(7):851–9.
- Behrens G, Matthews CE, Moore SC, Hollenbeck AR, Leitzmann MF. Body size and physical activity in relation to incidence of chronic obstructive pulmonary disease. CMAJ. 2014;186(12):E457–69.
- WHO. Obesity and Overweight Fact Sheet. Geneva (Switzerland): WHO; 2015.
- Brink CL van den (RIVM) BA (RIVM). How many people are overweight? Volksgezond Toekomst Verkenning, Natl Kompas Volksgezond Bilthoven RIVM, Dutch. 2016.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Gold Guid. 2016:1–94.
- López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–32.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet (London, England). 2011;378(9793):815–25.
- Eisner MD, Blanc PD, Sidney S, Yelin ED, Lathon PV, Katz PP, Tolstykh I, Ackerson L, Iribarren C. Body composition and functional limitation in COPD. Respir Res. 2007;8:7.
- Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–24.
- Montes de Oca M, Tálamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–50.
- Steuten LMG, Creutzberg EC, Vrijhoef HJM, Wouters EF. COPD as a multicomponent disease: Inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91.
- Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122(4):1256–1263.
- O’Donnell DE, Deesomchok A, Lam YMM, Guenette JA, Amornputtisathaporn N, Forkert L, Webb KA. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140(2):461–468.
- Vanfleteren LEGW, Spruit MA, Groenen M, Gaffron S, van Empel VPM, Bruijnzeel PLB, Rutten EP, Op 't Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–35.
- Divo MJ, Casanova C, Marin JM, et al. COPD comorbidities network. Eur Respir J. 2015;46(3):640–50.
- Cleutjens F, Triest F, Wilke S, Vanfleteren LEGW, Franssen FME, Janssen DJA, Rutten EP, Spruit MA, Wouters EF. New insights in chronic obstructive pulmonary disease and comorbidity. Am J Respir Crit Care Med. 2015;191(9):1081–2.
- Barr RG, Celli BR, Mannino DM, Petty T, Rennard SI, Sciurba FC, Stoller JK, Thomashow BM, Turino GM. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–55.
- Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–75.
- Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–62.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95–109.
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–9.
- Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163(12):1475–80.
- Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991–1999. COPD. 2005;2(1):35–41.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–107.
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, Poirier P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25(4):226–32.
- Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–11.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl 16):5–40.
- ATS, ERS. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
- Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61.
- Jones RCM, Dickson-Spillmann M, Mather MJC, Marks D, Shackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir Res. 2008;9:62.
- Walters JA, Haydn Walters E, Nelson M, Robinson A, Scott J, Turner P, Wood-Baker R. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J. 2011;20(4):396–402.
- Short ME, Goetzel RZ, Pei X, Tabrizi MJ, Ozminkowski RJ, Gibson TB, Dejoy DM, Wilson MG. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786–96.
- Austin EJ, Deary IJ, Gibson GJ, McGregor MJ, Dent JB. Individual response spread in self-report scales: personality correlations and consequences. Pers Individ Dif. 1998;24(3):421–438.
- Fan X. An exploratory study about inaccuracy and invalidity in Adolescent Self-Report Surveys. Field Methods. 2006;18(3):223–244.
- Torén K, Olin A-C, Lindberg A, et al. Vital capacity and COPD: the Swedish CArdioPulmonary bioImage Study (SCAPIS). Int J Chron Obstruct Pulmon Dis. 2016;11:927–33.
- Barros ARG de, Pires MB, Raposo NMF. Importance of slow vital capacity in the detection of airway obstruction. J Bras Pneumol. 2013;39(3):317–22.
- Nathell L, Nathell M, Malmberg P, Larsson K. COPD diagnosis related to different guidelines and spirometry techniques. Respir Res. 2007;8:89.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–61.
- Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639.
- Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70.
- Antonelli Incalzi R, Fuso L, De Rosa M, Forastiere F, Rapiti E, Nardecchia B, Pistelli R. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J. 1997;10(12):2794–800.
- Mihalache A, Fitting JW, Nicod LP. Chronic obstructive pulmonary disease and its links with cardiovascular risk factors. Rev Médicale Suisse. 2015;11(495):2151–2, 2154–6.
- Rossi FF, Pedone C, Antonelli Incalzi R. Chronic obstructive pulmonary disease and cardiovascular disease: role of the systemic inflammation. Recenti Prog Med. 2011;102(3):109–13.
- Alonso JLI. Chronic obstructive pulmonary disease and cardiovascular disease. Arch Bronconeumol. 2010;46 Suppl 3:18–22.
- Divo MJ, Cabrera C, Casanova C, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1(2):229–238.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370(9589):797–799.
- Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–55.
- Ortega FB, Sui X, Lavie CJ, Blair SN. Body mass index, the most widely used but also widely criticized index: would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2016;91(4):443–55.
- Chittal P, Babu AS, Lavie CJ. Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease? COPD J Chronic Obstr Pulm Dis. 2015;12(1):14–18.