Abstract
This article reviews the latest data pertaining to the Genuair®/Pressair® device, a breath-actuated, multi-dose dry-powder inhaler with a two-step inhalation process, which is marketed for the delivery of aclidinium alone or in combination with formoterol for long-term maintenance bronchodilation treatment in chronic obstructive pulmonary disease. It contains multiple feedback mechanisms to guide effective use, and safety features to avoid double-dosing or attempted use when empty. In addition to describing the design of Genuair®, the article will provide an update on inhaler operability and performance, focusing on recent patient preference and satisfaction studies. The evidence suggests that patients find Genuair® easy to use, with patients requiring less training and making fewer inhalation errors than with other inhalers, and that patient satisfaction with the device is high.
Introduction
Bronchodilators and inhaled corticosteroids are the cornerstone of chronic obstructive pulmonary disease (COPD) therapy, with inhalation being the preferred route of drug administration (Citation1). Inhalation allows therapeutic agents to be delivered directly to the airways, resulting in a high local concentration and low systemic concentration. Therefore, compared with systemic therapy, a more rapid therapeutic effect is observed, a lower dose is required and the potential for extrapulmonary side effects is reduced (Citation2). However, despite advances in device technology, inhalation device mishandling and poor inhaler technique remain common in patients with COPD (Citation3, Citation4). This can result in suboptimal dose delivery, and has consequently been shown to lead to reduced disease control, increased hospitalization and emergency room visits, and increased healthcare expenditure (Citation3, Citation4).
A wide variety of inhaler devices are available for delivering inhaled COPD medications, with pressurized metered-dose inhalers (pMDIs) and dry-powder inhalers (DPIs) the most commonly used (Citation5). pMDIs were first introduced more than 50 years ago and are still widely used today, as they are inexpensive, convenient and effective when used correctly. Device-handling errors may occur with conventional pMDIs due to poor hand-lung synchronization (Citation4), but these can generally be overcome using breath-actuated pMDIs or DPIs. A variety of single- and multi-dose DPIs are available, all of which are breath-actuated, thus eliminating the requirement for patient coordination of actuation with inhalation (). However, device-dependent critical errors can still occur, most commonly related to dose loading and preparation (Citation4). Additionally, patients must be able to generate a sufficient inspiratory flow rate to de-agglomerate the powder formulation into small respirable particles, which may be challenging for young children, the elderly and patients with severe airflow limitation or with poor inhalation technique (Citation6, Citation7). Therefore, in these patient populations, pMDIs may be the device of choice. Soft-mist inhalers represent a third type of pocket-sized inhaler and use liquid formulations similar to those in nebulizers; availability is currently limited to one device for use in COPD. It is important to note that device-independent errors, such as failure to breathe out before actuation, also occur in ∼50% of patients (Citation4). Therefore, regardless of the device used, it is vital that physicians provide appropriate guidance on correct inhaler use with regular follow up to ensure compliance (Citation1).
Table 1. Characteristics of available DPIs for delivering COPD medications (Citation16, Citation18, Citation44–49).
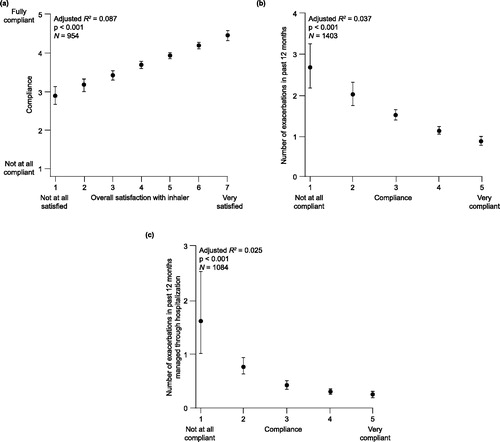
The most important consideration when tailoring COPD therapy to the individual patient is the choice of medication class, followed by the specific agent. However, as the inhalation device may impact both optimal drug delivery and patient adherence, the choice of inhaler is also an important consideration, as emphasized in the Global Initiative for Chronic Obstructive Lung Disease guideline (Citation8). There is a paucity of evidence comparing available inhalation devices, with the limited available data showing no clinically relevant differences in the efficacy and safety of devices in a clinical trial setting (Citation9–11). However, there are other parameters that influence the choice of inhaler, including patient age and ability to use the device correctly, manual dexterity, degree of breathlessness, cost and availability, dosing regimen, drug, administration time, and patient preference, acceptability and satisfaction (Citation2). In particular, patient preference, acceptability and satisfaction are strongly associated with therapeutic adherence in COPD () (Citation12). There is an association between poor adherence to COPD medication and worse clinical outcomes, including increased hospitalizations and mortality and reduced quality of life (Citation13).
Figure 1. Relationships between (a) patient-rated inhaler satisfaction and physician-assessed treatment compliance, and between compliance and (b) exacerbations, and (c) hospitalizations due to exacerbations in COPD patients (Citation12). COPD = chronic obstructive pulmonary disease. Reprinted from (Citation12) Copyright 2014, with permission from Elsevier.
