Abstract
Background: Chronic obstructive pulmonary disease (COPD) has been associated with an increased risk of type 2 diabetes (T2D). However, the mechanisms linking COPD and T2D is not fully understood and contradicting results are reported in the literature.
Aim: The aim of this study is to investigate whether COPD is associated with an increased risk of T2D.
Methods: A systematic review and meta-analysis of cohort and case-control studies were performed. Search for studies and data extraction was carried out by two authors independently. Study quality was assessed by NOS. Adjusted data were pooled using the random effects model to calculate summary odds ratios (ORs) with corresponding 95% confidence intervals (CIs).
Results: We identified four cohort studies and three case-control studies with a total of 1,369,560 participants of whom 42,716 were COPD patients. The quality of the studies was acceptable, with an average on 7.7 indicating overall good study quality. The meta-analysis on adjusted data from all seven studies showed that the COPD group had a higher risk of T2D compared with the non-COPD group: random effect OR = 1.17 (1.01–1.35), p = 0.03. No heterogeneity was found I2 = 0%. When including only studies diagnosing both COPD and T2D according to recommended guidelines the association did not remain statistically significant, OR =1.17 (0.96–1.42), p = 0.12.
Conclusion: This systemic review and meta-analyses showed that the association between COPD and T2D might be influenced by the diagnostic method and should be further investigated in studies using diagnostic definition according to guidelines. Nevertheless, physicians should be aware of comorbidities in COPD patients.
Keywords:
Introduction
Definition of COPD
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease characterised by chronic airway obstruction that interferes with normal breathing (Citation1). The cardinal symptoms of COPD are shortness of breath and cough with or without sputum. The COPD diagnosis is based on a spirometry test with an irreversible obstructive pattern and the presence of symptoms (Citation2, Citation3). In the majority of patients COPD is caused by inhalation of pollutants – particularly long-term tobacco smoking (Citation4), but also by chemical fumes or dust encountered in patients’ work environment (Citation5, Citation6).
Burden of COPD
A systematic review and meta-analysis from 2015 showed that the global prevalence of COPD was increased from 10.7% in 1990 to 11.7% in 2010 (7). COPD was the fifth leading cause of death in 2002 (Citation8), but has been estimated to become the third leading cause of death by 2030 (Citation8). COPD is associated with both impaired physical and mental functioning (Citation9). COPD poses a great burden in terms of mortality and healthcare-related expenses. A report from the World Economic Forum in 2011 showed that the global economic cost of COPD was estimated to be 2.1 trillion US dollars. The expected increase in the number of patients with COPD will double the expenses by 2030 (Citation10), and this might be explained by the prevalence of comorbidities in COPD-patients (Citation11, Citation12).
Comorbidities in COPD
COPD is associated with other systemic, metabolic and comorbid diseases, including cardiovascular diseases (Citation13), osteoporosis (Citation14), skeletal muscle dysfunction, lung cancer and type 2 diabetes (T2D) (Citation15, Citation16). These associations may be due to common underlying factors (Citation17, Citation18) such as ageing, smoking, physical inactivity and genetics. Exposure to toxic substances such as tobacco smoke and inhaled pollutants induces an ongoing inflammatory response in the lungs, which accumulate neutrophil granulocytes, macrophages and lymphocytes. The consequences of long-term smoking are well-known and it has been suggested that the association between COPD and these comorbidities can be explained by an induced effect from the lungs that causes pulmonary and systemic low-grade inflammation (Citation19).
Definition of T2D
Type 2 diabetes is a chronic disease (Citation20) characterised by ineffective use or production of insulin and a relative or absolute insulin secretory defect resulting in elevated blood glucose (Citation21). Uncontrolled blood glucose concentration in diabetes is a major factor leading to serious vascular complications (Citation20). According to current guidelines, the diagnosis should be based on a blood measurement of glycated haemoglobin (HbA1c) above 48 mmol/mol (6.5%) (Citation21).
