Abstract
Dual bronchodilation therapy represents the cornerstone for the treatment of COPD. A large retrospective study reports that adding a second long-acting bronchodilator in patients with COPD significantly increases the risk of heart failure. Nevertheless, retrospective studies are characterized by limitations including misdiagnosis and inaccuracy of recordkeeping. This study aimed to ascertain whether tiotropium/olodaterol (T/O) 5/5 μg fixed-dose combination (FDC) may modulate the risk of main cardiovascular outcomes in COPD patients enrolled in randomized controlled trials (RCTs). A meta-analysis (CRD42017070100) was performed by selecting RCTs reporting raw data from the ClinicalTrials.gov database concerning the impact of T/O 5/5 µg FDC vs. monocomponents on the occurrence of specific cardiovascular serious adverse events: arrhythmia, heart failure, myocardial infarction, and stroke. Data were reported as relative risk and 95% Confidence Interval, and the risk of publication bias assessed via Egger’s test. Eighty six full text articles were identified, and 10 RCTs published in 7 studies between 2015 and 2018 were included into the analysis. Data obtained from 12,690 COPD patients (44.47% T/O FDC, 55.53% monocomponents) were extracted. T/O 5/5 μg FDCs did not significantly modulate (p-value > 0.05) the risk of arrhythmia (1.02, 0.55 - 1.92), heart failure (0.88, 0.41 - 1.92), myocardial infarction (1.15, 0.70 - 1.87), and stroke (0.98, 0.44 - 2.16) vs. monocomponents. No significant publication bias affected the effect estimates of this meta-analysis. The results of this quantitative synthesis indicate that dual bronchodilation with T/O 5/5 μg FDC is characterized by an acceptable cardiovascular safety profile in COPD patients.
Introduction
The regular administration of dual bronchodilation therapy is extensively recognized as the cornerstone for the treatment of most patients suffering from chronic obstructive pulmonary disease (COPD) [Citation1–3]. A large retrospective nested case-control study indicated that [Citation4] new initiation of long-acting β2 adrenoceptor agonists (LABAs) or long-acting muscarinic antagonists (LAMAs) in COPD patients is associated with 1.5-fold increased severe cardiovascular (CV) risk, irrespective of prior CV disease status and history of exacerbations. Interestingly, another large retrospective cohort study [Citation5] focused on the main CV outcomes (i.e. myocardial infarction, stroke, heart failure, and arrhythmia) reported that adding a second long-acting bronchodilator in patients with COPD induced an increased risk of heart failure.
These observational studies [Citation4,Citation5] provide the evidence that both single and dual bronchodilation therapy may potentially increase the occurrence of specific CV comorbidities in COPD patients. However, these data could be biased by the typical limitations that affect retrospective studies such as potential misdiagnosis and inaccuracy of recordkeeping, as reported for the association of CV disease with chronic respiratory disorders [Citation6,Citation7].
In this respect, a recent meta-analysis of randomized controlled trials (RCTs) has shown that LABA/LAMA fixed-dose combination (FDC) therapy is characterized by an acceptable CV safety profile in COPD patients, although such a quantitative synthesis was performed overall on all the CV comorbidities and not on specific CV outcomes [Citation8].
To date there are five LAMA/LABA FDCs approved for the treatment of stable COPD: aclidinium/formoterol (A/F), glycopyrronium/formoterol (G/F), glycopyrronium/indacaterol (G/I), T/O, and umeclidinium/vilanterol (U/V). Several pivotal RCTs reported that all of these FDCs are more effective than monocomponents and do not increase the risk of serious adverse events (SAEs) that are characteristic of LAMAs and LABAs when used as monotherapy [Citation9,Citation10]. Nevertheless, the novel IBiS (novel Improved Bidimensional SUCRA [Surface Under the Cumulative Ranking Curve Analysis]) score indicated that each available LAMA/LABAFDC has a specific efficacy/safety profile, with T/O 5/5 μg being the most effective FDC characterized by a good CV safety profile [Citation11].
As recently published by Gershon et al. [Citation12], a well conducted meta-analysis of RCTs provides the highest level of evidence, greater than that obtained by single RCTs or observational studies. In fact when a meta-analysis is performed in agreement with the current guidelines [Citation13], it may increase the power and precision, detect reasons for differences in effect estimates, and settle controversies arising from apparently conflicting studies or generate new hypotheses [Citation14].
