Abstract
Chronic obstructive pulmonary disease (COPD) is a common multisystem inflammatory disease with ramifications involving essentially all organ systems. Pulmonary rehabilitation is a comprehensive program designed to prevent and mitigate these disparate systemic effects and improve patient quality of life, functional status, and social functioning. Although initial patient assessment is a prominent component of any pulmonary rehabilitation (PR) program, cardiopulmonary exercise testing (CPET) is not regularly performed as a screening physiologic test prior to PR in COPD patients. Further, CPET is not often used to assess or document the improvement in exercise capacity related to completion of PR. In this review we will describe the classic physiologic abnormalities related to COPD on CPET parameters, the role of CPET in Risk Stratification/Safety prior to PR, the physiologic changes that occur in CPET parameters with PR, and the literature regarding the use of CPET to assess PR results. Finally, we will compare CPET to 6MW in COPD PR, the common minimal clinically important difference (MCID) is associated with CPET, and the potential future roles of CPET in PR and Research.
COPD is a multisystem inflammatory disease
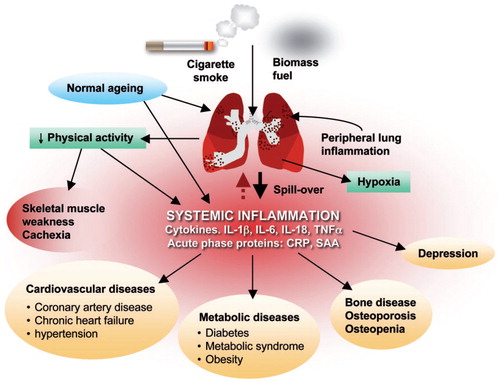
According to the Center for Disease Control (CDC), COPD is now the third leading cause of death in the United States with 15.7 million individuals that have been diagnosed with COPD. Cigarette smoking causes inflammatory changes in all systems of the body, resulting in a spectrum of cardiovascular and lung diseases, metabolic diseases such as diabetes and metabolic syndrome, osteoporosis, depression, reduced physical activity, and skeletal muscle weakness/cachexia. displays the pleotropic effects of cigarette smoke, as well as the longer-term manifestations in COPD.
Figure 1 Patients with COPD have lung inflammation that affects all organs of the body. Reproduced with permission from Boschetto et al. (Citation38) (License Number: 4467500227437, License date Nov 14, 2018. Licensed Content Publisher: John Wiley and Sons. Licensed Content Publication: Respirology)
The American Thoracic Society (ATS) and the European Respiratory Society (ERS) have adopted the following definition of Pulmonary Rehabilitation (PR):
“Pulmonary Rehabilitation is a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors” (Citation1).
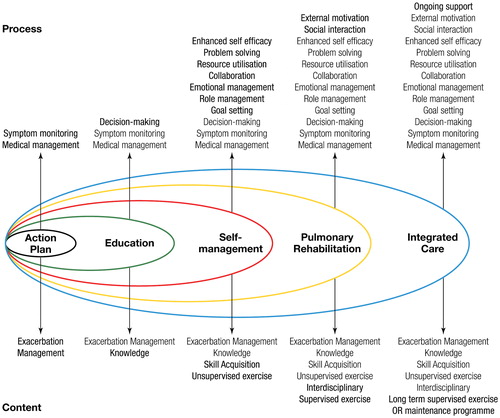
PR is one of many interventions targeted to improve the lives of patients with COPD, including education, self-management, smoking cessation, and integrated care ().
Figure 2 The spectrum of support available for patients with COPD. Reproduced with permission from Spruit et al. (Citation1). Reprinted with permission of the American Thoracic Society. Copyright© 2018 American Thoracic Society (Citation1). The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
What does the physiology of COPD exercise limitation look like in CPET?
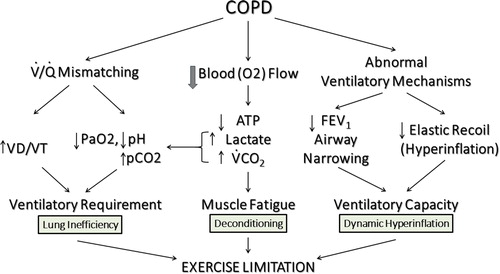
What pathophysiologic mechanisms limit exercise in patients with COPD as assessed by CPET? A normal sized adult walking at a moderate pace on a flat surface requires approximately 1 L/min of oxygen uptake. At this walking rate there is very little lactate generation, dyspnea, or muscle fatigue (Citation2), and an individual is able to sustain this activity nearly without limitation. However, in a patient with COPD () there are three important pathophysiologic processes
Figure 3 Pathophysiology of exercise impairment in COPD. The three most important mechanisms of exercise limitation in COPD are (1) Gas Exchange Inefficiency, (2) Muscle Deconditioning and (3) Dynamic Hyperinflation. Adapted from Wasserman et al. (Citation54)
gas exchange inefficiency of the lung,
deconditioning of the muscle, and
dynamic hyperinflation.