Burden of T2D
Obesity and physical inactivity are known to be the most important risk factors that drive the development of T2D, which has evolved into a major, global disease that affects more than 451 million people worldwide (both T1D and T2D) in the age of 18–99 and is projected to affect 693 million people by 2045 with huge social costs (Citation22, Citation23). The increase in diabetic patients within the last decades has occurred in all countries and in rural as well as urban areas (Citation23). In 2015, the number of deaths due to diabetes was estimated to be around 5 million (Citation23, Citation24) and by the increasing risk of developing cardiovascular and other diseases, T2D is estimated to be the seventh leading cause of death in 2030 (Citation25). Due to the serious effects on health, diabetes also imposes an economic burden on individuals and healthcare systems (Citation26). These financial costs are described in the report by The International Diabetes Federation (IDF) from 2017, who found that the global healthcare expenditures due to diabetes for people aged between 20 and 79 years was estimated to 673 billion USD in 2015. This number is expected to increase to 802 billion USD by 2040 (Citation23).
Systemic inflammation in COPD and T2D
The inflammatory state characterised by elevated biomarkers such as C-reactive protein (CRP), tumor necrosis factor (TNF) and interleukin 6 (IL-6) observed in COPD patients may be a mediator of subsequent development of type 2 diabetes (T2D) (Citation22, Citation27). It is well described that the same inflammatory cytokines related to low-grade systemic inflammation are elevated in both T2D and COPD. Even though COPD and T2D are different clinical, immunological and pathophysiological diseases, they still possibly share underlying etiological mechanisms that can explain the relationship between these diseases. Previous studies have reported contradicting results on the association between COPD and T2D (Citation19, Citation28–31), and might be explained by heterogeneous methodology. The current literature is in lack of a sufficient number of homogeneous studies in terms of methodology. A previous systematic review (Citation20) has discussed possible explanations for the association between T2D and COPD. However, no previous meta-analyses have been conducted to examine the association. Thus, the aim of this study was to systematically review the literature and perform meta-analyses to investigate whether COPD is associated with an increased risk of T2D.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines, and quality assessment of the included studies was done according to the Newcastle–Ottawa Scale (NOS) (Citation32, Citation33).
Data source and search strategy
Two biomedical databases, PUBMED and EMBASE were used in our search for published studies from inception to December 7, 2017, using MeSH terms for “chronic obstructive pulmonary disease” combined with MeSH terms for “type 2 diabetes mellitus.” The detailed search strategy is provided as Supporting Information Appendix A.
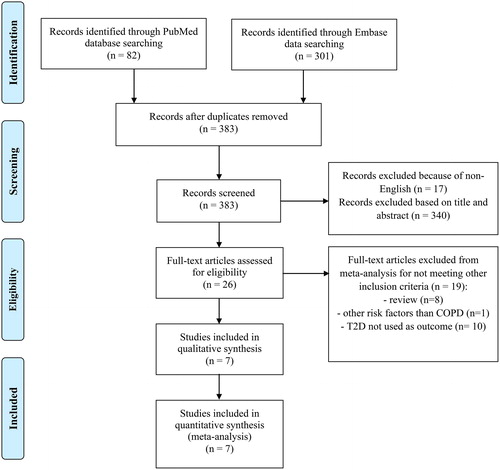
The search was subsequently supplemented by a manual screening of references of the selected and included papers. Searches were limited to human studies reported in English, Danish, Swedish or Norwegian. In addition, well-known studies that were unexpectedly found in the PUBMED and EMBASE search were manually included. An updated literature search was performed prior to submission (December 2017) to ensure that all significant new studies would be included in the meta-analyses. A study flow chart is shown in .
Study eligibility, inclusion and exclusion criteria
Studies were selected if they fulfilled our inclusion criteria: 1) RCTs, case–control, cohort, and cross-sectional with quantitative outcomes assessing the association between COPD and T2D; 2) human studies; 3) studies reporting results as odds ratios (OR), relative-risk (RR) or hazard ratio (HR) with 95% confidence intervals (CIs) or available data enabling calculations of the above; 4) full text in English, Danish, Swedish or Norwegian; and 5) hospital-diagnoses (the diagnosis given by a physician when admitted or discharged from the hospital) or self-reported physician diagnoses (patient confirming that the diagnosis was given by a physician) of COPD and T2D.
The following types of manuscripts were excluded: 1) Editorials, comments, qualitative studies, annual reports, systematic reviews; 2) studies with other than humans (e.g. animals); 3) studies reporting other than odds ratio (OR), relative risk (RR) or hazard ratio (HR); 4) any other diagnoses than hospital-diagnosed or self-reported physician; 5) full text in languages other than English, Danish, Norwegian, Swedish; 6) All eligibility inclusion and exclusion criteria are listed in .
Table 1. Full eligibility criteria used in the search strategy.