Therefore, considering the conflicting evidences regarding the CV safety of bronchodilator therapy, we have carried out a meta-analysis aimed to assess whether the favorable efficacy profile of T/O 5/5 μg FDC is accompanied by a safe CV profile with respect to the main CV outcomes in COPD patients enrolled in RCTs. In these patients we also investigated whether T/O 5/5 µg FDC may have an impact on mortality.
Materials and methods
Search strategy
This pair-wise meta-analysis has been registered in the international prospective register of systematic reviews (PROSPERO registration number: CRD42017070100; available at https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017070100), and performed in agreement with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols 2015 (PRISMA-P 2015 statement) () [Citation13].
Figure 1. PRISMA flow diagram for the identification of the full text articles included in the meta-analysis concerning the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia, heart failure, myocardial infarction, and stroke in COPD patients enrolled in RCTs. COPD: chronic obstructive pulmonary disease; CV: cardiovascular; FDC: fixed-dose combination; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs: randomized controlled trials; SAEs: serious adverse events; T/O: tiotropium/olodaterol.
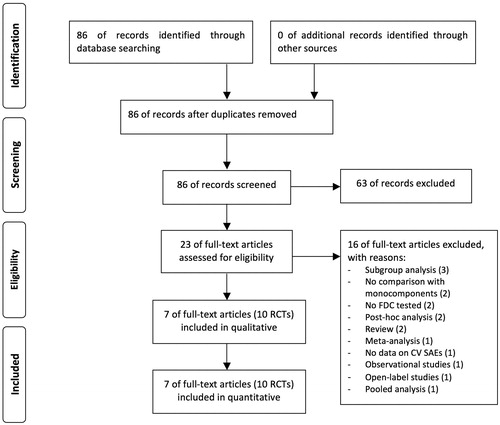
A comprehensive literature search was undertaken for full text articles written in English and investigating the impact of T/O FDC in COPD patients, in agreement with the following query: (“tiotropium bromide”[MeSH Terms] OR (“tiotropium”[All Fields] AND “bromide”[All Fields]) OR “tiotropium bromide”[All Fields] OR “tiotropium”[All Fields]) AND (“olodaterol”[Supplementary Concept] OR “olodaterol”[All Fields]) AND (combination[All Fields] OR combined[All Fields] OR (fixed-dose[All Fields] AND combination[All Fields]) OR FDC[All Fields]) AND (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR “copd”[All Fields]) AND (“loattrfull text”[sb] AND English[lang]). The final search strategy performed in agreement with OVID MEDLINE is reported in Table S1 of the supplemental online material. The search was performed in Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, Embase, EU Clinical Trials Register, Google Scholar, MEDLINE, Scopus, and Web of Science databases through March 2020, in order to provide for relevant studies published up to March 5th, 2020. Citations of previous published meta-analyses and relevant reviews were checked to select further pertinent studies, if any [Citation8,Citation15,Citation16].
Two reviewers independently checked the relevant RCTs identified from literature searches and databases. RCTs were selected in agreement with the previously mentioned criteria, and any difference in opinion about eligibility was resolved by consensus.
Study selection
The titles and abstracts of all the records identified in initial research were reviewed, and then a list of full text articles to be assessed for selection was defined. RCTs reporting raw data concerning the impact of T/O 5/5 µg FDC vs. monocomponents (T 5 µg and/or O 5 µg) on specific CV SAEs in patients suffering from COPD diagnosed by pulmonary function testing were selected. The availability of patient level data on ClinicalTrial.gov database was a requirement for selection. No study duration limit was applied. Full text articles not written in English, and/or not reporting original data from RCTs, and/or performed in non-COPD patients, and/or not comparing T/O 5/5 µg FDC with T 5 µg and/or O 5 µg, and/or not reporting specific data on CV SAEs in the ClinicalTrial.gov database were excluded. Two reviewers independently examined the RCTs and any difference in opinion about eligibility was resolved by consensus.