This article will review available data pertaining to the Genuair® inhalation device. Genuair® is a breath-actuated, multi-dose DPI, with a two-step inhalation process. It contains multiple feedback mechanisms to guide effective and correct use, including correct inhalation and sufficient peak inspiratory flow (PIF), and safety features to avoid double dosing or attempted use when empty (Citation14). Thus, the Genuair® inhaler supports appropriate inhalation maneuvers and indicates common technique error. Genuair® is marketed in the European Union and other countries for delivery of the long-acting muscarinic antagonist aclidinium bromide, either alone (Eklira®/Bretaris®) or in combination with the long-acting beta-agonist, formoterol (Duaklir®/Brimica®) for long-term maintenance bronchodilator treatment in COPD (Citation15, Citation16). Moreover, it is marketed in the USA as Pressair® for the delivery of aclidinium bromide (Tudorza®) (Citation17), and Duaklir® was recently approved by the US Food & Drug Administration. In addition to describing the design of Genuair®, this article will provide an update on inhaler operability and performance, with a particular focus on recent patient preference and satisfaction studies.
Methodology
A search of PubMed was performed using the search term ‘Genuair’. The search was limited to English-language publications published between April 2008 and December 2018. The reference lists of articles identified using this search strategy were also searched for relevant publications. All articles were screened for suitability. The following additional search was performed to provide additional context and background to the Genuair® data: ‘(“COPD” OR “chronic obstructive pulmonary disease”) AND (“device” OR “inhaler”) AND (“handling” OR “preference” OR “acceptability” OR “satisfaction” OR “adherence”)’.
Device resistance, inhalation flow rate and lung deposition
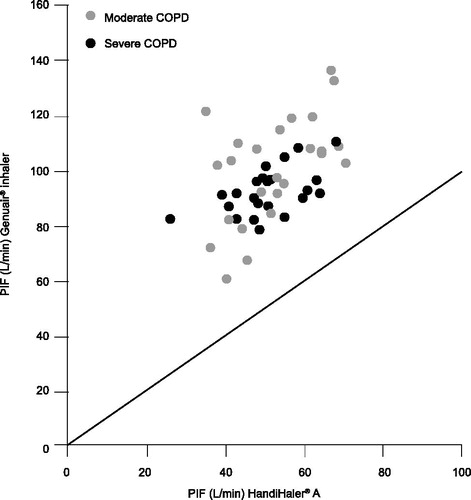
Genuair® is a medium-resistance device (Citation18, Citation19). In-vitro studies have shown that Genuair® delivers uniform, fine-particle doses of aclidinium bromide or aclidinium bromide/formoterol fumarate dihydrate throughout the life (Citation20), and shelf life (Citation21) of the inhaler. Fine-particle dose delivery is independent of flow rate in the therapeutic relevant range, as demonstrated by in-vitro studies within the typical working range of the device (35–95 L/min; technical test flow range as defined by in-vitro setup) (Citation22), and confirmed by in-vivo lung deposition data (Citation23). An open-label, randomized, crossover study assessing the inspiratory flow characteristics of Genuair® found that patients with moderate or severe COPD were able to generate sufficient inspiratory airflow through the Genuair® inhaler to inhale the full dose reliably and reset it ready for the next dose to be loaded (Citation19). This study also found that, in comparison with HandiHaler®, Genuair® has a lower flow resistance, meaning that a lower inspiratory effort is required to produce a comparable PIF ().
Figure 2. PIF through the Genuair® and HandiHaler® inhalers in patients with moderate or severe COPD (Citation19). Data points above the line reflect greater PIF through the Genuair® inhaler versus the HandiHaler®. COPD = chronic obstructive pulmonary disease; PIF = peak inspiratory flow. Reprinted from (Citation19) Copyright 2009, with permission from Elsevier.
An in-vitro comparison of the Genuair®, HandiHaler®, Respimat® and Breezhaler® devices demonstrated the highest airflow resistance with HandiHaler® and lowest with Respimat® and Breezhaler®. Genuair® delivered the highest number of optimal particles for inhalation (greatest sub-fractions of particles with mass median aerodynamic diameter 1–3 μm) at a medium flow rate, while Respimat® contained the highest sub-fractions of relatively coarse particles (3–6 μm) (Citation24). Particles with an aerodynamic diameter of ∼0.5–5 μm are likely to be deposited in the lower airways, whereas aerodynamic particles of >5 μm are more likely to impact in the oropharyngeal cavity (Citation25). The characterization of particle size delivered from Genuair®, based on these in-vitro studies, and its suitability for inhalation was confirmed by therapeutic efficacy demonstrated in phase III clinical trials of Eklira® and Duaklir® (Citation26–28).
