Abstract
Regular use of inhaled corticosteroids (ICS) is the standard of care for patients with persistent asthma and chronic obstructive pulmonary disease (COPD). Adherence to ICS is measured using the 10-item Medication Adherence Report Scale (MARS), a self-reported medication adherence assessment. However, data on the validity of this measure are limited. Data were obtained from two cohort studies that examined the association of health literacy with self-management behaviors among adults ages 65 and older with asthma and adults ages 40 and older with COPD. ICS adherence was objectively measured over a 4-week period using electronic monitoring devices. Adequate adherence by MARS assessment was defined as a score ≥4.5, and by electronic monitoring as ≥80% of doses prescribed. We assessed the criterion validity using correlations between self-reported adherence and electronic adherence. Receiver Operating Characteristic (ROC) curve analysis was performed between the two measures. Among patients with asthma, the continuous values for adherence measured by self-report and electronically were weakly correlated (r = 0.33, p < 0.001); similarly, the agreement between the dichotomized measures was weak (kappa 0.30, p=.49). Findings were similar for COPD patients: r = 0.26, p = 0.003; kappa 0.19, p = .60. Area under curve (AUC) values generated from ROC analysis was 0.69 and 0.61, for asthma and COPD patients, respectively. Commonly used measure for adherence performed weakly compared to electronic monitoring in separate populations of patients with asthma and COPD. Investigators measuring self-reported medication adherence among patients with these pulmonary diseases should consider using alternative instruments or using objective measures exclusively.
Background
Chronic obstructive pulmonary disease (COPD) and asthma are associated with significant morbidity and mortality, affecting approximately 6% and 7% of adults in the United States, respectively. Randomized controlled trials show that daily use of controller medications, including inhaled corticosteroids (ICS), long acting beta-agonists (LABA), and long-acting anti-muscarinic agents (LAMA) improves symptoms and quality of life, as well as reduces the risk of exacerbations for these diseases [Citation1–3]. Despite this, adherence to controller medications among patients with COPD and asthma remains low, ranging from 20% to 60% [Citation4–7]. Low adherence to controller medications is associated with worse patient outcomes and increased healthcare costs [Citation8–10]. Improving medication adherence for these and other chronic diseases is a major public health focus.
In order to improve their ability to support patients’ medication taking behaviors, clinicians and researchers first need to be able to accurately measure adherence. Unfortunately, medication adherence generally, and inhaler adherence specifically, are difficult to reliably measure. Existing methods of measuring adherence include self-reports, electronic dose measures, insurance claims, and blood or serum levels [Citation11, Citation12]. While electronic devices are considered the gold standard for measuring adherence to inhaled medications, patients may discharge doses without taking the medication and or they may have and use multiple devices for the same drug while only one of the devices is monitored [Citation13, Citation14]. Insurance claims are often used to determine medication possession ratios, a proxy for adherence, but they do not provide information about actual medication use [Citation15]. Serum drug levels are also considered a highly objective measure of medication use but are not available for inhaled medications.
Self-reported measures of medication adherence are particularly compelling because of the ease of administration and low cost, but they may be subject to biased responses owing to recall problems and social desirability [Citation17]. One widely used self-reported measure of inhaled medication adherence is the Medication Adherence Rating Scale (MARS) [Citation13, Citation16]. While commonly used, however, there are limited data on the validity of this scale [Citation5, Citation13, Citation17–22]. Moreover, advanced patient age presents a unique problem with self-reported adherence, regardless of the assessment, because of greater risk of inaccuracies in recall arising from cognitive impairment. Cognitive impairment is a sequela of both advanced COPD and asthma [Citation23, Citation24]. Despite the risk of recall bias, the validity of the MARS has not been previously examined among older adults.
In this study, we used data from 2 prospective cohorts of patients with asthma or COPD to assess the performance of the MARS compared to objective measures of inhaled medication adherence among older adults.
Methods
Study design
We conducted cross sectional analyses of data from two cohort studies of illness self-management behaviors among adults with asthma and COPD [Citation25, Citation26]. Although conducted independently, the recruitment protocol, data collection methods, and adherence measures of these studies were similar. In both studies, eligible participants were identified from daily queries of clinical records systems and were recruited from general medicine and pulmonary practices of the Mount Sinai Hospital in New York City, NY and the Northwestern University Hospital, in Chicago, IL. Interviews were conducted in English or Spanish by trained bilingual research assistants. Both studies were approved by the institutional review boards of the Icahn School of Medicine at Mount Sinai and the Feinberg School of Medicine of Northwestern University.