Data extraction
Data from included studies were extracted from published papers, and/or online supplementary files, and/or the repository database ClinicalTrial.gov. Data extraction was performed in agreement with the recommendations provided by the Cochrane Handbook for Systematic Reviews of Interventions [Citation17]. Data were extracted and checked for study characteristics and duration, patient characteristics, age, sex, smoking habit, forced expiratory volume in 1 s (FEV1), arrhythmia, heart failure, myocardial infarction, stroke, death, and Jadad score.
Endpoints
The primary endpoint of this meta-analysis was to assess the risk of CV SAEs of T/O 5/5 µg FDC in COPD patients, compared to the monocomponents included in the FDC. The secondary endpoint was the influence of T/O 5/5 µg FDC on mortality in COPD patients, compared to the monocomponents included in the FDCs.
Quality score, risk of bias and evidence profile
The Jadad score, with a scale of 1–5 (score of 5 being the best quality), was used to assess the quality of the papers concerning the likelihood of bias related with randomization, double blinding, withdrawals and dropouts [Citation18]. Studies were considered of low quality at Jadad score <3, of medium quality at Jadad score =3, and of high quality at Jadad score >3. Two reviewers independently assessed the quality of individual studies, and any difference in opinion about the quality score was resolved by consensus.
The test for heterogeneity (I2) was performed for primary endpoint to quantify the bias introduced by between-study dissimilarity, as previously reported [Citation19], and low, moderate, and high heterogeneity was assigned for I2 values of ≃25%, ≃50%, and ≃75%, respectively [Citation20].
The risk of publication bias was assessed for primary endpoint by applying the funnel plot and Egger’s test through the following regression equation: SND = a + b × precision, where SND represents the Standard Normal Deviate (treatment effect divided by its Standard Error [SE]), and precision represents the reciprocal of the standard error. Evidence of asymmetry from Egger’s test was considered to be significant at p-value < 0.1, and the graphical representation of 90% confidence bands is presented [Citation21].
The quality of the evidence for primary endpoint was assessed in agreement with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [Citation22].
Data analysis
The results of the pair-wise meta-analysis are expressed as relative risk (RR) and 95% confidence interval (95%CI). RCTs reporting at least one specific CV SAE including arrhythmia, heart failure, myocardial infarction, and stroke were included in the forest plots.
Since data were selected from a series of studies performed by researchers operating independently, and a common effect size cannot be assumed, the random-effects model was used in order to balance the study weights and adequately estimate the 95%CI of the mean distribution of effects on the investigated variable [Citation23]. In fact, although the mathematics behind the fixed-effects model is much simpler than that of the random-effects model, results of this quantitative synthesis cannot be generalized via fixed-effects model since the included studies were quite dissimilar [Citation24], as shown in . Therefore, the greater the degree of difference across the studies, the more important it becomes to employ the random-effects model [Citation25]. Moreover, since the follow-up duration was not consistent across the RCTs, the data have been normalized as a function of person-year [Citation26,Citation27]. This method, supported by the Cochrane Collaboration and successfully used in recent meta-analyses [Citation14,Citation19–21] involves the conversion of the measures into a common metric (events per person-time) prior to meta-analyzing the data, leading to improved estimates of effect, precision, and clinical interpretability of results [Citation19,Citation20].
Table 1. Patient demographics, baseline and study characteristics.
Subset analyses were performed with regard to the effect of the class of monocomponents included in the FDC (LABA or LAMA).
Meta-regression analysis was performed for primary endpoints to examine the source of significant (p-value < 0.05) heterogeneity between-studies (I2) and, eventually, identify potential effect modifiers [Citation28].
A pooled analysis was performed to calculate the frequency (% and 95%CI) of AEs, ranked in agreement with the “European Medicine Agency, section 4.8: Undesirable effects”, as follows: very common ≥1/10, common ≥1/100 to <1/10, uncommon ≥1/1000 to <1/100, frequency not known if not calculable from the available data [Citation29].
OpenMetaAnalyst software was used for performing the meta-analysis, OpenEpi [Citation30] software for the pooled analysis, GraphPad Prism (CA, US) software to graph the data, and GRADEpro GDT to assess the quality of evidence [Citation19,Citation22]. The statistical significance was assessed for p-value < 0.05.
