Abstract
The treatment of chronic obstructive pulmonary disease (COPD) and concomitant diseases requires several medications. Yet there is little data on how the pharmacological burden progressed over time among older individuals with COPD. We aimed to: 1) describe the proportion of older adults with COPD in Quebec, Canada, that were exposed to polypharmacy (≥10, ≥15 or ≥20 medications/year) between 2000 and 2015; 2) calculate the proportion of individuals receiving specific prescriptions for COPD during this period. We conducted a population-based cohort study with the Quebec Integrated Chronic Disease Surveillance System. Individuals aged ≥66 years with COPD and covered by the universal public drug plan were included. We calculated the total number of drugs used at least once by each individual during each of the studied years, and used age-standardized proportions to compare proportions of users between the years. The average number of drugs used increased from 12.0 in 2000 to 14.8 in 2015. The proportion of individuals exposed to polypharmacy increased (≥10 drugs: 62.0% to 74.6%;≥15 drugs: 31.2% to 45.4%; ≥20 drugs: 12.3% to 22.4%). The proportion of individuals receiving long-acting bronchodilators increased from 18.7% in 2000 to 69.6% in 2015. The use of short-acting bronchodilators decreased from 81.5% to 67.9%, and that of inhaled corticosteroids from 60.6% to 26.0%. The proportion of users of methylxanthines decreased from 15.0% to 1.9%. Older individuals with COPD are increasingly exposed to polypharmacy. Identifying which polypharmacy is beneficial is a priority.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) affects around 15% of the population over 65 years of age (Citation1). COPD is associated with increased health care use and poor quality of life (Citation2). Fortunately, a variety of medications are now available to improve lung function and reduce complications, including exacerbations (Citation3).
Older individuals with COPD also present numerous co-morbidities (Citation4,Citation5), which increases the probability of using several medications, known as polypharmacy. Such complex medication regimens have substantial impact on compliance, side effects, and drug-drug and drug-disease interactions (Citation4,Citation6). Yet the pharmacological burden that COPD individuals have been facing in the last decade is not well defined. We therefore aimed at reporting the prevalence of polypharmacy and illustrating the particular trends of COPD-related medication use among older patients with COPD in the province of Quebec, Canada, between 2000 and 2015.
Methods
Data source and population
We used the Quebec Chronic Disease Surveillance System (QICDSS). This database is composed of five health administrative databases grouping data about physician services fees, pharmaceutical services, hospitalization data (Med-Echo), registration plan, and death registry. The pharmaceutical database provides information on drugs dispensed, including drug names, dispensing dates, and number of days’ supply. Around 90% of the older population in Quebec is covered by the public drug plan (Citation7). The QICDSS permits the identification of chronic diseases with validated algorithms (Citation7) and the calculation of a co-morbidity score (Citation8).
COPD definition
We used a validated definition of COPD (Citation9) to identify individuals with the disease. The International classification of diseases (ICD) 9th revision codes 491–492 and 496, and the ICD-10-CA codes J41-44 were used to ascertain cases. To be included, an individual had to have two diagnosis of COPD in the physician database in a two year-period or one primary or secondary diagnosis in the hospital database. There was no restriction on age as the population included only individuals 66 years and older. We did not apply further inclusion or exclusion criteria to the definition (such as smoking patterns, which are not available in the database, or the presence of other respiratory conditions). This definition has a sensitivity of 85% (95%CI: 77.0–91.0) and a specificity of 78.4% (95% CI: 73.6–82.7) (Citation9) and is notably used by public health authorities to estimate the burden of COPD (Citation7,Citation10,Citation11). Nonetheless, in order to increase the positive predictive value, we restricted our analysis to individuals who had used a medication specific to COPD in the year before or in the year studied.
We included individuals based on their fulfilment of COPD definition every fiscal year, from 2000 to 2015 (Fiscal year begins April 1st and ends March 31st). An individual can therefore meet the case definition criteria a year, but not be included the following year. This allowed us to further reduce the number of false positive cases over the years. Individuals also had to be alive and covered by the universal public drug plan throughout the studied year to assess the total number of medications used during this time period.