With regards to gas exchange inefficiency, COPD patients display various combinations of hypoxia, hypercapnia, acidosis, and mismatching the ventilation to perfusion (VA/Q) with increased dead space to tidal volume ratio (VD/VT), all resulting in increased ventilatory requirements. The COPD patient is also prone to muscle fatigue, has diminished blood flow, reduced aerobic ATP generation, excess lactic acidosis, and additional CO2 load from bicarbonate buffering of the excess lactate. Finally, the process of dynamic hyperinflation causes increased work of breathing, high airway resistances, airflow obstruction, increased dead space ventilation, and decreased elastic recoil all reducing the ability of the COPD patient to deliver and meet the required ventilatory capacity for the work rate. Therefore, the combination of inefficient gas exchange, muscle deconditioning, and progressive failure of the ventilatory pump attributable to dynamic hyperinflation produces marked exercise limitation and progressive dyspnea during exercise (Citation3).
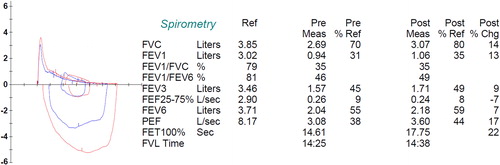
Bearing these important pathophysiological processes in mind, let’s examine a typical individual CPET response to exercise in a COPD patient (age 57, height 174 cm, weight 73.6 km, BMI 24.4) with severe obstruction in resting spirometry ().
Figure 4 Pre- and Post-Bronchodilator spirometry of a patient with severe COPD. Pre/Post Bronchodilator responses indicating severe (0.94 L, 30% of predicted) reduction in FEV1 and a very low FEV1/FVC (35%). There is a bronchodilator response that meets ATS/ERS criteria (>12% and 200 mL in either FEV1 or FVC). Further lung function results were: The total lung capacity (TLC) is normal (6.57 L or 96% predicted) and the diffusing capacity for carbon monoxide (DLco) is 107 mL/min/mmHg or 37% of predicted (not shown). Reference values for spirometry were according to Hankinson (Citation55), for the lung volumes were according to the ECSC (Citation56) and for the diffusion capacity by Miller et al. (Citation57). Reprinted with permission of the American Thoracic Society. Copyright© 2018 American Thoracic Society (Citation57). American Review of Respiratory Disease is an official journal of the American Thoracic Society.
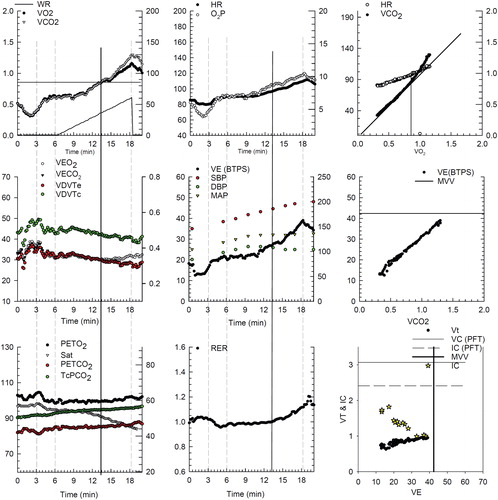
The CPET results for this patient are graphically shown in . The patient performed a 5 W/min ramp pattern exercise on a cycle ergometer to the limit of tolerance. The patient has a reduced peak oxygen uptake (∼1.1 L/min) and severely reduced exercise capacity (60 w of work on a cycle ergometer). His peak ventilation is 38.5 L/min and his predicted MVV from PFTs is ∼42 L/min. Therefore, he reaches a very high percentage (>90%) of his predicted peak ventilation during exercise and his breathing reserve is well less than 14 L/min, all suggesting ventilatory limitation. Further, the inspiratory capacity falls during exercise consistent with dynamic hyperinflation. There is significant oxygen desaturation, the ventilatory equivalents for oxygen and carbon dioxide are elevated (∼32) and the patient has baseline hypercapnia that worsens with exercise, again suggesting severe ventilatory limitation. Using transcutaneously determined arterialized capillary pCO2 to estimate VD/VT, it is well above 0.3 throughout exercise (∼0.42). The lactic acidosis threshold, O2 pulse, and heart rate response suggest that the exercise is not cardiovascular limited. The blood pressure response to exercise is slightly exuberate at peak exercise (200/100 mmHg).