Results
Studies characteristics
Eighty six full text articles were identified and sixty three were excluded by assessing the title and abstract; sixteen out of the twenty three eligible studies were excluded by considering each whole paper and in agreement with reasons reported in . Finally, data obtained from 12,690 COPD patients (44.47% treated with T/O 5/5 µg FDC, 43.60% treated with T 5 µg, 11.93% treated with O 5 µg) were extracted from 7 studies including 10 RCTs published between 2015 and 2018 [Citation31–37].
Detailed patient demographics, baseline and characteristics of the studies included in this meta-analysis are reported in . Briefly, all the studies were Phase 3 RCTs characterized by a Jadad score ≥3 and lasting between 6 weeks and 52 weeks. Both the FDC and monocomponents were administered once daily via the Soft Mist Inhaler device Respimat®. The age of enrolled patients ranged from 61.1 years and 69.8 years, with post bronchodilator FEV1 (% predicted) values between 44.6% and 59.4%.
Primary endpoint
Raw data concerning the specific CV SAEs investigated in this study (arrhythmia, heart failure, myocardial infarction, and stroke) occurred during the RCTs have been extracted from files of the ClinicalTrials.gov database.
The overall pairwise meta-analysis indicated that T/O 5/5 µg FDC did not significantly (p-value > 0.05) increase the risk of arrhythmia (RR 1.02, 95%CI 0.55 – 1.92; I2 8.01%, p = 0.37; ), heart failure (RR 0.88, 95%CI 0.41 – 1.92; I2 15.46%, p = 0.31; ), myocardial infarction (RR 1.15, 95%CI 0.70 – 1.87; I2 0%, p = 0.70; ), and stroke (RR 0.98, 95%CI 0.44 – 2.16; I2 0%, p = 0.88; ) in COPD patients, compared with monocomponents.
Figure 2. Forest plot of pairwise meta-analysis of the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia (A), heart failure (B), myocardial infarction (C), and stroke (D) in COPD patients. Each forest plot reports also the subset analysis with regard to the effect of the class of monocomponents included in the FDC (T/O 5/5 μg FDC vs. T 5 μg or vs. O 5 μg). COPD: chronic obstructive pulmonary disease; FDC: fixed-dose combination; LABA: long-acting β2 adrenoceptor agonist; LAMA: long-acting muscarinic antagonist; O: olodaterol; T: tiotropium.
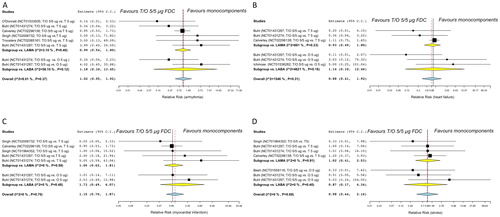
The subset analysis showed that there was no significant (p-value > 0.05) difference between T/O 5/5 μg FDC and T 5 μg or between T/O 5/5 μg FDC and O 5 μg on the risk of arrhythmia, heart failure, myocardial infarction, and stroke ().
The pooled analysis showed that the frequency of the investigated CV SAEs was generally uncommon ().
Table 2. Pooled analysis of the frequency of specific CV SAEs (arrhythmia, heart failure, myocardial infarction, and stroke) in COPD patients treated with T/O 5/5 μg FDC and their monocomponents. Data were extracted from RCTs in the repository database ClinicalTrials.gov that reported at least one specific CV SAEs in the investigated arms. The frequencies were ranked in agreement with EMA guideline.
No significant heterogeneity was detected in the pair-wise meta-analysis and, thus, no meta-regression analysis was performed. The analysis of risk of bias carried out via the visual inspection of funnel plot evidenced neither dispersion nor asymmetry (), as confirmed by the lack of statistical significance (p-value > 0.1) resulting from the Egger’s ().
Figure 3. Publication bias assessment via funnel plots (left panels) and Egger’s test (right panels) for the impact of T/O 5/5 μg FDC vs. monocomponents on the risk of arrhythmia (A and B), heart failure (C and D), myocardial infarction (E and F), and stroke (G and H) in COPD patients. COPD: chronic obstructive pulmonary disease; FDC: fixed-dose combination; O: olodaterol; T: tiotropium.