Selection process
The selection of included studies was conducted initially by reviewing titles and abstracts, which resulted in 26 potential studies for inclusion (SMR; ). A second reviewer (HM) repeated this process independently of the first reviewer (SMR). The first (SMR) and last (HM) author settled any disagreements before any studies were included in or excluded from the systematic review.
Data extraction
The following information from each study was extracted: first author’s name, year of publication, country of study, study design, ascertainment of COPD, ascertainment of T2D, number of participants/patients in both the non-COPD group and the COPD group, mean age, mean BMI, sex and quality assessment score. All information from the included studies is shown in .
Table 2. Characteristics of the studies included in the meta-analysis.
Quality assessment of included studies
The NOS (Citation32) was used to evaluate the study quality by classifying it into three domains: selection, comparability and exposure or outcome for included case-control studies and included cohort studies. Each study was assigned from 0 to 9 stars to judge its quality. A study was awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars were awarded for comparability. The total number of stars determines the quality of the included study. Scores from 0 to 3 stars indicate poor study quality, 4 to 6 stars indicate acceptable study quality and 7 to 9 stars indicate good study quality. Any disagreements on quality assessment between the first (SMR) and last (HM) author were discussed until a consensus was reached. Studies awarded from 0 to 3 stars were excluded from the meta-analysis due to poor study quality.
Statistical analysis
Data were pooled using the software programme RevMan to calculate summary adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs). A Mantel–Haenszel analysis with random effect model was performed and forest plots were graphically displayed. To strengthen the liability of the results, the meta-analyses was based on adjusted data by conducting a generic inverse variance outcome analysis using RevMan (Citation34). In this method, the study estimates and calculated standard error for each study are entered in the software. The weight given to each study is the inverse of the variance of the effect estimate. This method gives larger studies more weight than smaller studies and thereby reduces the uncertainty of the pooled effect result.
In addition, we conducted several subgroup analyses; 1) comparing cohort and case-control studies, 2) comparing studies using COPD diagnoses according to current recommendations and studies using questionnaire-based COPD diagnoses, 3) studies using diagnostic methods according to guidelines for both COPD and T2D. If any results were significant, we estimated the difference between the estimates of the subgroups according to tests of interaction. A p-value <0.05 indicates a significant difference between the subgroups (Citation35). To examine both clinical and statistical heterogeneity, the I2 statistic was used. The risk of publication bias was assessed graphically by means of a funnel plot (Citation36). Sensitivity analyses were performed to by excluding one study at a time to see if there were any significant changes in the estimated results in the forest plot. The sensitivity analyses were made using the software programme Comprehensive Meta-Analysis. Technical details on the software programme used in this article are found in Supporting Information Appendix B.
Results
Through database searches we identified 383 articles (). After exclusion of nonrelevant studies, we identified 26 potentially relevant studies. After reading the full text of these studies, 19 studies were excluded, as they did not meet the eligibility criteria (). Excluded studies were either reviews (n = 8), assessed other risk factors than COPD (n = 1), or T2D was not a reported outcome (n = 10). Subsequently, 7 studies (Citation19, Citation28–31, Citation37, Citation38) were included for the meta-analyses (or included in the review; ).
Characteristics of included studies
All relevant characteristics of the included studies in the meta-analysis are shown in . The quality assessment of included studies did not result in exclusion of any studies due to poor quality. Quality scores ranged from 7 to 9. The included studies were either case-control (n = 3) or cohort (n = 4) studies with a total number of 1,369,560 individuals and published between 2004 and 2015. They included data from different populations, that is, Europeans (Citation19, Citation29, Citation37), Americans (Citation30, Citation31) and Asians (Citation28, Citation38).
The included cohort studies are both prospective (n = 2) and retrospective (n = 2) studies with COPD patients (n = 12,112) and non-COPD patients (n = 137,188), with a total of 149,300 patients. Case-control studies (n = 3) included a total of 1,220,260 patients distributed as COPD (n = 30,604 patients) and non-COPD (n = 1,189,656 patients).