Figure 5 Progressive ramp-incremental test on the cycle ergometer on a patient with severe COPD and dynamic hyperinflation. Panel 1 (top left), is oxygen uptake (VO2), carbon dioxide output (VCO2), and work rate as a function of time (3 min of rest, 3 min of unloaded cycling, and 12 min of exercise with 5 W/min increments, followed by 2 min of recovery). Panel 2 (top middle), is heart rate and oxygen pulse (O2P) as a function of time. Panel 3 (top right), is heart rate and VCO2 as a function of VO2. This panel is used to identify the lactic acidosis threshold using the V-slope method (Citation58) (in this example, approximately 0.85 L/min). Panel 4 (middle left), ventilatory equivalents for oxygen (VE/VO2) and carbon dioxide (VE/VCO2) as well as the VD/VT (calculated using the transcutaneously determined PaCO2) are displayed. The left y axis is the ventilatory equivalents and the right y axis is the transcutaneous VD/VT (VDVTc, green filled circles). VD/VT is markedly elevated throughout exercise with a nadir of 0.44. In panel 5 (center graph), the ventilation and blood pressures are displayed. The patient had an end-exercise blood pressure of 200/100 mmHg. In panel 6 (middle, right), ventilation as a function of VCO2 is displayed. The VE/VCO2 slope is approximately 26 L/min. Panel 7 (bottom left) displays the end tidal oxygen (PETO2), carbon dioxide (PETCO2), the oxygen saturation (Sat), and the transcutaneous pCO2 (TcPCO2). End tidal and transcutaneous CO2 are markedly elevated in this graph (56 and 48 mmHg, respectively). Oxygen saturation was progressively decreasing during exercise to a nadir of ∼85%. Panel 8 (bottom center), is the respiratory exchange ratio (RER) as a function of time. Panel 9 (bottom right), displays inspiratory capacity (golden stars) falling as a function of increasing ventilation. As such respiratory rate increases to almost 40 at end exercise with a tidal volume that does not exceed 1 liter. Finally, the exercise ventilation is essentially the MVV from 40 X FEV1.
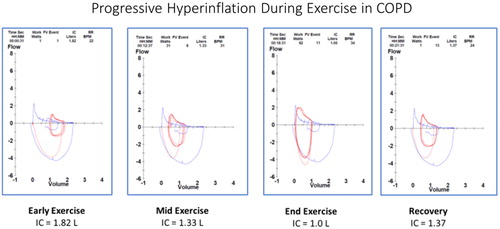
Comparing the flow volume loops during exercise () in the same COPD patient as during progressive exercise, we see that at rest severe airflow obstruction is present (blue outline showing the resting flow volume loop). However, the superimposed tidal breathing (red lines) early in exercise are at a slightly increased Functional Reserve Capacity (FRC). As exercise progresses, the tidal breathing loops move progressively towards Total Lung Capacity (TLC) and end-expiratory lung volume (EELV) increases resulting in progressive loss of inspiratory capacity. These changes all suggest dynamic hyperinflation as a significant contributor to exercise limitation in this patient. Interventions that reduce dynamic hyperinflation, reduce ventilatory requirements, improve gas exchange, and/or reduce muscle fatigue/deconditioning should improve the functional status of this patient. All of these physiologic goals are addressed in pulmonary rehabilitation (PR) (Citation4). It has been shown that a substantial increase in exercise endurance is associated with a decrease in dynamic hyperinflation as a response to dynamic exercise training (Citation5).
Figure 6 Dynamic hyperinflation during progressive exercise in a patient with COPD. This patient is the same patient as and . Panel 1 (early exercise) The flow volume loop (red line) compared to the resting flow volume loop (blue line). Total lung capacity (TLC) is on the left, residual volume (RV) is on the right. Panel 2 (mid exercise) The flow volume loops have moved to the left (towards TLC) consistent with dynamic hyperinflation. Panel 3 (peak exercise) The entire flow volume loop is shifted leftward with a marked reduction in expiratory flow compared to inspiratory flow. Of note, both the peak spontaneous expiratory and inspiratory flow rates at peak exercise approach that were obtained pre-exercise during the resting spirometry. All of these changes begin to resolve in early recovery (panel 4).