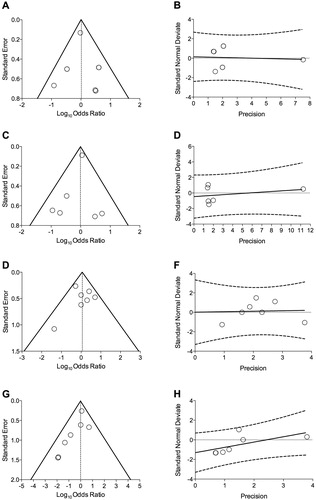
The GRADE analysis reported high quality of evidence (++++) concerning the effect estimates resulting from the pairwise meta-analysis on the risk of arrhythmia, heart failure, myocardial infarction, and stroke induced by T/O 5/5 μg FDC vs. monocomponents in COPD patients.
Secondary endpoint
Raw data concerning all cause mortality occurred during the RCTs have been extracted from files of the ClinicalTrials.gov database. Three studies reported at least one event of death [Citation31,Citation33,Citation36] and were included in the meta-analysis.
The pairwise meta-analysis indicated that T/O 5/5 µg FDC did not significantly (p-value > 0.05) increase the risk of death (RR 0.92, 95%CI 0.70 – 1.22; I2 0%, p = 0.84) in COPD patients, compared with monocomponents.
Discussion
The results of this pairwise meta-analysis indicate that T/O 5/5 µg is a safe FDC to treat COPD patients when compared with monocomponents. Specifically, the findings show that combining T 5 µg with O 5 µg does not increase the potential risk of arrhythmia, heart failure, myocardial infarction, and stroke that can affect the LABAs and LAMAs when administered as monocomponents. These results are confirmed by the subset analyses, in which there was no difference in the risk of specific CV SAEs when comparing T/O 5/5 µg FDC with either T 5 µg or O 5 µg. As expected, also the risk of death was not increased by T/O 5/5 µg FDC vs. monocomponents.
The findings resulting for the specific CV SAEs investigated in this quantitative synthesis are characterized by a high quality of evidence, as confirmed by the GRADE analysis. This means that we are very confident that the true effect lies close to that of the estimate of the effect; in other words, further research is very unlikely to change our confidence in the estimate of effect [Citation38]. Such a reliability of the effect estimates was mainly due to the quality of the RCTs as reported by Jadad score, the lack of heterogeneity, and the absence of publication bias confirmed by the qualitative assessment of funnel plots and the quantitative analysis of Egger’s test.
Interestingly, the data originating by the meta-analysis of RCTs on the specific CV safety profile of T/O 5/5 µg FDC vs. monocomponents are generally consistent with data from real-world settings published by Suissa et al. [Citation5], in which the addition of a second long-acting bronchodilator as recommended by COPD treatment guidelines appears to be safe. However, in the same study the authors [Citation5] highlighted that there was a small, although significant, increase in the risk of heart failure ranging from RR 1.14 (95%CI 1.03 – 1.26) to RR 1.16 (95%CI 1.03 – 1.30) for dual bronchodilation therapy vs. monotherapy. However, it should be noted that the COPD population included in RCTs could be different from the real-world population using bronchodilator therapy. In fact real-world observational investigations are designed to not exclude COPD patients with comorbidities or have limitations with inclusion criteria regarding the severity of disease, upper age limit, or smoking history [Citation39,Citation40].
The results of this meta-analysis showed neither a statistical significance nor a trend toward significance in the increased risk heart failure, with RR effect estimate value at 0.88 (95%CI 0.41 – 1.92). However, it cannot be omitted that a detailed assessment of the cases reported in shows an increase in the heart failure frequency when the LAMA is included in the therapy. Thus, we agree with Suissa et al. [Citation5] that the modest increase of heart failure frequency may warrant further investigation [Citation5], but it seems that attention should be focused not specifically on LAMA/LABA FDCs, but generally on bronchodilation therapy based on antimuscarinic agents. In this regard, recent quantitative syntheses have shown that each LAMA/LABA FDC is characterized by a specific efficacy/safety profile, with T/O and A/F FDCs being the safest FDCs and those including glycopyrronium (i.e. G/F and G/I) as having the less favorable CV safety profile [Citation8,Citation11].