The asthma study included adults aged 60 years and older with physician diagnosis of moderate or worse persistent asthma. We excluded individuals who had a > 10 pack-year smoking history, a diagnosis of COPD or other chronic obstructive lung disease, and dementia and uncorrectable visual impairment. The COPD study enrolled patients ≥40 years old with a physician diagnosis of COPD who were English or Spanish-speaking. We excluded individuals with diagnoses of other chronic respiratory diseases and dementia or any condition profoundly affecting cognition.
Measurement of self-reported medication adherence
In both studies, adherence to ICS, LABA or LAMA (or combinations) was assessed with the 10-item MARS (Appendix A, supplementary material). The MARS queries patients about medication taking behaviors, including intentional (“I avoid using it if I can”) and unintentional (“I forget to use it”) nonadherence and includes both general questions about medication use (“I use it regularly every day”) as well as items about use under specific circumstances (“I only use it when I feel breathless”). MARS was designed with questions framed as negative statements to limit social desirability bias. Responses are scored on a 5-point Likert scale from very often (1) to never (5). An overall score for the MARS is calculated as the average of the 10 item-specific scores; range is 0-5 with higher scores indicating higher adherence. In a previous study of adults with asthma, a MARS score dichotomized at 4.5 or greater had a sensitivity of 84% and a specificity of 69% against an electronic measure of adherence (defined as 80% or more of doses taken) [Citation15]. We also evaluated the MARS-5 [Citation27, Citation28] a 5-item version of the MARS, which has one item that assesses unintentional nonadherence and four items that assess intentional nonadherence.
In both studies, the MARS was administered during baseline interviews by the research assistant. The MARS was administered rather than self-completed because it was one of several assessments conducted during the 1.5 to 2 h-long interviews.
Objective measurement of medication adherence
The objective measures of adherence included 1) electronic monitoring devices and 2) dose counts from analog counters. [Citation14, Citation29] For electronic devices in both studies, research assistants fit an electronic monitor to metered dose inhalers (MDI) and dry powder inhalers (DPI) and provided the study subjects with instructions to return the monitors after a 4-week period in a postage-paid envelope they provided. Patients also received calls to remind them to return the devices. In the COPD study, the Doser Electronic Monitor (Meditrack, MA) was used for MDI and the Smartdisk (Nexus6, Franklin, OH) for DPI. Only the Doser CT was used in the asthma study. Dose counts from analog counters were documented only for patients using an inhaler device for which an electronic monitor was not available. At the baseline interview, the research coordinators noted the date and number of remaining doses on the device’s analog counter or by the number of non-punctured dose capsules (tiotropium inhaler) and again documented date and doses used at a follow-up interview. The electronic monitors were affixed to the patient’s inhaler when a new inhaler was started (e.g. when their inhaler prescription was renewed). This could occur any time between the baseline interview and 30-days hence.
From these objective measurements, adherence was calculated as the total number of doses used during the 4-week period divided by the number of doses prescribed. Adequate adherence was defined as ≥80% of total doses prescribed, per convention [Citation30, Citation31].
Sociodemographic characteristics
Data were also collected in both studies on sociodemographic and health status variables that are associated with asthma or COPD medication adherence, including age, sex, race, educational attainment, household income, English language proficiency, and general health. These data were obtained by self-report. Additionally, data were collected on asthma or COPD medical histories, including steroid use, prior history of hospitalization and emergency department visits.
Asthma control was assessed with the validated 5-item, Asthma Control Questionnaire [Citation32]. Items are scored on a seven-point scale from 0 (completely controlled) to 6 (very poorly controlled). COPD severity was evaluated using the COPD Severity Score, a validated instrument that assesses respiratory symptoms, systemic corticosteroid use, other COPD medications, home oxygen use, hospitalizations and history of intubation [Citation9, Citation33]. Scores range from 0 to 25 with greater scores indicating a greater disease severity.
Statistical analysis
Separate analyses were performed using data from the asthma and COPD studies. In bivariate analyses, the t-test, chi-square and Wilcoxon rank sum tests were used to compare the characteristics of patients who underwent objective adherence monitoring (including electronically measurements and dose counts) and those who did not. We assessed the internal consistency of the English and Spanish language versions of the instrument by calculating Cronbach’s α.