Analysis of morphological changes of the flow volume loops during exercise in COPD
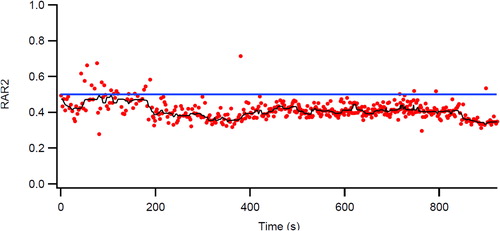
Porszasz et al. have invented an ingenious method for analyzing the flow volume wave forms during cardio-pulmonary exercise testing (Citation6, Citation7). This method involves assessing the rectangular area ratio (RAR) of each breath during a CPET. For a rectangular box, a line from the top left to the bottom right would yield an area ratio of 0.5 on each side of the line. A person without flow limitation has an RAR of about 0.6 at rest and during exercise this value approximates 0.8, whereas those with progressive airflow limitation, this ratio falls very often below 0.5, indicating that the expiratory flow-volume curve became concave. The phenomenon has been shown to be associated with dynamic hyperinflation (Citation7) and that this improves with bronchodilation therapy; at the same time, exercise endurance improves (Citation8). In (same patient as ) the RAR is below 0.5 even during rest, suggesting that the expiratory flow/volume curve is concave. More importantly, during exercise RAR falls further below 0.4 and stays very low even during recovery. RAR values have been shown to be responsive to pharmacologic bronchodilator therapy by improving RAR during constant work rate exercise, presumably by reducing dynamic hyperinflation (Citation8).
Figure 7 Breath-by-breath representation of the dynamic changes in the rectangular area ratio (RAR) during exercise. At the start of ramp pattern exercise in the same patient as in , the RAR value is consistently <0.5 even at rest. However, as exercise progresses it falls below 0.4, indicating severe airflow obstruction that is associated with dynamic hyperinflation (decreased inspiratory capacity and increasing end expiratory lung volumes, EELV) (compare with and ).
What is the role of CPET in COPD pulmonary rehabilitation?
Detailed physiologic assessment
Moving from an individual CPET response in COPD to the role of CPET in PR, clinicians must identify where CPET adds important clinical information to the patient assessment at the start and end of PR. PR should be considered in a COPD patient experiencing marked symptoms of dyspnea, exercise limitation, reduction in activities of daily living/inactivity, reduced quality of life, recent COPD exacerbation, deconditioning, increased healthcare utilization, or loss of lean body mass/cachexia (Citation1, Citation9–20). PR assessment is a comprehensive examination of a broad range of domains, including physical functioning, psychological, pharmaceutical, pulmonary function, dietary, psychosocial, smoking status, and current social functioning.
This review will focus on the physiologic assessment of the patient using exercise testing (CPET) pre/post PR. Traditionally, most PR programs have used the six-minute walk (6MW) as the physiologic assessment of choice (Citation21, Citation22). In fact, 6MW distance at the start of PR does predict subsequent mortality (Citation23), however, the average change in 6MW distance pre/post PR is only 43.9 m according to a recent Cochrane review (Citation24).
Although 6MW gives a very limited assessment of the exercise performance pre/post exercise testing, CPET is much more comprehensive with respect to interrogating the cardiac (heart rate, peak oxygen uptake, lactic acidosis threshold, oxygen pulse, 12 lead ECG), pulmonary (ventilation as compared to MVV, inspiratory capacity, pulmonary gas exchange, saturation, dynamic hyperinflation, flow volume loops), muscle (power, lactic acidosis), perception of dyspnea (BORG Scores for shortness of breath and leg discomfort), and overall motivational function during exercise. CPET also allows for the objective evaluation of the integration of these important functions.
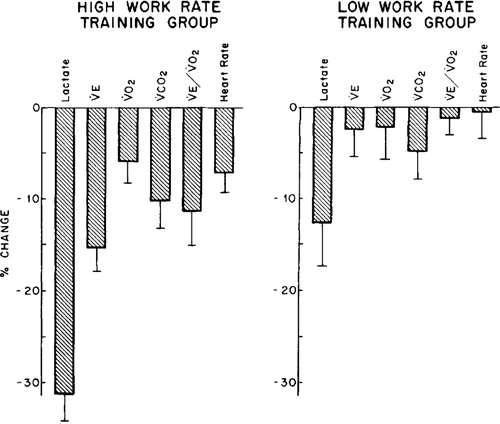
When CPET has been carefully studied in research settings pre/post rehabilitation (Citation25) the authors found that high intensity interval cycle ergometer training for 6 weeks resulted in marked reductions in lactate values (31%), minute ventilation (15%), ventilatory equivalent for oxygen (12%), heart rate (8%), oxygen uptake (6%), and carbon dioxide output (11%) at the same exercise intensity. In addition, these variable changes were even more marked when high intensity exercise was compared to lower intensity PR training ().