Polypharmacy and medication use
We defined polypharmacy as the yearly use of at least 10 different medications (Citation12). We also conducted analysis using thresholds of 15 and 20 medications. We compiled the number of medications prescribed to each individual through a fiscal year by using the common denominations (chemical name) of the drugs. We assessed whether or not individuals used the following COPD-related medications, as defined by at least one billing in the studied year: long-acting bronchodilators, short-acting bronchodilators, inhaled corticosteroids, oral corticosteroids, methylxanthines, roflumilast, antibiotics and smoking cessation medications. Chronic and acute use of medications were not differentiated, as we only evaluated the presence of at least one claim of the respective medications through the year studied.
Statistical analysis
We calculated the mean number of medications used by each individual each year. We also calculated the proportion of individuals exposed to at least 10 different medications through the year, at least 15 or at least 20 as well as the proportion of those using the COPD related medications. In order to make comparisons, we calculated age-and sex-standardized proportions according to the 2001 Quebec population. T-tests were used to compare continuous variables and we tested trends, adjusted for sex and age, using Poisson regression models with robust error variance estimator. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Ethics
The use of the QICDSS has been approved by the custodians of the databases, the provincial Public Health Research Ethics Board and the Quebec Commission protecting access to information.
Results
The number of included individuals varied from 68,532 in 2000 to 117,005 in 2015 (). Over the years, the population comprised an increasing number of women, older individuals, and individuals with more co-morbidities.
Table 1. Characteristics of the Older Adults with COPD in Quebec, Canada, and their Use of Medication between 2000 and 2015.
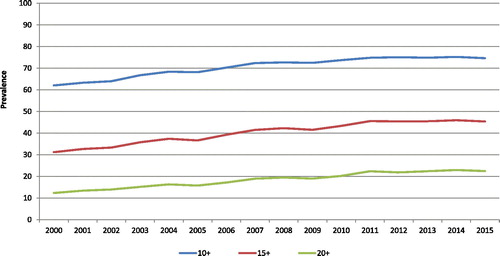
The mean yearly number of medications increased from 12.0 in 2000 to 14.8 in 2015 (p < 0.0001) (not shown). Polypharmacy simultaneously increased during the period (, ). From 2000 to 2015, the proportion of individuals using ≥10 medications per year increased by 12.6% (from 62.0% to 74.6%) with a relative increase of 1.3% per year ([95% confidence interval (CI):1.2;1.4], p < 0.0001); ≥15 medications, by 14.2% (from 31.2% to 45.4%) with a relative yearly increase of 2.7% ([CI:2.6;2.8], p < 0.0001) and ≥20 medications, by 10.1% (from 12.3% to 22.4%) with a relative yearly increase of 4.3% ([CI:4.1;4.4], p < 0.0001).
Figure 1. Age- and sex-standardized proportions of older adults with COPD in Quebec, Canada, exposed to three levels of polypharmacy, between 2000 and 2015.
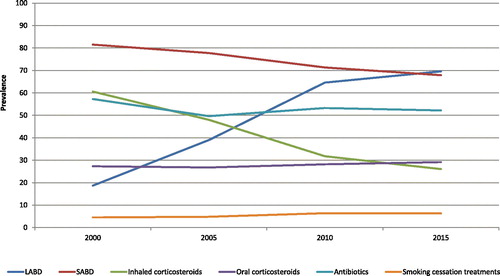
Patterns of COPD-related medication use over time differed according to the medications (, ). The proportion of long-acting bronchodilators users increased over the years (18.7%–69.6%), with a relative increase of 7.0% per year ([CI:6.9;7.1], p < 0.0001), whereas the proportion of short-acting bronchodilators users decreased (81.5%–67.9%), with a relative decrease of 1.2% per year ([CI:1.1;1.2], p < 0.0001). Inhaled corticosteroids were common in early years (60.6%) but their use decreased drastically to reach 26% in 2015; translating in a relative decrease of 5.9% per year ([CI:6.0;5.8], p < 0.0001). On the other hand, oral corticosteroids, antibiotics and smoking cessation medications remained fairly stable. The use of methylxanthine decreased over the years, whereas the use of roflumilast slightly increased after its marketing date before reaching a low, but stable, level.