Criterion validity was determined by calculating the Spearman correlation for the association of the continuous MARS score and the percent of prescribed doses determined from the objective measures of adherence. We also calculated the kappa statistic for agreement between the dichotomous measures of self-reported adherence (MARS ≥4.5) and objectively measured adherence (≥80% of doses taken). Receiver Operating Characteristic (ROC) curve analysis of the continuous value for MARS scores against the dichotomized outcome of objective adherence was also performed. The above set of analyses was repeated with the MARS-5. Statistical tests were performed using SAS statistical software version 9.3 (SAS institute Cary, NC).
Results
Subject characteristics
In the asthma cohort, 452 patients completed the baseline survey; 407 were on controller medications, among whom 341 (84%) completed the MARS questionnaire. Among 241 (53%) patients who underwent objective monitoring, 201 had an electronic monitor for a DPI (84%), 40 (16%) were monitored by review of the inhaler’s analog dose counter. In the COPD cohort, 393 patients completed the baseline survey; 357 were on controller medications, among whom 342 (87%) completed the MARS. Among the 189 COPD patients who had objective monitoring, 98 (52%) had an electronic monitor for a DPI, 10 (5%) had an electronic monitor for a MDI, and 81 (43%) were monitored by review of the inhaler’s analog dose counter. There were no significant differences in either cohort between the sociodemographic characteristics of patients who underwent objective adherence monitoring and those who did not, although asthmatic patients with objective monitoring data were more likely than those without it to have used oral steroids in the past year () and COPD patients with objective monitoring were more likely to have had a hospital admission for COPD exacerbation in the past year ().
Table 1. Demographic and clinical characteristics of patients with asthma.
Table 2. Demographic and clinical characteristics of patients with chronic obstructive pulmonary disease.
The mean age of patients in the asthma and COPD cohorts was 67.5 years () and 67.9 years (), respectively. In both cohorts, the majority of patients were female (asthma, 84% and COPD, 58%), non-Hispanic black (asthma, 31% and COPD, 45%), and Hispanic (asthma, 39% and COPD, 16%).
Medication adherence rates
In the asthma cohort, rates of objective adherence were 70%, the mean MARS score was 4.07 (0.75) and MARS scores ≥4.5 were observed for 130 (38.0%) patients (). MARS scores in the asthma cohort were greater for patients who underwent objective monitoring (4.15 [0.70] than those who did not 3.87 [0.84], p = 0.005).
Table 3. Mean Medication Adherence Report Scale Scores, by adherence as determined by objective measures.
For COPD patients, the rates of objective adherence were 71%, the mean MARS score was 4.12 (0.71), and MARS scores ≥4.5 were observed for 134 (39.2%) (). Scores on the MARS did not differ significantly between the subgroups of patients who did and did not undergo objective monitoring (4.09 [0.72] vs. 4.14 [0.70], respectively; p = 0.62). Scores on the MARS also did not differ significantly between the subgroups of patients whose adherence was assessed by dose counters vs. electronic monitor (4.07 [0.77] vs. 4.2 [0.64], respectively; p = 0.27).
Table 4. Sensitivity and Specificity of the Medication Adherence Report Scale at different threshold values among patients with asthma.
Table 5. Sensitivity and Specificity of the Medication Adherence Report Scale at different threshold values among patients with COPD.
Internal validity
In the asthma cohort overall, the MARS had a Cronbach’s α of 0.79. For the MARS administered in English or Spanish interviews, Cronbach’s α was 0.80 and 0.76, respectively. In the COPD cohort, Cronbach’s α for MARS was 0.75 for all patients, and for the subgroups of English and Spanish-language interviews, 0.75 and 0.68, respectively.
Associations of MARS scores with objectively measured adherence
MARS scores were significantly correlated with objective measures of adherence for both asthma (r = 0.33, p < 0.001) and COPD (r = 0.26, p = 0.0003) patients. There was poor agreement between the dichotomized MARS score and objective adherence measure for the asthma cohort (kappa 0.30, p = 0.49) and COPD cohort (kappa 0.19, p = 0.60).