Figure 8 Changes in physiologic responses to identical exercise tasks induced by two different intensity training strategies in patients with COPD (high and low work rate training). Original Legend to Figure: Changes in physiologic responses to an identical exercise task (high constant work rate test0 produced by two exercise training strategies in patients with COPD. Left panel. High work rate training group (n = 11) Right panel. Low work rate training group (n = 8). Reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society (Citation25). American Review of Respiratory Disease is an official journal of the American Thoracic Society.
The changes observed in oxygen uptake, work rate, ventilation, ventilatory equivalents, heart rate, and VD/VT are displayed in . In general, using ramp pattern exercise to assess physiological changes pre/post PR, the oxygen uptake, work rate, and ventilation increase by about 10 to 36% with a slightly less increase in heart rate (6%). Comparing isotime values on ramp exercise pre/post PR (all at 40 W), there is indistinguishable change in oxygen uptake, however ventilation, ventilatory equivalents, heart rate and VD/VT all decrease after PR. During constant work rate testing (all at 32 W, ∼80% of peak exercise) pre/post PR identical oxygen uptakes are observed, however ventilation, ventilatory equivalents, heart rate and VD/VT all decrease post PR.
In a follow up study by Casaburi, et al. regarding CPET and PR (Citation47) designed to look at high intensity exercise training (6 weeks of PR) on progressively increasing cycle ergometry exercise and constant work rate cycle exercise in patients with severe airflow obstruction (FEV1 ∼1 L or ∼35% predicted), identical work rates (40 W on the ramp, 32 W for the constant work rate), the VD/VT (using pCO2 from arterialized capillary blood in calculation) decreased from 0.54 ± 0.007 pre PR to 0.47 ± 0.007 post PR on the incremental test and from 0.53 ± 0.007 to 0.46 ± 0.007 on the constant work rate testing, a 13% change. This change in VD/VT was likely related to a slower respiratory rate (33 ± 7 vs 27 ± 6) and a larger tidal volume pattern (0.99 ± 0.30 vs 1.10 ± 0.31) as well as improvements in pulmonary blood flow (less Zone I lung from dynamic hyperinflation) (Citation26). All of these changes improve pulmonary gas exchange during exercise.
Table 1. CPET variables on cycle ergometry that change with careful study of pulmonary rehabilitation (PR) data from Casaburi (Citation25, Citation47) and Emtner (Citation27).
Oxygen supplementation in general allowed a greater increase in training work rate, a better increase in maximal exercise tolerance and more pronounced changes in physiological variables with a more dramatic change in exercise endurance tested at the same work rate pre- and post-training (Citation27). Interestingly, in the same study, there was a dramatic improvement in dynamic hyperinflation (Citation5).
A recent review by Puente-Maestu et al. evaluating the interventional efficacy of various exercise tests, demonstrated that ramp type CPETs in COPD patients post PR demonstrated a modest increase in peak VO2 of 0.1 to 0.5 L/min, or about 10–40% of baseline, with a mean improvement of ∼11%. The mean change in peak work rate in watts (W) was 6.8 W from PR, and that the MCID for peak work rate was 4 W (Citation28).
In the same review, when constant work rate exercise testing (CWRET) based upon the initial ramp pattern CPET was evaluated pre/post rehabilitation at intensities of 75–80% of incremental peak work rate (WRpeak), the improvements in the limit of tolerance (tlim, 10 studies of PR) ranges from 100 to 700 s, with a mean increase of ∼350 s or ∼100% increase from the pre PR baseline. Obviously, this change on the constant work rate cycle test is much more dramatic than peak VO2 or peak WR from a ramp pattern test. Puente-Maestu also demonstrates that PR is a more beneficial nonpharmacological intervention in COPD patients than Heliox, Oxygen, or Non-Invasive Ventilation during exercise, and that the mean MCID thresholds for CWRET is an improvement of 105 s or 33% from baseline pre-PR (Citation28).
Cardiopulmonary exercise testing and pulmonary rehabilitation: safety, risk stratification, and exercise intensity
In their clinical therapeutics article, Casaburi and ZuWallack (Citation4) stated the following regarding evaluation of the potential patient for PR prior to enrollment: “stress test for cardiovascular evaluation, perhaps in the form of a cardiopulmonary exercise test, should be performed.” (Citation4) CPET is preferred compared to PFTs and 6MWT as the degree of organ dysfunction assessment across multiple domains is superior. Current guidelines recommend (Citation29) CPET as it “provides valuable information before exercise training to determine safety and to optimize training intensity.” (Citation18, Citation30) Guidelines and thought leaders in COPD PR strongly recommend a complete physiologic assessment of the patient pre/post PR using CPET for safety reasons alone, separate from the substantial and important physiologic information.