Most studies (Citation19, Citation28, Citation29, Citation37, Citation38) used guidelines or recommendations from BTS, ATS/ERS, ICD-10 codes or GOLD to ascertain COPD. Rana et al. (Citation30) used a questionnaire for the assessment of respiratory status, followed by spirometry to confirm the diagnosis according to GOLD recommdations. One study (Citation31) used a questionnaire-based definition for the ascertainment of COPD. Determination of T2D was defined as either prescription for glucose-lowering medicine as oral hypoglycemic agents or insulin (Citation37, Citation38) or fasting blood glucose >7.0 mmol/L (Citation38). Rana et al. (Citation30) used a questionnaire-based definition of T2D or symptoms combined with fasting glucose >7.8 mmol/L or random glucose >11.1 mmol/L. Two studies (Citation29, Citation31) used a questionnaire survey to ascertain T2D.
Association between COPD and T2D
The association between COPD and T2D is illustrated in a Forest-plot in .
Figure 2. Forest-plot showing the association between COPD and T2D. Generic inverse variance outcome analysis with random effect on outcome of all included studies with adjusted data.
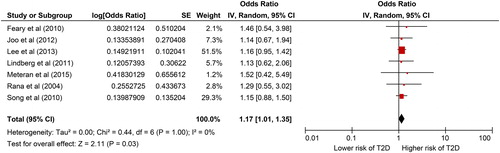
Results from a meta-analysis including all seven studies using adjusted data showed that the COPD group had a higher risk of T2D compared with the non-COPD group: random effect OR =1.17 (1.01–1.35), p = 0.03. The I2 was low =0%.
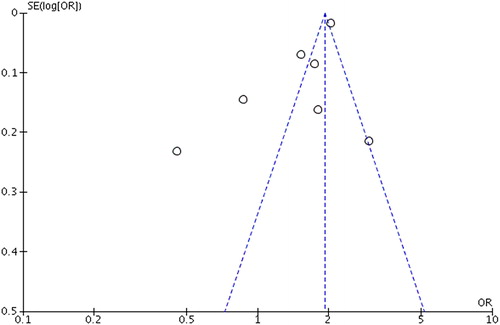
Visual inspection of the funnel plot did not indicate bias () and statistical tests were not applied as there were only few (<10) studies included. Results from the sensitivity analysis (figure not included) showed that the risk of T2D was not significantly affected by the exclusion of any of the seven included studies.
Figure 3. Funnel plot of standard error by log odds ratio of all included studies in the meta-analysis.
A subgroup analysis comparing studies diagnosing COPD according to current recommendations (Citation19, Citation28, Citation29, Citation37, Citation38) and studies using questionnaire-based diagnoses (Citation30, Citation31) () showed no significant association between COPD and T2D in neither group.
Figure 4. Forest plot showing the association between COPD and T2D. Generic inverse variance outcome analysis with random effect on the outcome of subgroup analysis comparing studies using COPD diagnosis according to guidelines versus studies with COPD diagnosis based on questionnaire.
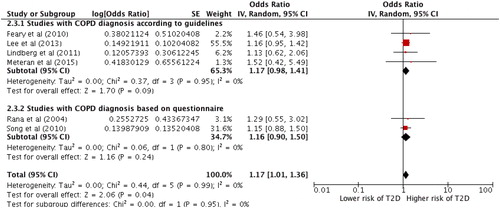
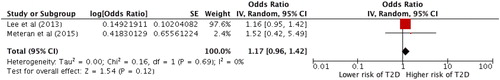
Another subgroup analysis including only studies using diagnostic methods for both COPD and T2D according to recommended guidelines showed an increased risk of T2D in patients with COPD, however, the result was not statistically significant, random effect OR =1.17 (0.96–1.42), p = 0.12 ().
Figure 5. Forest plot showing the association between COPD and T2D. Generic inverse variance outcome analysis on studies using diagnostic methods according to guidelines for both COPD and T2D.
Lastly, a subgroup analysis separating cohort studies (Citation28–31) and case-control studies (Citation19, Citation37, Citation38) did not find any association between COPD and T2D (figure not included).
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to investigate whether COPD is associated with an increased risk of T2D. The overall result from the seven included studies on adjusted data showed that patients with COPD have a 17% increased risk of T2D compared to patients without COPD. A sensitivity analysis showed that the pooled odds ratio was not significantly altered when excluding any of the examined studies, which indicates a robust analysis. The increased risk of COPD in T2D was also observed in subgroup analyses comparing studies using different methodology to diagnose COPD and T2D, however, the association did not remain statistically significant.