Sensitivity and specificity of MARS for adherence
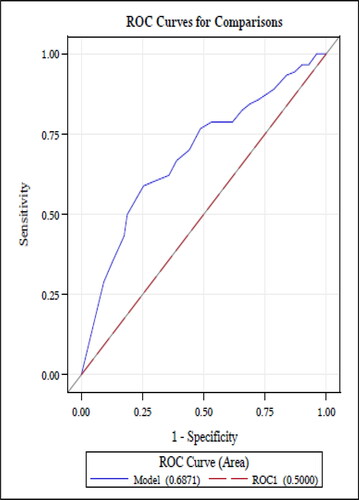
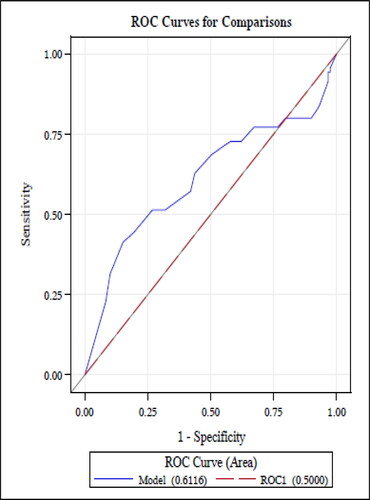
The receiver operator characteristic (ROC) curve for MARS scores among patients with asthma is shown in . The area under the curve (AUC) was 0.69. Similar performance was observed for the MARS among COPD patients (AUC 0.61; ). Among asthma patients, the sensitivity and specificity were 60% (95% CI: 51.0–70.1%) and 71% (95% CI: 64.0–79.0%), respectively. The sensitivities and specificities of the MARS across a range of threshold values are shown in (asthma) and Citation5 (COPD). Among COPD patients, the sensitivity and specificity of the MARS for adequate adherence using a threshold score of ≥4.5 was for 51% (95% CI: 40.2–65.0%) and 68% (95% CI: 60.0–76.4%), respectively. In the asthma cohort, the threshold for MARS that achieved the highest levels of both sensitivity and specificity was ≥4.0, with a sensitivity and specificity of 80% (95% CI: 72.0%-87.3%) and 48% (95% CI: 39.0–56.0%), respectively. In the COPD cohort, the optimal threshold for MARS was also ≥4.0, with a sensitivity and specificity of 74% (95% CI: 62.4–83.2%) and 42% (95% CI: 33.1–51.0%), respectively.
Mars-5
In the asthma cohort, Cronbach’s α for MARS-5 was 0.71 overall, and 0.73 for the English version alone and 0.67 for the Spanish version alone. In the COPD cohort, Cronbach’s α for MARS-5 was 0.65 overall, and 0.66 for English and 0.49 for Spanish. MARS-5 scores and objectively measured adherence correlated weakly in both the asthma cohort (r = 0.29, p = 0.009) and COPD cohort (r = 0.23, p = 0.002). Among asthma patients, MARS-5 had a sensitivity and specificity of 86% (95% CI: 78.2–98.2%) and 33% (95% CI: 27.0%-41.0%), respectively. Among COPD patients, the sensitivity and specificity of the MARS-5 at a threshold of ≥4.5 was 74% (95% CI: 62.4–83.2%) and 34.2% (95% CI: 26.2–43.4%), respectively.
Discussion
This study sought to evaluate the validity of MARS as a tool for measuring self-reported adherence to ICS use among English and Spanish-speaking adults with COPD and asthma. We found that the MARS had good internal validity when measured among patients with COPD or asthma. However, the MARS was weakly correlated with objectively measured medication adherence, and achieved low levels of sensitivity and specificity for both COPD and asthma. Our study confirms the findings of previously published studies that demonstrated good internal validity of the MARS [Citation13], but is not consistent with studies that evaluated the criterion validity of MARS [Citation13, Citation34].
Very few studies have reported comparisons of data from the MARS and objective measures for inhaled medications. In a study of asthmatic patients under the age of 60, we identified a moderate correlation between data from MARS and electronic monitors (r = 0.42, p < 0.001), similar to the performance observe for the MARS in the current study [Citation13]. In a study of COPD patients, other investigators reported a considerably weaker correlation between the MARS and electronic monitors (r = 0.10; p = 0.01) [Citation14]. Another study on this topic was subject to a methodological issue that seriously threatens the validity of their findings. The study assessed the correlation of MARS scores and prescription refill records for ICS use among asthmatics ages 18 to 45 years and reported similar correlation of self-reported and objective adherence measurement (r = 0.46, p < 0.01) [Citation34]. However, the MARS was administered by the patients’ physicians, raising the specter of social desirability bias in the patients’ self-reports.