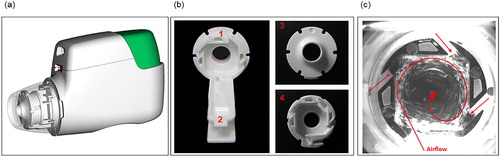
Lung deposition following administration via Genuair® was investigated in healthy subjects (Citation23). A single dose of aclidinium 200 μg, radiolabeled with 99mTc, was administered at a targeted PIF rate of 90 L/min in 12 healthy males (18–63 years old), quantifying drug deposition in the lungs and oropharynx, as well as amounts retained in the inhaler. The mean PIF rate (± standard deviation [SD]) was 79.0 ± 9.4 L/min, and the mean percentages of the metered dose deposited in the whole lung and oropharynx were 30.1 ± 7.3% and 54.7 ± 7.2%, respectively. Deposition of aclidinium occurred in all six lung zones, but was highest in the most central zone (Citation23). In addition, there was no correlation between whole lung deposition and PIF rate over a range of 66–99 L/min. This further demonstrates the flow-rate independency of fine particle delivery to the lungs, and may be due, in part, to the novel design of the cyclone unit (classifier technology) adjacent to the mouthpiece of the Genuair® device (). This unit is intended to aid particle de-agglomeration and efficiently generate and deliver fine particles by utilizing the inspiratory energy from the patient’s airflow (Citation23, Citation29).
Figure 3. Structure and mechanism of the cyclone unit of the Genuair® device. Effective particle deagglomeration and powder aerosol generation is provided by the cyclone unit; (a) shows the position of the cyclone unit inside the Genuair® mouthpiece (transparent view); (b) shows the cyclone base (b1), powder channel (b2), and front (b3) and back (b4) view of the cyclone unit; (c) shows particle activities in the cyclone after an inhalation time of ∼0.25 s.
Genuair® design and usability
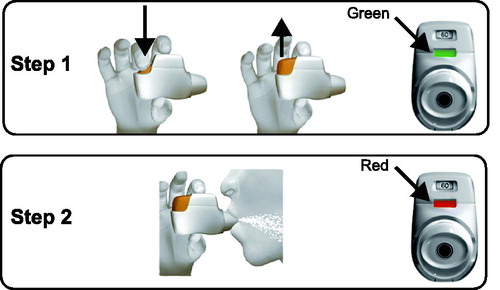
The Genuair® device is a breath-actuated, multi-dose DPI that is preloaded with 30 or 60 doses. It has a two-step inhalation process (), minimizing the potential for patient handling errors, and a combination of features that promote safety and ease of use () (Citation16, Citation30). These features include a safety mechanism to prevent the release of a second dose into the delivery inhalation channel, avoiding accidental inhalation of a double dose, and a lock-out mechanism to block the use of the device after the last dose has been dispensed (Citation16, Citation30). The device also includes optical and acoustic signals to guide effective medication uptake; a colored control window changes from red to green when the dose has been dispensed and is ready to be inhaled, and changes back to red, along with an audible click, when the dose has been inhaled correctly (Citation16, Citation30). Performance quality is reliably maintained in the event of common patient handling effects, such as dosing in different orientations, cleaning the mouthpiece before use, or after dropping the device (Citation20).
Figure 4. Genuair inhalation process (Citation16). Step 1: after removing the cap, the patient must hold the inhaler horizontally and press the button all the way down to load the dose. The control window will then change from red to green to indicate that the medicine is ready to be inhaled. The button can then be released. Step 2: after breathing out thoroughly, the patient must take a strong, deep breath through the mouthpiece to actuate the dose. The control window will change to red, indicating the medicine has been inhaled correctly.