Discussion
Our results show how polypharmacy is common among older people with COPD and is steadily increasing between 2000 and 2015.
Other studies have suggested high rates of polypharmacy among older COPD patients. In the UK, Hanlon et al. indicated that 52.0% of their cohort of community-dwelling adults (median age: 62 years) received >5 medications (Citation4). In Italy, the proportion was 59.5% among the 22,505 patients (64% ≥65 years old) in primary care (Citation13). Our results contrast with the proportion of individuals exposed to polypharmacy in the general population. In Canada, 27% of adults older than 65 years received at least 10 different medication classes in 2014 (Citation12), while it was 75% among our COPD population. To our knowledge, this is the first study to portray a population-based evaluation of medication use over fifteen years among COPD patients. It allows for the visualization of the increase of medications and is a first step towards the objective of rationalizing medication use among older patients with COPD to find the best risk to benefit ratio.
The increase in the total number of medications has consequences. Together with increasing management complexity, it leads to possible drug–disease and drug–drug interactions and more side effects (Citation6,Citation14). Because of pharmacodynamic and pharmacokinetics changes associated with aging (Citation15), older people are more susceptible to such adverse events. Iatrogenic risk associated with polypharmacy is thus a genuine concern for older adults with COPD. The growing trends we have identified in our study raise questions about the safety of these therapies. In fact, polypharmacy is a complex phenomenon, involving a wide variety of medication combinations, which has not yet been fully described and understood (Citation16). Nevertheless, it has been associated with myriad negative outcomes in older populations, such as hospitalizations, falls or frailty (Citation17–20). Quality may be at stake: one in ten older COPD patients could receive potentially inappropriate medications (Citation21), while clinical guidelines are not always followed (Citation22). To ensure optimal pharmacotherapy, there may be a necessity to identify what medications provide the greatest benefits, the lowest risks and the better quality of life. Finding such optimal therapy may prove difficult (Citation23) and should involve the patient.
Pharmacological management of co-morbidities may lead to significant medication burden for older adults with COPD. The study population had an average of three concomitant diseases. Although the number of co-morbidities varies considerably between studies (Citation24), multimorbidity is a constant concern. In a group of 826 patients with COPD in Turkey, 84.5% had at least one co-morbidity (Citation25). In another study involving 1,659 patients in the United States and Spain, the average number of co-morbidities was 6 (SD:3.5, range 0–21) (Citation26). The greater presence of co-morbidities in this last study can be explained by the difference in population (patients were recruited in pulmonary clinics) and by the methodology (co-morbidities were recorded from direct questioning and review of medical records).
With such multimorbidity, optimizing pharmacological treatment reveals challenging in older adults and oldest old individuals with COPD (Citation27). In addition, they often have a limited functional reserve and frailty that will also affect treatment choices (Citation27). In order to improve physical functioning with medications, it is necessary to carry out a holistic evaluation, taking into account the patient’s cognitive and functional status and co-morbidities together with their willingness to be treated. If polypharmacy proves inevitable for many patients, it may be necessary to make rational choices and prioritize certain medications. Indeed, the escalation in medication use that we have described over the years is unlikely to be sustainable, either from the perspective of the individual or of society.
Our study showed different trends in COPD medication use. Such trends have been described elsewhere. In a population-based Danish study, the overall use of long-acting bronchodilators among adults increased between 2000 and 2016, while the use of inhaled corticosteroids (alone or fixed-dose combinations) remained relatively stable (Citation28). According to the authors, the prompt acceptance of long-acting muscarinic antagonists as a new treatment for COPD drove the increase in the use of long-acting bronchodilators, which is likely analogous to the phenomenon we observed in our population. The authors also hypothesized that the stable use of inhaled corticosteroids among bronchodilators users in their study is in fact explained by a decline in the use of this therapy among COPD patients (since they could not differentiate indications for therapies). This concurs with the reduction in inhaled corticosteroids we observed, which itself is coherent with the increased awareness about safety issues of inhaled corticosteroids in this population (Citation29,Citation30), and with guidelines recommendations (Citation31). Finally, similar to our findings, Kwak et al. found a low average prescription rate for smoking cessation therapies: between 2007 and 2012, 3.64% of their active smokers with COPD used these medications (Citation32).