Studies of MARS for assessment of adherence with non-inhaled medications have shown generally weak results. For antihypertensive medication use among primary care, the MARS had low sensitivity (8.5%) but high specificity (97.4%) compared with prescription refill data [Citation15]. A study conducted among patients with mental health disorders found that MARS scores were weakly correlated with serum concentrations of their psychotropic mediations (r = 0.25, p=.01) [Citation12]. Recruitment of patients for the latter study was performed by their clinicians raising concerns about referral bias.
There are two important potential explanations for the low level of agreement we observed between MARS scores and objectively measured adherence. First, patients may have overestimated medication adherence due to issues with recall [Citation5]. Data for this study were from cohorts of older adults with COPD or asthma, populations more likely to experience problems with memory than younger populations and patients without these conditions [Citation23, Citation24]. Second, COPD and asthma patients may have and use multiple inhalers for the same drug; however objective monitoring was conducted with only one inhaler. These explanations point to the persistent challenges of accurately and reliably measuring adherence to inhaler medications among patients with COPD and asthma, and possibly for medications delivered through other routes as well.
This study has limitations worth noting. First, the electronic measurement of adherence was used only with a subset of patients in both studies, thus analog dose counter data were included. However, in subgroup analyses similar results were obtained for comparisons of electronic monitors and dose counters with MARS scores. Second, the MARS captured patient’s general behaviors at the time of the baseline interview whereas electronic monitors prospectively measured adherence, and electronic monitors could have been affixed to patient’s inhaler devices any time between the day the MARS was administered and up to 30-days hence. . Patients could have had varied medication adherence over this course of time, but this cannot be confirmed and data from other studies suggest that there is little variation in medication adherence as measured by the MARS over 6 months [Citation35]. Third, the asthma and COPD studies were conducted among predominantly African-American and Hispanic older adults in inner-city Chicago and New York, so findings may have limited generalizability to other populations. Additionally, a majority of subjects in both the asthma and COPD studies were women, further limiting generalizability.
The Medication Adherence Rating Scale is a convenient and widely used self-reported measure of medication adherence. We found, however, that it and its abbreviated version (MARS-5) were only weakly associated with objective measurement of adherence to inhaled medications for asthma and COPD. Given this finding, as well as the mixed reports of performance of the MARS compared with objective measures elsewhere in the research literature, investigators should consider using objective measurements of medication adherence when their studies require assessment of medication taking behaviors. Additional research should be considered to determine the settings, including patient populations and clinical conditions, in which the MARS has greatest validity.
Declaration of Interest
The authors have no potential conflicts of interest to disclose.
Additional information
Funding
References
- Employment and Activity Limitations Among Adults with Chronic Obstructive Pulmonary Disease — United States. 2013. Available from:https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6411a1.htm.).
- Summary Health Statistics Tables for U.S. Adults: National Health Interview Survey, 2015. Table A-2. 2015. Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2015_SHS_Table_A-2.pdf. )
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017. Available from: http://goldcopd.org.
- Dolce JJ, Crisp C, Manzella B, et al. Medication adherence patterns in chronic obstructive pulmonary disease. Chest 1991;99(4):837–841. doi:10.1378/chest.99.4.837.
- George J, Kong DC, Thoman R, et al. Factors associated with medication nonadherence in patients with COPD. Chest 2005;128(5):3198–3204. doi:10.1378/chest.128.5.3198.
- Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it's not just black and white. J Allergy Clin Immunol.2003;111(6):1219–1226. doi:10.1067/mai.2003.1479.
- Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149(2_pt_2):S69–S76. doi:10.1164/ajrccm/149.2_Pt_2.S69.
- van Boven JF, Chavannes NH, van der Molen T, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med.2014;108(1):103–113. doi:10.1016/j.rmed.2013.08.044.
- Miravitlles M. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59(5):387–395. doi:10.1136/thx.2003.008730.
- Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014. doi: 10.1002/14651858.CD002990.pub3.
- Haynes RB, Taylor DW, Sackett DL, et al. Can simple clinical measurements detect patient noncompliance? Hypertension 1980;2(6):757–764. doi:10.1161/01.HYP.2.6.757.