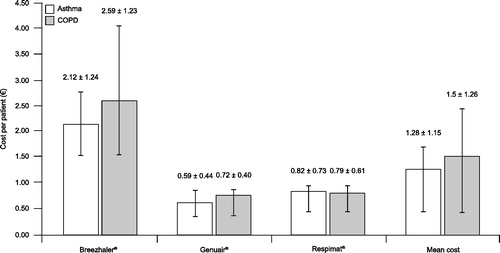
The time spent training patients to achieve the first correct inhalation, and the number of attempts required for patients to achieve this, represent important indicators of the true usability of an inhalation device (Citation31–33). In a study of commonly used inhalation devices in outpatients with asthma (n = 175) or COPD (n = 158), the mean (± SD) number of attempts to achieve the first correct actuation was lower with Genuair® (1.6 ± 0.8) and Respimat® (1.6 ± 1.0) compared with Handihaler® (2.5 ± 1.1) and Breezhaler® (2.6 ± 1.1). Of patients using Genuair®, 56% performed a successful actuation on the first attempt, compared with 62%, 19% and 18% with the Respimat®, Handihaler® and Breezhaler® devices, respectively. Likewise, the total time required to achieve the first correct actuation was lower with Genuair® (2.5 ± 1.6 min) and Respimat® (2.8 ± 2.3 min), compared with Handihaler® (4.9 ± 2.7 min) and Breezhaler® (10.3 ± 5.0 min) (Citation31, Citation32). In a randomized crossover study, inhaler operability with Genuair® and Respimat® were evaluated in patients with COPD and who had no prior experience of either device (n = 54). In this study, fewer rounds of instruction were required to achieve a correct inhalation with Genuair® (mean ± SD, 1.83 ± 1.28) versus Respimat® (2.74 ± 1.23) (p < 0.001). Twenty-four (44.4%) patients required only one round of instruction to correctly perform the inhalation procedure with Genuair®, compared with two (3.7%) patients with the Respimat® device (Citation34). Time spent training patients to achieve the first proper inhalation can also be characterized in economic terms. Using a cost per patient based on nurses’ time spent demonstrating and attending patients’ maneuvers, Breezhaler® was found to be three to four times more expensive than Genuair® or Respimat® () (Citation33). The outcomes of these studies may be explained by the clear feedback signals from Genuair® indicating proper inhalation flow, as this feature supports patient training by providing insight during inhaler use for both the patient and the healthcare provider. Consequently, patient errors, such as poor inhaler techniques, are revealed clearly and can be corrected or improved. That being said, one consideration regarding the acoustic and visual feedback mechanism is that patients need to understand the nature of the feedback and the related trigger factor (i.e. the inspiratory air-flow level of the inhalation maneuver). This must be understood for the patient to improve their inhalation technique, particularly in the case that feedback signals do not appear. Therefore, as with other DPIs, to avoid mistakes and confusion, patients must be carefully instructed on how they should perform a correct inhalation; for example, to firstly exhale thoroughly and then to inhale forcefully for as long as possible with the lips tightly around the mouthpiece. This guidance on proper inhaler use and reiteration of patient training supervised by health-care providers remains an important therapeutic success factor (Citation3).
Figure 5. Cost per patient (mean ± standard deviation) of the time spent by the nurse explaining inhaler operability (Citation33). COPD = chronic obstructive pulmonary disease. Republished with permission of Dove Medical Press, from The economic impact of educational training assessed by the Handling Questionnaire with three inhalation devices in asthma and Chronic Obstructive Pulmonary Disease patients. Dal Negro R, Povero M. ClinicoEconomics and Outcomes Research 2016;8:171–176.
Studies have demonstrated that the rate of handling errors, including critical errors, is also generally lower with Genuair® compared with other inhalation devices. In a crossover study comparing Genuair® and Handihaler® (n = 105), significantly fewer patients made one or more critical errors with Genuair® compared with HandiHaler® (10.5% vs 26.7%; p < 0.0001) (Citation35). Critical errors included those that impeded delivery of sufficient dose or drug deposition in the lungs, such as blowing into the inhaler when the dose is loaded, or shaking the inhaler with the mouthpiece facing the ground after priming. Similarly, in a randomized crossover study (n = 54), the mean (± SD) number of inhalation technique errors was significantly lower for Genuair® than Respimat® (0.44 ± 0.80 and 1.28 ± 1.53, respectively; p < 0.001) (Citation34). In an open-label crossover study comparing Genuair® with Breezhaler® (n = 123), the proportion of patients making one or more critical errors was low with both inhalers (3.3% vs 7.3%) although the difference was not statistically significant (Citation36). In a randomized, closed-label study of Genuair®, Ellipta® and Breezhaler® in healthy subjects with no prior experience of using DPIs (n = 130), the critical error rate was evaluated after subjects received written instructions or a visual demonstration. The number of subjects committing one or more critical errors (defined as errors preventing subjects from inhaling any medication) after reading the instructions only was highest with Breezhaler® (73.8%), followed by Genuair® (53.8%) and Ellipta® (25.4%). However, following a visual demonstration, the number of subjects committing critical errors was substantially reduced (Breezhaler®, 23.1%; Ellipta®, 9.2%; Genuair®, 8.5%), highlighting the importance of receiving appropriate inhaler-use guidance (Citation37).
As a consequence of the low rate of handling errors, correct operation rate with Genuair® is high. In a randomized crossover study (n = 54), correct operation rate was significantly higher for Genuair® than for Respimat® (96.0 ± 7.4% and 89.1 ± 12.8%, respectively; p < 0.001) (Citation34). Similarly, in a prospective, cross-over study in patients with COPD who were currently using HandiHaler® (n = 98), patients were less likely to have adequate inhaler technique with Respimat® compared with Genuair® (odds ratio 0.49; 95% confidence interval 0.26, 0.95); however, no significant differences in the likelihood of adequate inhaler technique were observed between Genuair® and Breezhaler® (Citation38). Additionally, in a randomized, crossover study (n = 48), the number of correct inhalations achieved was higher with Genuair® than with HandiHaler® (97.2% vs 73.6%) (Citation19). In this study an inhalation was defined as correct if all required inhalation steps had been performed after written and verbal instruction. For the Genuair® inhaler, these steps were: removing the mouthpiece cap, pressing and releasing the button to load a single dose into the powder inhalation chamber, and achieving a successful inhalation (as confirmed by the audible click and the control window changing from green to red). For the HandiHaler® the required steps were: removing a capsule from a package, opening the mouthpiece and placing the capsule in the chamber, closing the mouthpiece and pressing a side button to pierce the capsule, inhaling through the mouthpiece to deliver the medication (confirmed by hearing the capsule vibrate, tasting the powder and seeing the empty capsule), opening the mouthpiece and discarding the used capsule.