However, the current evidence provides further data confirming the CV safety of LAMAs. In fact in another large observational study always published by Suissa et al. in 2017 [Citation41], the initiation of COPD treatment with T or with a LABA was associated with similar risk of arrhythmia, heart failure, myocardial infarction, and stroke, and a significantly lower risk of pneumonia with T compared with LABAs. Interestingly, also in the large retrospective nested case-control study carried out by Wang et al. [Citation4] the new initiation of LABAs or LAMAs in COPD patients induced the same risk of heart failure and other CV diseases. Notably, the 4-year Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial has demonstrated that T not only is safe by a CV viewpoint, but in patients treated with T the risk of heart failure and myocardial infarction was even reduced than in those that received placebo.
The main strength of this study is related with the high quality of evidence resulting from the meta-analysis and, thus, certainly the obtained findings can be valid for populations of COPD patients with characteristics that are consistent with those of patients enrolled in RCTs. Furthermore, this meta-analysis has been conducted in agreement with the current guidelines [Citation13] and registered in PROSPERO as indicated by the PRISMA-P 2015 Explanation and Elaboration statement [Citation42]. Nevertheless, it should not be forgotten that only a small proportion (≃14%) of subjects with COPD can be eligible in real-life for inclusion in RCTs [Citation43,Citation44], and this discrepancy represents the main weakness of our study.
Meta-analyses, as well as RCTs and observational studies, are characterized by intrinsic limitations [Citation45–47]. Thus, taken together the results of this meta-analysis along with those of the current evidences raised from large RCTs and large observational studies, the results of this research should be interpreted by clinicians in agreement with the medical characteristics of each COPD patient.
Conclusion
In this meta-analysis we have demonstrated that, although some specific LABA/LAMA FDCs may increase the risk of CV SAEs, T/O 5/5 µg FDC is characterized by an acceptable CV safety profile vs. monocomponents, with no effect on the risk of arrhythmia, myocardial infarction, stroke, and mortality and limited not significant impact on the frequency of heart failure in COPD patients.
Declaration of interest
PR reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, grants from Zambon, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from Mundipharma, outside the submitted work.
BLR reports no conflict of interest.
MC reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma, personal fees from Ockham Biotech, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, personal fees from Lallemand, personal fees from Mundipharma, personal fees from Pfizer, outside the submitted work.
MGM reports personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, personal fees from Chiesi Farmaceutici, personal fees from Almirall, personal fees from ABC Farmaceutici, personal fees from GlaxoSmithKline, outside the submitted work.
LC reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, non-financial support from AstraZeneca, grants from Chiesi Farmaceutici, grants from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma, personal fees from Ockham Biotech, outside the submitted work.
Additional information
Funding
References
- Thomas M, Halpin DMG, Miravitlles M. When is dual bronchodilation indicated in COPD? COPD. 2017;12:2291–2305. doi:10.2147/COPD.S138554.
- GOLD. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD – 2020 Report; 2020. [Last accessed 2020 Mar 7]. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
- Matera MG, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators revisited. Pharmacol Rev. 2020;72(1):218–252. doi:10.1124/pr.119.018150.
- Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178(2):229. doi:10.1001/jamainternmed.2017.7720.
- Suissa S, Dell'Aniello S, Ernst P. Concurrent use of long-acting bronchodilators in COPD and the risk of adverse cardiovascular events. Eur Respir J. 2017;49(5):1602245. doi:10.1183/13993003.02245-2016.
- Rogliani P, Cazzola M, Calzetta L. Cardiovascular disease in chronic respiratory disorders and beyond. J Am Coll Cardiol. 2019;73(17):2178–2180. doi:10.1016/j.jacc.2018.11.068.
- Carter P, Lagan J, Fortune C, et al. Association of cardiovascular disease with respiratory disease. J Am Coll Cardiol. 2019;73(17):2166–2177. doi:10.1016/j.jacc.2018.11.063.
- Rogliani P, Matera MG, Ora J, et al. The impact of dual bronchodilation on cardiovascular serious adverse events and mortality in COPD: a quantitative synthesis. COPD. 2017;12:3469–3485. doi:10.2147/COPD.S146338.