- Jonsdottir H, Opjordsmoen S, Birkenaes AB, et al. Medication adherence in outpatients with severe mental disorders: relation between self-reports and serum level. J Clin Psychopharmacol. 2010;30:169–175. doi:10.1097/JCP.0b013e3181d2191e.
- Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy, Asthma Immunol. 2009;103:325–331. doi:10.1016/S1081-1206(10)60532-7.
- Tommelein E, Mehuys E, Van Tongelen I, et al. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48(5):589–595. doi:10.1177/1060028014522982.
- van de Steeg N, Sielk M, Pentzek M, et al. Drug-adherence questionnaires not valid for patients taking blood-pressure-lowering drugs in a primary health care setting. J Eval Clin Pract.2009;15(3):468–472. doi:10.1111/j.1365-2753.2008.01038.x.
- Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. Journal of Psychosomatic Research.1999;47(6):555–567. doi:10.1016/S0022-3999(99)00057-4.
- Horne R, Weinman J. Self-regulation and Self-management in Asthma: Exploring The Role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychology & Health.2002;17:17–32. doi:10.1080/08870440290001502.
- Bowskill R, Clatworthy J, Parham R, et al. Patients' perceptions of information received about medication prescribed for bipolar disorder: implications for informed choice. J Affect Disord.2007;100(1-3):253–257. doi:10.1016/j.jad.2006.10.018.
- Clatworthy J, Bowskill R, Parham R, et al. Understanding medication non-adherence in bipolar disorders using a necessity-concerns framework. J Affect Disord.2009;116(1/2):51–55. doi:10.1016/j.jad.2008.11.004.
- Mahler C, Hermann K, Horne R, et al. Assessing reported adherence to pharmacological treatment recommendations. Translation and evaluation of the Medication Adherence Report Scale (MARS) in Germany. J Eval Clin Pract. 2010;16(3):574–579. doi:10.1111/j.1365-2753.2009.01169.x.
- Mardby AC, Akerlind I, Jorgensen T. Beliefs about medicines and self-reported adherence among pharmacy clients. Patient Educ Couns 2007;69:158–164.
- Tibaldi G, Clatworthy J, Torchio E, et al. The utility of the Necessity–Concerns Framework in explaining treatment non-adherence in four chronic illness groups in Italy. Chronic Illn.2009;5(2):129–133. doi:10.1177/1742395309102888.
- Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J.2010;35(4):913–922. doi:10.1183/09031936.00125109.
- Ray M, Sano M, Wolf MS, et al. Asthma control and cognitive function in a cohort of elderly adults. J Am Geriatr Soc. 2015;63(4):684–691. doi:10.1111/jgs.13350.
- Wisnivesky JP, Krauskopf K, Wolf MS, et al. The association between language proficiency and outcomes of elderly patients with asthma. Ann Allergy, Asthma Immunol. 2012;109:179–184. doi:10.1016/j.anai.2012.06.016.
- Krauskopf K, Federman AD, Kale MS, et al. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. COPD 2015;12(2):151–164. doi:10.3109/15412555.2014.922067.
- Butler JA, Peveler RC, Roderick P, et al. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation 2004;77(5):786–789. doi:10.1097/01.TP.0000110412.20050.36.
- Ediger JP, Walker JR, Graff L, et al. Predictors of medication adherence in inflammatory bowel disease. Am J Gastroenterol. 2007;102(7):1417–1426. doi:10.1111/j.1572-0241.2007.01212.x.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi:10.1056/NEJMra050100.
- DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care.2002;40:794–811. doi:10.1097/00005650-200209000-00009.
- Williams LK, Joseph CL, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–1159. doi:10.1016/j.jaci.2007.08.020.
- Juniper EF, O'Byrne PM, Ferrie PJ, et al. Measuring Asthma Control. Am J Respir Crit Care Med. 2000;162(4):1330–1334. doi:10.1164/ajrccm.162.4.9912138.
- Omachi TA, Yelin EH, Katz PP, et al. The COPD severity score: a dynamic prediction tool for health-care utilization. COPD 2008;5(6):339–346. doi:10.1080/15412550802522700.
- Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res.2008;64(1):47–54. doi:10.1016/j.jpsychores.2007.07.016.
- Mora PA, Berkowitz A, Contrada RJ, et al. Factor structure and longitudinal invariance of the Medical Adherence Report Scale–Asthma. Psychol Health.2011;26(6):713–727. doi:10.1080/08870446.2010.490585.