These study results are further supported by the fact that Genuair® is able to detect the most common technique error, which is low inhalation flow rate (Citation7), and clearly indicates this to the patient, offering the opportunity to improve inhalation technique.
Patient preference, acceptability and satisfaction
Several randomized crossover studies in patients with COPD have demonstrated higher patient satisfaction, willingness to continue and preference with Genuair® compared with other available inhalation devices. The key patient satisfaction results from these studies are summarized in , and the key patient preference results are summarized in .
Figure 6. Overall inhaler device preference in two open-label, crossover studies (Citation35, Citation36) and one double-blind, double-dummy study (Citation39) comparing device performance in patients with COPD. ***p < 0.001; ****p < 0.0001. COPD = chronic obstructive pulmonary disease.
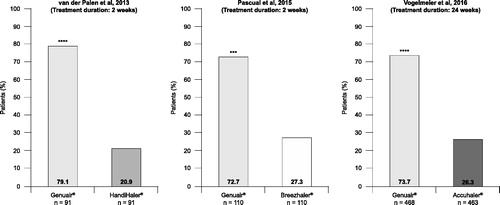
Table 2. Patient satisfaction and willingness to continue with Genuair® compared with other inhalation devices: results from randomized crossover studies (Citation34–37, Citation39).
The previously mentioned prospective, crossover study in patients with COPD who were currently using HandiHaler® (n = 98) also evaluated initial device preferences (after reading instructions and seeing a demonstration video, but before using the device) and preferences after testing the devices (Genuair®, Breezhaler®, and Respimat®). Before testing, there were no significant differences between initial preference; however, after initial testing, 30.6% preferred Genuair®, 22.4% preferred Respimat®, 17.3% preferred Breezhaler®, and 29.6% preferred their current device (HandiHaler®). In studies of 2–24 weeks in duration, a significantly higher proportion of patients preferred Genuair® compared with HandiHaler® (Citation35), Breezhaler® (Citation36), and Accuhaler® (Citation39) (). A real-world study assessing the performances of three DPIs (Genuair®, HandiHaler® and Breezhaler®) in outpatients with asthma (n = 175) or COPD (n = 158) produced similar findings (Citation32). Of the 127 patients who tested all three DPIs, >50% preferred the Genuair® device and perceived it to be the easiest to use.
Patients with COPD are often elderly and have multiple co-morbidities, including general muscle weakness and deficits in vision and cognition. The age-related physical challenges faced by this patient population can confound inhaler competence (Citation40); therefore, patient satisfaction in elderly patients is of particular importance. An Italian public survey found the Genuair® device to be well accepted and easy to use by elderly patients with COPD (n = 438) (Citation41, Citation42). In total, 88% of patients rated the Genuair® device as good/excellent when asked “Is it easy to understand how to use the device?” and “Is it easy to learn to use the device?”. The device was considered “practical/handy” and “easy to use” by 93% and 89% of patients, respectively. No differences in mean satisfaction scores were detected between the groups according to age, educational level, previous use of devices or the presence of hand arthritis/arthrosis. The acceptability of the device was emphasized by the willingness of 87% of elderly respondents to use Genuair® if they required inhaled therapy (Citation41).