Strengths and limitations
The major strength of this study is the systematic review and meta-analyses conducted according to international guidelines plus the very large sample size allowing us to quantitatively evaluate the association between COPD and T2D. The validity of the main meta-analysis was tested by subgroup analyses comparing case-control and cohort studies. Further, the association between COPD and T2D was also examined comparing studies using different methods to diagnose COPD and T2D. In addition, this review has adhered to a very thorough and broad search strategy only excluding non-English and non-Scandinavian studies. The included studies represent individuals from an extended geographical area, and the included seven studies were published within the last 13 years. Finally, the results seem robust as any of the sensitivity analysis did not change the overall result.
Nevertheless, this study has limitations. The total number of included studies is relatively small with a low level of heterogeneity (I2=0%). As a rule of thumb, tests for funnel plot asymmetry according to the Cochrane Handbook for Systematic Reviews of Interventions, should be used only, when there are at least 10 studies included in the meta-analysis, because when there are fewer studies, the power of the tests is too low to distinguish chance from real asymmetry (Citation39).
The use of self-reported diseases in research has been met with skepticism. The use of self-reported diabetes seems to be reasonably accurate (Citation40) and for COPD in a selected population (health professionals). However, some of the authors of this study, have recently shown that the overlap between self-reported COPD and clinical COPD (according to ERS/ATS recommendations) was lower than one third in a large population-based study (Citation41). These possible misclassifications may have had an impact on the overall outcome of this meta-analysis and might explain the difference between the outcome in studies using questionnaire-based diagnoses and studies using diagnoses according to guidelines.
We used The NOS for quality assessment (Citation32). The quality assessment of the included studies was acceptable, with an average of 7.7, indicating good overall study quality when rated based on the NOS. Nonetheless, there are no official guidelines for distinguishing between high and low ratings of study quality.
Association between COPD and T2D
Chronic obstructive pulmonary disease has been associated with many comorbid diseases (Citation13–16, Citation42). Among these comorbid diseases, type 2 diabetes is suggested as being much more frequent in patients with COPD compared with non-COPD patients (Citation43). The underlying mechanism that links these two diseases is still not fully established, but there are some possible explanations in the literature. One suggests that, as in many other chronic medical conditions, local and systemic inflammation may play a key role. Both COPD and T2D are associated with systemic inflammation indicated by increased levels of e.g. CRP (Citation44) and cytokines such as TNF, IL-6, IL-1β (Citation45, Citation46), and ongoing systematic inflammation can lead to oxidative stress with an increased risk of developing subsequent diseases.
Oxidative stress is another potential factor that is suggested to contribute to the association between COPD and T2D. Oxidative stress reflects a disturbance between the production of reactive oxygen species (free radicals) and antioxidant defences. Studies suggest that increased oxidative stress can be a risk factor for the onset of T2D (Citation47). In addition, most patients with COPD are known to have hypoxia, which is also associated with an excessive oxidative state (Citation48), which may result in dysfunctional beta-cells in pancreas causing T2D, but this is only shown in studies with focus on obstructive sleep apnea (Citation49). Even though an association between COPD and T2D may exist, these conclusions are based on biomarkers and there could be several other pathological pathways of which we are not yet aware.
Another suggested risk factor to explain the association between COPD and T2D is the use of corticosteroids in the medical treatment of COPD (Citation50, Citation51). The treatment of COPD involves lifestyle modifications as well as various pharmacotherapies (Citation52). Suissa et al. conducted a population-based cohort study to examine whether the use and dosage of inhaled corticosteroids (ICS) were associated with an increased risk of developing diabetes in COPD patients. They found a 34% increased risk of diabetes onset with ICS and a 64% increased risk with high-dose ICS (fluticasone-equivalent doses ≥1,000 μg/day) (Citation53). In addition, studies have shown that patients with both COPD and T2D and who are prescribed with ICS have a significantly higher increase in HbA1c values compared with those who have been prescribed non-ICS therapies (Citation54). Furthermore, patients with COPD and comorbid T2D displayed a 94% increase in diabetes-related hospitalisation when treated with a total corticosteroid-defined daily dose of ≥0.83/day. Lower corticosteroid dosages (DDD <0.83/day) were not associated with an increased risk of diabetes-related hospitalisation (Citation55).
Lifestyle factors have also been suggested as common risk factors for both diseases. While most studies have adjusted for smoking, few, if any, studies have adjusted for intake of alcohol and physical activity, but used BMI as an indicator of health status. It is well known that COPD patients are markedly inactive compared to healthy elderly individuals (Citation47). While the association between BMI and COPD is not fully elucidated, a high BMI is associated with an increased risk of T2D (Citation19).