What is the physiologic effect of exercise training on exercise capacity as assessed by CPET?
Limb muscle dysfunction is an important systemic consequence of COPD and PR can have an important positive effect on muscle biology (). Exercise rehabilitation in a COPD Pulmonary Rehabilitation program improves muscle function in the exercising limb as well as the morphology of the muscle (Citation11, Citation31–33).
Figure 9 Morphological and structural alterations reported in limb muscles in patient with COPD. Reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society. Maltais et al. (Citation11).
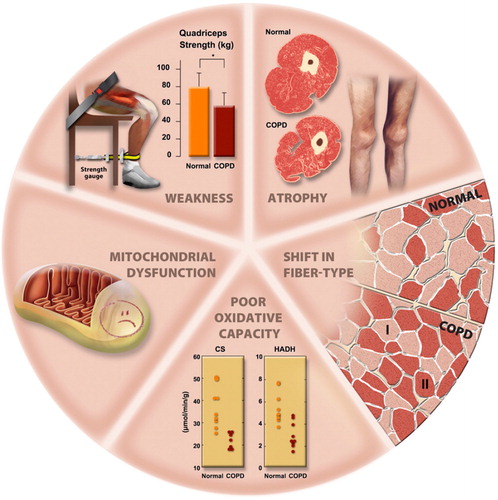
Specifically, with regards to the effect of PR on the exercising limbs with aerobic exercise training, muscle fibers can be divided into slow (S or Type I) and fast (F or Type II) types. Fast twitch fibers can be low (FF, high glycolytic) or high fatigue resistant (FR, high oxidative potential). PR causes Type IIb fibers (fast twitch, glycolytic, low oxidative potential, FF) to undergo structural and biochemical changes to more closely mirror Type IIa fibers (fast twitch, glycolytic, higher capacity for oxidative metabolism, FR) (Citation34). Positive effects in Type I fibers (slow twitch, oxidative muscle fibers) are also observed (Citation11, Citation35). These changes combine to reduce the amount of lactic acid produced at any particular exercise intensity and allow more exercise to be performed prior to ventilatory limitation and dynamic hyperinflation.
The relative benefits and disadvantages of CPET (either cycle or treadmill) versus six minute walk (6MW) in PR are expressed in . Although 6MW is frequently used as the initial (and final) assessment of exercise tolerance in PR, it suffers from not having much physiologic data to isolate the specific organ system limiting exercise performance. In addition, the safety level of a patient exercising in a controlled laboratory (treadmill or cycle) is superior to a 6MW done in a corridor. Finally, CPET can provide additional data for customizing the PR program to the patient at hand. With regards to 6MW, it is advantageous in that it is easy to explain, does not require sophisticated equipment, and walking may be more real world than a cycle ergometer test. However, 6MW has no measurements beyond heart rate and pulse oximetry, just lately, the pulse oximetry is supported by devices that can record it, but unless these devices are being used, both heart rate measurement and pulse oximetry are done prior and after the test, or sporadically if the personnel reads off the display of the device. Unless the laboratory utilizes a portable gas exchange device (e.g., COSMED or OXYCON), there is no measurement of gas exchange, ventilation, EKG or inspiratory capacities to assess dynamic hyperinflation. Finally, provider supervision is optimal for CPET and is rarely provided for 6MW.
Table 2. CPET vs 6MW in COPD PR—pro/con table.
Many studies have focused on the minimal clinically important difference (MCID), i.e., how large an effect is required to be perceptible and important to the patient (Citation28, Citation36, Citation37). Some of the important values regarding physiologic testing, 6MW, and CPET are summarized in . These assessments/measurements/physiologic testing should be considered important patient centered outcomes in the design of any PR intervention.
Table 3. Minimal clinically important differences, MCID for CPET and PR
Future role of CPET in COPD PR and research
What does the future hold for PR and CPET? The authors envision more PR programs routinely adopting CPET since it is a more sophisticated, comprehensive, and definitive method to physiologically assess the patient prior to and after PR. 6MWT is not sensitive to the important physiologic changes that occur with PR, nor can it detect safety issues such as ischemia, arrhythmias, or desaturation, all common to smokers with heart and lung disease prior to an intensive PR program (Citation38). It is important to acknowledge that to a certain extent, CPET and the 6MWT may complement each other and both can meaningfully inform PR programs for the benefit of patients. However, we must emphasize that the 6MWT is self-paced, therefore is not a maximal test and because of the very restricted instrumentation, it does not provide information about physiological changes usually characteristic for the effect of exercise training that is part of PR. Rather, other than being informative about the habitual walking intensity, it does not provide specific information about the physiological mechanisms limiting exercise.