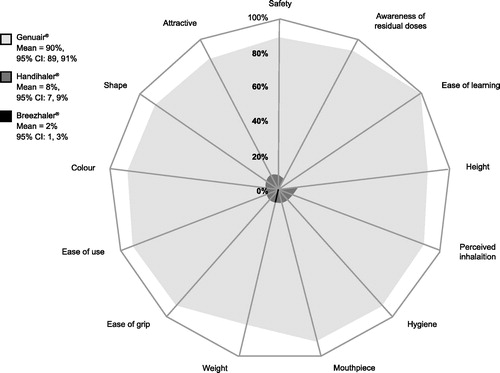
Several studies have directly assessed the individual attributes of Genuair® from a patient perspective. During a prospective, non-interventional study (n = 795), up to 90% of patients with COPD considered the individual attributes of the Genuair® inhaler to be very good or good, with the highest-rated feature cited as ‘ease of dose preparation’ (rated as very good by 72% of patients), followed by ‘control mechanism for correct inhalation’ (70%), ‘handling’ (67%), ‘ease of use’ (67%), ‘understanding of handling’ (65%), ‘comfort’ (62%) and ‘grip’ (60%) (Citation43). A randomized, double-blind phase IIIb study evaluated the efficacy of aclidinium bromide versus placebo and tiotropium, along with inhaler preference (Genuair® or HandiHaler®), in patients with moderate-to-severe COPD (n = 414). Over 75% of patients preferred Genuair® to HandiHaler® for each of the individual inhaler attributes assessed, including ease of use, convenience, ease of learning to use, ease of holding, ease of operating, ease of dose preparation, and feedback to indicate correct inhalation. Inhaler preference appeared to be independent of whether active medication or placebo was administered via the inhalers (Citation44). In the previously mentioned real-world study assessing the performances of Genuair®, HandiHaler® and Breezhaler®, when different aspects of preference and usability were assessed, Genuair® was the most preferred in terms of appearance, comfort, safety and convenience () (Citation32). A study using a computer-assisted telephone survey collected real-world data on inhaler use from patients with mild to very-severe COPD using Genuair® (n = 194), Breezhaler® (n = 186), Ellipta® (n = 191), or Respimat® (n = 201). The proportion of patients who were confident or very confident of having taken the full dose, an inhaler attribute cited as important by patients with COPD (Citation45), was high with all devices: 84%, 93%, 80% and 76% with Genuair®, Breezhaler®, Ellipta® and Respimat®, respectively (Citation46). Finally, the previously mentioned randomized, closed-label study in 130 healthy subjects naïve to inhaler operation also compared patient satisfaction with individual attributes of Genuair®, Ellipta® and Breezhaler®. Again, Genuair® demonstrated higher patient satisfaction than both Ellipta® and Breezhaler® in terms of confidence to have inhaled the entire dose (p ≤ 0.001 and p ≤ 0.05). Genuair® also demonstrated significantly higher satisfaction than Ellipta® in terms of clarity to indicate correct dose preparation and inhalation (p ≤ 0.05 and p ≤ 0.01, respectively), and convenience of carrying (p ≤ 0.01), and significantly higher patient satisfaction than Breezhaler® in terms of ease of dose preparation (p ≤ 0.01). There were no significant differences between Genuair® and either Ellipta® or Breezhaler® in terms of comfort, ease of operation or handling time (Citation37).
Figure 7. Response to the question “which device offers the most convenience in terms of…?”: the patient’s opinion (Citation32). CI = confidence interval. Reproduced from (Citation32) Creative Commons Attribution 4.0 International License. For licence, please see http://creativecommons.org/licenses/by/4.0/
Taken together, these findings suggest high preference and satisfaction with Genuair® in various patient populations, including elderly patients and those with airflow limitation. As patient preference and satisfaction are strongly associated with therapeutic adherence in COPD (Citation12), the improved patient satisfaction reported with Genuair® in comparison with other devices could potentially lead to improved adherence, and consequently, more effective therapy. However, in the available studies, as the devices contained placebo or different therapeutic compounds, they were unable to evaluate whether differences in device performance impact clinical outcome. Further studies would be required to directly evaluate this association. Furthermore, the available studies have a number of additional limitations. The studies were generally of a short duration, with only a brief time interval between training and assessment; thus, the results may not provide an accurate reflection of clinical practice and may underestimate the rate of handling errors. Additionally, it is difficult to perform comparative studies of inhaler devices in a randomized and blinded manner, which has the potential to introduce bias. Lastly, there is no common validated method to assess patient satisfaction and preferences. Therefore, head-to-head comparisons of different devices are required. Studies evaluating the efficacy and safety of different devices using the same therapeutic compound would be difficult to conduct since formulations vary with available devices. However, studies linking ease of device use to efficacy data would be particularly useful in further differentiating available inhalation devices.
Conclusions
Inhaler choice is an important consideration and should take into account patient characteristics, such as age, ability to use the device, manual dexterity, and degree of breathlessness. Inhaler choice is driven by the availability of the drug regimen; for example, Genuair® is currently approved for the delivery of the long-acting muscarinic antagonist aclidinium bromide, either alone or in combination with the long-acting beta-agonist, formoterol, and therefore, would not be available for patients who require a different treatment regimen. Alongside efficacy and safety, the critical success factors for inhaled COPD therapy are usability, patient preference, acceptability and satisfaction. The evidence base suggests that patients find Genuair® easy to use, with patients requiring less training and making fewer inhalation errors than with other inhalers, and patient satisfaction with the device is high. The Genuair® inhaler supports correct use and inhalation by indicating common technique errors, and thereby provides an effective therapy.
Declaration statement
Helgo Magnussen received lecture fees from AstraZeneca, Boehringer Ingelheim, ndd, and Novartis. Beatrix Fyrnys and Roland Greguletz are employees of Sofotec GmbH, a member of the AstraZeneca Group.
Additional information
Funding
References
- Global initiative for chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report [Internet]. 2018 [cited 2018 Apr 1]. Available from: http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf.