This study has limitations. First, the case definition suffers from limitations inherent to the use of administrative databases. Indeed, the presence of COPD could not be confirmed clinically because we did not have access to spirometry results and it was not possible to do further tests with the study population. Some of the symptoms attributed to COPD may be due to alternative conditions such as asthma (Citation33) and chronic heart failure (Citation34), inducing a possible misclassification bias. The limited specificity of the case definition can therefore lead to a number of false positives. However, this case definition applied in the QICDSS revealed a prevalence of COPD consistent with other population-based data that included spirometry measures (Citation10). Yet, the fact that the definition predominantly identifies moderate to severe COPD (Citation10) coupled with the fact that we only included people who had recently used a COPD medication, may have resulted in the presence of a more severely affected population. This may have overestimated the number of individuals exposed to polypharmacy, but as the same criterion was applied over the years, the impact should be uniform in the study period. Second, only one claim was required to evaluate use of medications. Our definition of polypharmacy did not imply simultaneous use of medications, which may theoretically result in individuals using large number of medications through the year, but none at the same time. Nonetheless, we applied the same definition over the year and used population-based data that allowed us to perform trends. On the other hand, we have underestimated the medications COPD patients are exposed to because we did not include over the counter products as they are not reimbursed under the drug plan. Finally, we did not assess adherence to treatment. While it would have provided a more thorough portrait of the quality of medication use, this analysis was beyond the objectives of the study.
Conclusion
The proportion of older individuals with COPD using at least 10 medications per year increased gradually to reach nearly three quarters in 2015. There is a need to explore if this increase in medications translated into benefits for the patients and the healthcare system. In general, there is a need to fully apprehend medications used by these patients, in order to reduce the burden polypharmacy may generate and ensure patients fully benefit from pharmacotherapy.
Disclosure statement
No financial interest or benefit has arisen from the direct applications of our research.
Additional information
Funding
References
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605.
- Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18:67. doi: 10.1186/s12931-017-0548-3.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD); Global Strategy for the Diagnosis, Management and Prevention of COPD. [Internet]. [cited 2019 Jan 25]. Available from: https://goldcopd.org.
- Hanlon P, Nicholl BI, Jani BD, et al. Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross-sectional UK Biobank study. BMJ Open. 2018;8:e018404. doi: 10.1136/bmjopen-2017-018404.
- Chetty U, McLean G, Morrison D, et al. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67:e321–e328. doi: 10.3399/bjgp17X690605.
- Nagewo NA, Gibson PG, Wark PA. Treatment burden, clinical outcomes, and comorbidities in COPD: an examination of the utility of medication regimen complexity index in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2929–2942. doi: 10.2147/COPD.S136256.
- Blais C, Jean S, Sirois C, et al. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chronic Dis Inj Can. 2014;34:226–235.
- Simard M, Sirois C, Candas B. Validation of the combined comorbidity index of Charlson and Elixhauser to predict 30-day mortality across ICD-9 and ICD-10. Med Care. 2018;56:441–447. doi: 10.1097/MLR.0000000000000905.
- Gershon AS, Wang C, Guan J, et al. Identifying individuals with physician-diagnosed COPD in health administrative databases. Can Respir J. 2009;6:388–394. doi: 10.1080/15412550903140865.
- Doucet M, Rochette L, Hamel D. Incidence, prevalence, and mortality trends in chronic obstructive pulmonary disease over 2001 to 2011: A public health point of view of the burden. Can Respir J. 2016;2016:1. doi: 10.1155/2016/7518287.
- Feely A, Lix LM, Reimer K. Estimating multimorbidity prevalence with the Canadian Chronic Disease Surveillance System. Health Prom Chronic Dis Prev Can. 2017;37:215–222. doi: 10.24095/hpcdp.37.7.02.
- Canadian Institute for Health Information. Drug use among seniors in Canada, 2016. [Internet].Ottawa, ON: CIHI; 2018. [cited 2019 Jan 28]. Available from: https://www.cihi.ca/sites/default/files/document/drug-use-among-seniors-2016-en-web.pdf.