Another plausible explanation for the comorbidity between COPD and T2D is genetic factors. Specifically, it has been shown that CRP levels in COPD patients are controlled by genetic factors (Citation56). Meteran et al. (Citation20) examined the relationship between COPD and T2D and showed that genetic factors can explain 43% of the relationship between COPD and T2D. These results did not reach statistical significance, but this was most likely due to a small sample size.
A larger study with twin pairs discordant for T2D will shed light on the role of genetic pleiotropy.
Ascertainment of COPD and T2D using current recommandations versus questionnaires
In the main finding of these meta-analyses we have distinguished between studies using questionnaire-based diagnoses and studies using diagnostic methods according to guidelines for both COPD and T2D. A Subgroup analysis comparing studies using COPD diagnosis according to current recommendations versus studies with COPD diagnosis based on questionnaire did not reach statistical significance. The results indicate that the diagnostic methods used to diagnose both COPD and T2D might influence on the possible association between COPD and T2D.
The most ideal analysis for assessing association between COPD and T2D will include studies using objective measurements in the diagnostic methods according to guidelines for both COPD and T2D. With these criteria our meta-analysis are left with only two comparable studies, where no statistical significance was found.
Conclusion
In conclusion, the meta-analysis based on adjusted data from seven studies showed that patients with COPD have a 17% increased risk of T2D compared with non-COPD patients. However, the association did not remain statistically significant in a subgroup analysis including only studies using diagnostic methods for both COPD and T2D according to guidelines. Based on the current studies, the association between COPD and T2D seems to be influenced by the diagnostic tool used in the respective studies. We were only able to identify two studies diagnosing COPD and T2D according to recommended guidelines. Therefore, further studies using guideline-recommended disease definitions for both disease will bring new insight.
Declaration of Interest
The authors report no conflicts of interest.
| Abbreviations | ||
| ATS | = | American Thoracic Society |
| BTS | = | British Thoracic Society |
| CI | = | Confidence intervals |
| COPD | = | Chronic obstructive pulmonary disease |
| CRP | = | C-reactive protein |
| ERS | = | European Respiratory Society |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| HR | = | Hazard ratio |
| ICD | = | International Classification of Diseases |
| IL-6 | = | Interleukin 6 |
| IL-1β | = | Interleukin 1 beta |
| NOS | = | Newcastle–Ottawa Scale |
| OR | = | Odds ratio |
| PRISMA-P | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RR | = | 6-minute walking test |
| TNF | = | Tumor necrosis factor |
| T2D | = | Type 2 diabetes |
References
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2017. https://goldcopd.org.
- van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J. 2010;19(4):326–334.
- Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375(9):871–878.
- Omland O, Wurtz ET, Aasen TB, et al. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scand J Work Environ Health. 2014;40(1):19–35. doi:10.5271/sjweh.3400.
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Global Health. 2015;5(2):020415.
- World Health Organization. Burden of COPD. http://www.who.int/respiratory/copd/burden/en/. Accessed September 20, 2018.
- Rodríguez-González Moro JM, Izquierdo IJ, Antón E, de Lucas P, Martín A; MUVICE Study Group. Health-related quality of life in outpatient women with COPD in daily practice: the MUVICE Spanish study. Respir Med. 2009:103(9):1303–1312.
- Bloom DE, Cafiero ET, Jané-Llopis E, et al. The Global Economic Burden of Non-communicable Diseases. Geneva: World Economic Forum. A report by the World Economic Forum and the Harvard School of Public Health; 2011.
- Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest 2015;148(1):138–150.
- McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007;62(5):411–415.
- Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities Study): a cohort study. Lancet 1999;353(9165):1649–1652.
- Bolton CE, Ionescu AA, Shiels KM, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(12):1286–1293.
- Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD 2008;5(4):235–256. doi:10.1080/15412550802237531.
- Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–S40.
- Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi:10.1183/09031936.03.00405703.
- Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007;370(9589):797–799.
- Meteran H, Backer V, Kyvik KO, Skytthe A, Thomsen SF. Comorbidity between chronic obstructive pulmonary disease and type 2 diabetes: a nation-wide cohort twin study. Respir Med. 2015;109(8):1026–1030.