- Vincken W, Dekhuijzen PR, Barnes P, et al. The ADMIT series - issues in inhalation therapy. 4) How to choose inhaler devices for the treatment of COPD. Prim Care Respir J. 2010;19:10–20. doi: 10.4104/pcrj.2009.00062.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49:1601794. doi: 10.1183/13993003.01794-2016.
- Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105:1099–1103. doi: 10.1016/j.rmed.2011.03.012.
- Lavorini F, Pistolesi M, Usmani OS. Recent advances in capsule-based dry powder inhaler technology. Multidiscip Respir Med. 2017;12:11.
- Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1333–1343. doi: 10.1164/rccm.201604-0733OC.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2018 [Internet]. 2018 [cited 2018 Apr 1]. Available from: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf.
- Brocklebank D, Ram F, Wright J, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5:1–149.
- Dahl R, Kaplan A. A systematic review of comparative studies of tiotropium Respimat® and tiotropium HandiHaler® in patients with chronic obstructive pulmonary disease: does inhaler choice matter? BMC Pulm Med. 2016;16:135.
- Ram FS, Brocklebank DM, Muers M, et al. Pressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(1):CD002170.
- Chrystyn H, Small M, Milligan G, et al. Impact of patients' satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108:358–365. doi: 10.1016/j.rmed.2013.09.021.
- van Boven JF, Chavannes NH, van der Molen T, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108:103–113. doi: 10.1016/j.rmed.2013.08.044.
- van der Palen J. Genuair® in chronic obstructive pulmonary disease: a novel, user-friendly, multidose, dry-powder inhaler. Ther Deliv. 2014;5:795–806. doi: 10.4155/tde.14.49.
- electronic Medicines Compendium (eMC). Eklira Genuair 322 micrograms inhalation powder: summary of product characteristics (SmPC) [Internet]. 2012 [cited 2018 Apr 1]. Available from: https://www.medicines.org.uk/emc/product/4302.
- electronic Medicines Compendium (eMC). Duaklir Genuair 340 micrograms/12 micrograms inhalation powder: summary of product characteristics (SmPC) [Internet]. 2014 [cited 2018 Mar 12]. Available from: https://www.medicines.org.uk/emc/product/3562.
- U.S. Food & Drug Administration (FDA). TUDORZATM PRESSAIRTM (aclidinium bromide inhalation power): highlights of prescribing information [Internet]. 2012 [cited 2018 Jul 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202450s000lbl.pdf.
- Krüger P, Ehrlein B, Zier M, et al. Inspiratory flow resistance of marketed dry powder inhalers (DPI). Eur Respir J. 2014;44(Suppl. 58):(Abstract 4635).
- Magnussen H, Watz H, Zimmermann I, et al. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respir Med. 2009;103:1832–1837. doi: 10.1016/j.rmed.2009.07.006.
- Plugge J, Basaldella U, Fyrnys B, et al. Drug product performance after simulated patient handling of an inhalation powder using and LAMA/LABA combination in a dry powder inhaler. Thorax. 2016;71(Suppl. 3):A247 (Abstract P289).
- Linne-Geyer S, Stahl K, Pieper T. In-use stability of aclidinium bromide 400 μg/formoterol fumarate dihydrate 12 μg inhalation powder in a dry powder inhaler. Thorax. 2016;71(Suppl. 3):A249–A251 (Abstract P291). doi: 10.1136/thoraxjnl-2016-209333.434.
- Block K, Floger S, Fyrnys B, et al. Delivered dose and fine particle dose of aclidinium bromide 200 µg via the Genuair® inhaler are independent of flow rate within the working range of the device. Eur Respir J. 2010;36(Suppl. 54):2037 (Abstract).
- Newman SP, Sutton DJ, Segarra R, et al. Lung deposition of aclidinium bromide from Genuair, a multidose dry powder inhaler. Respiration. 2009;78:322–328. doi: 10.1159/000219676.
- Gjaltema D, Hagedoorn P, Grasmeijer F, et al. Comparative in vitro performance of the new drug aclidinium in a novel multidose dry powder inhaler. Eur Respir J. 2013;42(Suppl. 57):(Abstract P3384).
- Heyder J, Gebhart J, Rudolf G, et al. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci. 1986;17:811–825. doi: 10.1016/0021-8502(86)90035-2.
- Sethi S, Kerwin E, Watz H, et al. AMPLIFY: a randomized, Phase III study evaluating the efficacy and safety of aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2019;14:667–682. doi: 10.2147/COPD.S189138.
- Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur. Respir. J. 2012;40:830–836. doi: 10.1183/09031936.00225511.
- Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir. Res. 2011;12:55.
- Greguletz R, Arlt M, Anke S, et al. The Genuair® inhaler: a novel multidose dry powder inhaler for the delivery of various types of inhalation powder. Am. J. Respir. Crit. Care Med. 2009;179:A2461.