- Vertrano DL, Bianchini E, Onder G, et al. Poor adherence to chronic obstructive pulmonary disease medications in primary care: role of age, disease burden and polypharmacy. Geriatr Gerontol Int. 2017;17:2500–2506. doi: 10.1111/ggi.13115.
- Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. doi: 10.1186/s12916-015-0322-7.
- Reeve E, Wiese M, Mangoni A. Alterations in drug disposition in older adults. Expert Opin Drug Metab Toxicol. 2015;11:491–508. doi: 10.1517/17425255.2015.1004310.
- Sirois C, Simard M, Gagnon ME, et al. Mixed bag polypharmacy: methodological pitfalls and challenges of this exposure definition. Curr Epidemiol Rep. 2019. https://doi.org/10.1007/s40471-019-00214-4
- Sganga F, Landi F, Ruggiero C, et al. Polypharmacy and health outcomes among older adults discharged from hospital: results from the CRIME study. Geriatr Gerontol Int. 2015;15:141–146. doi: 10.1111/ggi.12241.
- Payne RA, Abel GA, Avery AJ, et al. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77:1073–1082. doi: 10.1111/bcp.12292.
- Fried TR, O'Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62:2261–2272. doi: 10.1111/jgs.13153.
- Gutierrez-Valencia M, Izquierdo M, Cesari M, et al. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84:1432–1444. doi: 10.1111/bcp.13590.
- Graf J, Lucke T, Herrera R, et al. Compatibility of medication with PRISCUS criteria and identification of drug interactions in a large cohort of patients with COPD. Pulm Pharmacol Ther. 2018;49:123–129. doi: 10.1016/j.pupt.2018.01.011.
- Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi: 10.2147/COPD.S62750.
- Sirois C, Lunghi C, Laroche M-L, et al. The delicate choice of optimal basic therapy for multimorbid older adults: a cross-sectional survey. Res Social Adm Pharm. 2019;15:761–766. doi: 10.1016/j.sapharm.2018.09.008.
- Houben-Wilke S, Triest FJJ, Franssen FME, et al. Revealing methodological challenges in chronic obstructive pulmonary disease studies assessing comorbidities: a narrative review. Chronic Obstr Pulm Dis. 2019;6:166–177. doi: 10.15326/jcopdf.6.2.2018.0145.
- Eroglu SA, Gunen H, Yakar HI, et al. Influence of comorbidities in long-term survival of chronic obstructive pulmonary disease patients. J Thorac Dis. 2019;11:1379–1386. doi: 10.21037/jtd.2019.03.78.
- Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC.
- Corsonello A, Scarlata S, Pedone C, et al. Treating COPD in older and oldest old patients. Curr Pharm Des. 2015;21:1672–1689.
- Reilev M, Pottegård A, Davidsen JR, et al. Seventeen-year nationwide trends in use of long-acting bronchodilators and inhaled corticosteroids among adults: a Danish Drug Utilization Study. Basic Clin Pharmacol Toxicol. 2018;123:58–64. doi: 10.1111/bcpt.12978.
- Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908.
- Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. PMID:24130228. doi: 10.1136/thoraxjnl-2012-202872.
- Bourbeau J, Bhutani M, Hernandez P, et al. CTS position statement: pharmacotherapy in patients with COPD – an update. Can J Respir Crit Care Sleep Med. 2017;1:222–241. doi: 10.1080/24745332.2017.1395588.
- Kwark MJ, Kim J, Bhise V, et al. National trends in smoking cessation medication prescriptions for smokers with chronic obstructive pulmonary disease in the United States, 2007-2012. J Prev Med Public Health. 2018;51:257–262. doi: 10.3961/jpmph.18.119.
- Price D, Brusselle G. Challenges of COPD diagnosis. Expert Opin Med Diagn. 2013;7:543–556. doi: 10.1517/17530059.2013.842552.
- Güder G, Störk S. COPD and heart failure: differential diagnosis and comorbidity. Herz. 2019. doi: 10.1007/s00059-019-4814-7.