- Glaser S, Kruger S, Merkel M, Bramlage P, Herth FJ. Chronic obstructive pulmonary disease and diabetes mellitus: a systematic review of the literature. Respiration 2015;89(3):253–264. doi:10.1159/000369863.
- Roglic G; World Health Organization. Global Report on Diabetes. Geneva, Switzerland: World Health Organization; 2016.
- Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003;52(7):1799–1805. doi:10.2337/diabetes.52.7.1799.
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. PharmacoEconomics 2015;33(8):811–831.
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi:10.1164/ajrccm.153.2.8564092.
- Lee CT, Mao IC, Lin CH, Lin SH, Hsieh MC. Chronic obstructive pulmonary disease: a risk factor for type 2 diabetes: a nationwide population-based study. Eur J Clin Invest. 2013;43(11):1113–1119.
- Lindberg A, Larsson LG, Ronmark E, Lundback B. Co-morbidity in mild-to-moderate COPD: comparison to normal and restrictive lung function. COPD 2011;8(6):421–428.
- Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27(10):2478–2484. doi:10.2337/diacare.27.10.2478.
- Song Y, Klevak A, Manson JE, Buring JE, Liu S. Asthma, chronic obstructive pulmonary disease, and type 2 diabetes in the Women's Health Study. Diabetes Res Clin Pract. 2010;90(3):365–371. doi:10.1016/j.diabres.2010.09.010.
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxford.htm, Published 2009. Accessed September 20, 2018.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
- Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions version 5.1.0: The Cochrane Collaboration. http://handbook.cochrane.org/, Published 2011; Updated March 2011. Accessed September 20, 2018.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–634.
- Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 2010;65(11):956–962. doi:10.1136/thx.2009.128082.
- Joo H, Park J, Lee SD, Oh YM. Comorbidities of chronic obstructive pulmonary disease in Koreans: a population-based study. J Korean Med Sci. 2012;27(8):901–906. doi:10.3346/jkms.2012.27.8.901.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0: The Cochrane Collaboration. Published 2011; Updated March 2011. http://handbook.cochrane.org/.
- Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56(2):148–154.
- Meteran H, Miller MR, Thomsen SF, Christensen K, Sigsgaard T, Backer V. The impact of different spirometric definitions on the prevalence of airway obstruction and their association with respiratory symptoms. ERJ Open Res. 2017;3(4).
- Sode BF, Dahl M, Nordestgaard BG. Myocardial infarction and other co-morbidities in patients with chronic obstructive pulmonary disease: a Danish nationwide study of 7.4 million individuals. Eur Heart J. 2011;32(19):2365–2375. doi:10.1093/eurheartj/ehr338.
- Cazzola M, Bettoncelli G, Sessa E, Cricelli C, Biscione G. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2010;80(2):112–119.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286(3):327–334.
- Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52(3):812–817. doi:10.2337/diabetes.52.3.812.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59(7):574–80. doi:10.1136/thx.2003.019588.
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622.
- MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):50–60. doi:10.1513/pats.200411-056SF.
- Pallayova M, Lazurova I, Donic V. Hypoxic damage to pancreatic beta cells--the hidden link between sleep apnea and diabetes. Med Hypotheses. 2011;77(5):930–934.
- Spies CM, Strehl C, van der Goes MC, Bijlsma JWJ, Buttgereit F. Glucocorticoids. Best Pract Res Clin Rheumatol. 2011;25(6):891–900. doi:10.1016/j.berh.2011.11.002.
- Herth FJ, Bramlage P, Muller-Wieland D. Current perspectives on the contribution of inhaled corticosteroids to an increased risk for diabetes onset and progression in patients with chronic obstructive pulmonary disease. Respiration 2015;89(1):66–75. doi:10.1159/000368371.
- Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life Sci. 2003;72(14):1549–1561.
- Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001–1006.
- Price DB, Russell R, Mares R, et al. Metabolic effects associated with ICS in patients with COPD and comorbid type 2 diabetes: a historical matched cohort study. PLoS One. 2016;11(9):e0162903. doi:10.1371/journal.pone.0162903.
- Slatore CG, Bryson CL, Au DH. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med. 2009;122(5):472–428. doi:10.1016/j.amjmed.2008.09.048.
- Hersh CP, Miller DT, Kwiatkowski DJ, Silverman EK. Genetic determinants of C-reactive protein in COPD. Eur Respir J. 2006; 28(6):1156–1162.