With regards to research, the important questions regarding CPET and PR involve the mechanisms of improvement in functional capacity (lungs, muscles, motivation, etc.) when pulmonary function remains essentially unchanged pre/post PR. If these mechanisms of exercise improvement can be illuminated, they can potentially be augmented and specifically targeted for improvement, either through combining PR with pharmacologic therapy, or by intensifying the effect of PR during the program to achieve improved outcomes from the exercise performed (Citation12, Citation39–42). Indeed, as regenerative medicine moves forward (Citation43–46), CPET is the ideal assessment mechanism to document physiologic improvement (including the organ system(s) responsible) in COPD patients undergoing exercise therapy (PR) after regenerative interventions.
Conflict of interest
Dr. Stringer reports grants from AstraZeneca, Boehringer-Ingelheim, Astellas, other support from Allergan-Syneos, Wolters Kluwer - Lippincott Williams & Wilkins, all outside the submitted work.
Dr. Marciniuk reports grants from Boehringer-Ingelheim, GlaxoSmithKline, Lung Association of Saskatchewan, Novartis, AstraZeneca, Canada Health Infoway, Canadian Institutes of Health Research, Schering-Plough; personal fees and other from Canadian Foundation for Healthcare Improvement, other from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Saskatchewan Health Authority, Health Canada, University of Saskatchewan, all outside the submitted work.
References
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–64.
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema KE, Sun XG, Whipp BJ. Principles of exercise testing and interpretation: including pathophysiology and clinical applications. 5th ed. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011, 572 p.
- Butcher SJ, Lagerquist O, Marciniuk DD, Petersen SR, Collins DF, Jones RL. Relationship between ventilatory constraint and muscle fatigue during exercise in COPD. Eur Respir J. 2009;33:763–770 doi: 10.1183/09031936.00014708.
- Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med 2009;360:1329–1335 doi: 10.1056/NEJMct0804632.
- Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest 2005;128:2025–2034
- Ma S, Hecht A, Varga J, Rambod M, Morford S, Goto S, Casaburi R, Porszasz J. Breath-by-breath quantification of progressive airflow limitation during exercise in COPD: a new method. Respir Med 2010;104:389–396 doi: 10.1016/j.rmed.2009.10.014.
- Varga J, Casaburi R, Ma S, Hecht A, Hsia D, Somfay A, Porszasz J. Relation of concavity in the expiratory flow-volume loop to dynamic hyperinflation during exercise in COPD. Respir Physiol Neurobiol 2016;234:79–84
- Porszasz J, Carraro N, Cao R, Gore A, Ma S, Jiang T, Maltais F, Ferguson GT, O’Donnell DE, Shaikh A. Effect of tiotropium on spontaneous expiratory flow–volume curves during exercise in GOLD 1-2 COPD. Resp Physiol Neurobiol 2018;251:8–15 doi: 10.1016/j.resp.2018.02.006.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012 doi: 10.1056/NEJMoa021322.
- Garvey C, Bayles MP, Hamm LF, Hill K, Holland A, Limberg TM, Spruit MA. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36:75–83 doi: 10.1097/HCR.0000000000000171.
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–62
- Marciniuk DD, Brooks D, Butcher S, Debigare R, Dechman G, Ford G, Pepin V, Reid D, Sheel AW, Stickland MK, et al. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease–practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J 2010;17:159–168 doi: 10.1155/2010/425975.
- Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, Ford G, Bourbeau J, O'Donnell DE, Maltais F, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18:69–78 doi: 10.1155/2011/745047.
- Moore E, Newson R, Joshi M, Palmer T, Rothnie KJ, Singh S, Majeed A, Soljak M, Quint JK. Effects of pulmonary rehabilitation on exacerbation number and severity in people with COPD: an historical cohort study using electronic health records. Chest 2017;152:1188–1202
- Moore E, Palmer T, Newson R, Majeed A, Quint JK, Soljak MA. Pulmonary rehabilitation as a mechanism to reduce hospitalizations for acute exacerbations of COPD: a systematic review and meta-analysis. Chest 2016;150:837–859
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413 doi: 10.1164/rccm.200508-1211ST.
- Rehabilitation BTSSoCSoP. Pulmonary rehabilitation. Thorax 2001;56:827–834
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007;131:4S–42S
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192:1373–1386 doi: 10.1164/rccm.201510-1966ST.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38 doi: 10.1164/rccm.200408-1109SO.