- Chrystyn H, Niederlaender C. The Genuair® inhaler: a novel, multidose dry powder inhaler. Int. J. Clin. Pract 2012;66:309–317. doi: 10.1111/j.1742-1241.2011.02832.x.
- Dal Negro RW, Povero M. Acceptability and preference of three inhalation devices assessed by the Handling Questionnaire in asthma and COPD patients. Multidiscip Respir Med. 2016;11:7.
- Dal Negro RW, Povero M. Dry-powder inhalers in patients with persistent airflow limitation: usability and preference. Multidiscip Respir Med. 2016;11:31.
- Dal Negro RW, Povero M. The economic impact of educational training assessed by the handling questionnaire with three inhalation devices in asthma and chronic obstructive pulmonary disease patients. Clin. Outcomes Res. 2016;8:171–176.
- Ohbayashi H, Kudo S, Ishikawa M. Inhaler operability and patient satisfaction regarding Genuair® and Respimat® inhalers for chronic obstructive pulmonary disease: a randomized crossover study. Pulm Ther. 2017;3:173–185. doi: 10.1007/s41030-017-0038-2.
- van der Palen J, Ginko T, Kroker A, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin Drug Deliv. 2013;10:1023–1031. doi: 10.1517/17425247.2013.808186.
- Pascual S, Feimer J, De Soyza A, et al. Preference, satisfaction and critical errors with Genuair and Breezhaler inhalers in patients with COPD: a randomised, cross-over, multicentre study. NPJ Prim Care Respir Med. 2015;25:15018.
- Man KN, Tian Z, Lam DC-L, et al. Satisfaction, preference and error occurrence of three dry powder inhalers as assessed by a cohort naïve to inhaler operation. Int J Chron Obstruct Pulmon Dis. 2018;13:1949–1963. doi: 10.2147/COPD.S152285.
- Bournival R, Coutu R, Goettel N, et al. Preferences and inhalation techniques for inhaler devices used by patients with chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2018;31:237–247. doi: 10.1089/jamp.2017.1409.
- Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48:1030–1039. doi: 10.1183/13993003.00216-2016.
- Ding B, Small M, Scheffel G, et al. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936. doi: 10.2147/COPD.S154525.
- Blasi F, Canonica GW, Centanni S, et al. Genuair® usability test: results of a national public survey of the elderly. COPD. 2016;13:367–371. doi: 10.3109/15412555.2015.1067675.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients? Respir Res. 2017;18:19.
- Marth K, Schuller E, Pohl W. Improvements in patient-reported outcomes: a prospective, non-interventional study with aclidinium bromide for treatment of COPD. Respir Med. 2015;109:616–624. doi: 10.1016/j.rmed.2015.02.004.
- Beier J, Kirsten A-M, Mróz R, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled phase IIIb study. COPD. 2013;10:511–522. doi: 10.3109/15412555.2013.814626.
- Hawken N, Torvinen S, Neine M-E, et al. Patient preferences for dry powder inhaler attributes in asthma and chronic obstructive pulmonary disease in France: a discrete choice experiment. BMC Pulm Med. 2017;17:99.
- Price D, Keininger DL, Viswanad B, et al. Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey. Int J Chron Obstruct Pulmon Dis. 2018;13:695–702. doi: 10.2147/COPD.S149404.
- Electronic Medicines Compendium (eMC). Spiriva 18 microgram inhalation powder, hard capsule: summary of product characteristics (SmPC) [Internet]. 2016 [cited 2018 Feb 5]. Available from: https://www.medicines.org.uk/emc/product/1693/smpc.
- Electronic Medicines Compendium (eMC). Ultibro Breezhaler: summary of product characteristics (SmPC) [Internet]. 2013 [cited 2018 Mar 12]. Available from: https://www.medicines.org.uk/emc/product/3496/smpc.
- U.S. Food & Drug Administration (FDA). ADVAIR DISKUS 100/50, 250/50 and 500/50 (fluticasone propionate and salmeterol) inhalation powder: highlights of prescribing information (PI) [Internet]. 2014 [cited 2018 Apr 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021077s042lbl.pdf.
- Electronic Medicines Compendium (eMC). Anoro Ellipta 55 micrograms/22 micrograms inhalation powder, pre-dispensed: summary of product characteristics (SmPC) [Internet]. 2014 [cited 2018 Mar 12]. Available from: https://www.medicines.org.uk/emc/product/5423/smpc.
- Electronic Medicines Compendium (eMC). Fostair NEXThaler 100/6: summary of product characteristics (SmPC) [Internet]. 2014 [cited 2018 Feb 5]. Available from: https://www.medicines.org.uk/emc/product/3317/smpc.
- Electronic Medicines Compendium (eMC). Symbicort Turbohaler 400/12, inhalation powder: package leaflet [Internet]. 2017 [cited 2018 Feb 5]. Available from: https://www.medicines.org.uk/emc/files/pil.6775.pdf.