- Laboratories ACoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117
- Salzman SH. The 6-min walk test: clinical and research role, technique, coding, and reimbursement. Chest 2009;135:1345–1352
- Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 2007;132:1778–1785
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;4:CD003793
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18
- Rossi A, Aisanov Z, Avdeev S, Di Maria G, Donner CF, Izquierdo JL, Roche N, Similowski T, Watz H, Worth H, et al. Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respir Med. 2015;109:785–802
- Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168:1034–1042 doi: 10.1164/rccm.200212-1525OC.
- Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, O'Donnell DE, Onorati P, Porszasz J, Rabinovich R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47:429–460
- ATS/ACCP. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277
- Lareau SC, ZuWallack R, Carlin B, Celli B, Fahy B, Gosselink R, Jones P, Larson JL, Meek P, Rochester C, et al. Pulmonary rehabilitation-1999. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:1666–1682
- Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Bérubé C, Carrier G, Maltais F. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:896–901 doi: 10.1164/ajrccm.159.3.9807034.
- O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157:1489–1497 doi: 10.1164/ajrccm.157.5.9708010.
- Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–212 doi: 10.1016/S0002-9343(00)00472-1.
- Andersen P, Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977;99:123–125
- Maltais F, LeBlanc P, Jobin J, Bérubé C, Bruneau J, Carrier L, Breton MJ, Falardeau G, Belleau R. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:555–561 doi: 10.1164/ajrccm.155.2.9032194.
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255 doi: 10.1164/rccm.201310-1863PP.
- Man-Son-Hing M, Laupacis A, O'Rourke K, Molnar FJ, Mahon J, Chan KB, Wells G. Determination of the clinical importance of study results. J Gen Intern Med. 2002;17:469–476
- Boschetto P, Beghé B, Fabbri LM, Ceconi C. Link between chronic obstructive pulmonary disease and coronary artery disease: implication for clinical practice. Respirology 2012;17:422–431 doi: 10.1111/j.1440-1843.2011.02118.x.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005;127:809–817
- Chavoshan B, Fournier M, Lewis MI, Porszasz J, Storer TW, Da X, Rambod M, Casaburi R. Testosterone and resistance training effects on muscle nitric oxide synthase isoforms in COPD men. Respir Med. 2012;106:269–275 doi: 10.1016/j.rmed.2011.07.018.
- Evans RA, Dolmage TE, Mangovski-Alzamora S, Romano J, O'Brien L, Brooks D, Goldstein RS. One-legged cycle training for chronic obstructive pulmonary disease. A pragmatic study of implementation to pulmonary rehabilitation. Ann Am Thorac Soc. 2015;12:1490–1497 doi: 10.1513/AnnalsATS.201504-231OC.
- Marciniuk DD, Butcher SJ, Reid JK, MacDonald GF, Eves ND, Clemens R, Jones RL. The effects of helium-hyperoxia on 6-min walking distance in COPD: a randomized, controlled trial. Chest 2007;131:1659–1665
- Geiger S, Hirsch D, Hermann FG. Cell therapy for lung disease. Eur Respir Rev. 2017;26:170044
- Oh DK, Kim YS, Oh YM. Lung regeneration therapy for chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2017;80:1–10
- Sun Z, Li F, Zhou X, Chung KF, Wang W, Wang J. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis. 2018;10:1084–1098 doi: 10.21037/jtd.2018.01.46.
- Wecht S, Rojas M. Mesenchymal stem cells in the treatment of chronic lung disease. Respirology 2016;21:1366–1375
- Casaburi R, Porszasz J, Burns MR, Carithers ER, Chang RS, Cooper CB. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:1541–1551 doi: 10.1164/ajrccm.155.5.9154855.
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2:111–124
- Mahler DA, Witek TJ. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005;2:99–103
- Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, Borg scale, and visual analog scale. COPD 2005;2:105–110
- Jones PW. St. George's respiratory questionnaire: MCID. COPD 2005;2:75–79
- Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F, Group NETTNR. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784–790 doi: 10.1183/09031936.00063810.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Rev Port Pneumol. 2008;14:579–583 doi: 10.1016/S0873-2159(15)30266-X.
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Sietsema K, S. X-G, Whipp BJ. Principles of exercise testing and interpretation: pathophysiology and clinical applications. Baltimore, Maryland: Lippincott Williams & Wilkins; 2012
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187
- Castellsague J, Burgos F, Sunyer J, Barbera JA, Roca J. Prediction equations for forced spirometry from European origin populations. Barcelona Collaborative Group on Reference Values for Pulmonary Function Testing and the Spanish Group of the European Community Respiratory Health Survey. Respir Med. 1998;92:401–407 doi: 10.1016/S0954-6111(98)90282-7.
- Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127:270–277
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027