- Matera MG, Rogliani P, Calzetta L, et al. Safety considerations with dual bronchodilator therapy in COPD: an update. Drug Saf. 2016;39(6):501–508. doi:10.1007/s40264-016-0402-4.
- Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646.
- Rogliani P, Matera MG, Ritondo BL, et al. Efficacy and cardiovascular safety profile of dual bronchodilation therapy in chronic obstructive pulmonary disease: A bidimensional comparative analysis across fixed-dose combinations. Pulm Pharmacol Ther. 2019;59:101841. doi:10.1016/j.pupt.2019.101841.
- Gershon AS, Jafarzadeh SR, Wilson KC, et al. Clinical knowledge from observational studies. everything you wanted to know but were afraid to ask. Am J Respir Crit Care Med. 2018;198(7):859–867. doi:10.1164/rccm.201801-0118PP.
- Moher D, Shamseer L, Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.
- Deeks J, Higgins J, Altman D. Chapter 9–Analysing data and undertaking meta-analyses: Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. In: Cochrane handbook for systematic reviews of interventions version. Vol. 5; 2011.
- Rogliani P, Calzetta L, Matera MG, et al. Inhaled therapies and cardiovascular risk in patients with chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2019;20(6):737–750. doi:10.1080/14656566.2019.1570133.
- Rogliani P, Ora J, Matera MG, et al. The safety of dual bronchodilation on cardiovascular serious adverse events in COPD. Expert Opin Drug Saf. 2018;17(6):589–596. doi:10.1080/14740338.2018.1472232.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_6_4_meta_regression.htm. The Cochrane Collaboration. 2011;9.6.4 Meta-regression.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4.
- Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Soft. 2012;49(5):1–15. doi:10.18637/jss.v049.i05.
- Calzetta L, Crupi R, Roncada P, et al. Clinical efficacy of bronchodilators in equine asthma: Looking for minimal important difference. Equine Vet J. 2020;52(2):305–313. doi:10.1111/evj.13137.
- Calzetta L, Roncada P, di Cave D, et al. Pharmacological treatments in asthma-affected horses: A pair-wise and network meta-analysis. Equine Vet J. 2017;49(6):710–717. doi:10.1111/evj.12680.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026.
- Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24(137):451–461. doi:10.1183/16000617.00002215.
- DeCoster J. Meta-analysis notes; 2004. [accessed 2017 June 14. Available from: http://www.stat-help.com/meta.pdf.
- Turner JR, Durham TA. Meta-methodology: conducting and reporting meta-analyses. J Clin Hypertens. 2014;16(2):91–93. doi:10.1111/jch.12215.
- Shen Y, Cai W, Lei S, et al. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2013;11(3):131230073230003–131230073230008. doi:10.3109/15412555.2013.858315.
- Calzetta L, Matera MG, Braido F, et al. Withdrawal of inhaled corticosteroids in COPD: a meta-analysis. Pulm Pharmacol Ther. 2017.
- Cazzola M, Rogliani P, Calzetta L, et al. Impact of Mucolytic Agents on COPD Exacerbations: A Pair-wise and Network Meta-analysis. COPD. 2017;14(5):552–563. doi:10.1080/15412555.2017.1347918.
- European Commission. A guideline on summary of product characteristics (SmPC); 2009. [accessed 2017 June 22]. Available from: http://ec.europa.eu/health//sites/health/files/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf
- Sullivan KM, Dean A, Soe MM. OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep. 2009;124(3):471–474. doi:10.1177/003335490912400320.
- Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344.
- Troosters T, Maltais F, Leidy N, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–1032. doi:10.1164/rccm.201706-1288OC.
- O'Donnell DE, Casaburi R, Frith P, et al. Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD. Eur Respir J. 2017;49(4):1601348. doi:10.1183/13993003.01348-2016.
- Ichinose M, Kato M, Takizawa A, et al. Long-term safety and efficacy of combined tiotropium and olodaterol in Japanese patients with chronic obstructive pulmonary disease. Respir Investig. 2017;55(2):121–129. doi:10.1016/j.resinv.2016.09.004.
- Beeh KM, Westerman J, Kirsten AM, et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;32:53–59. doi:10.1016/j.pupt.2015.04.002.
- Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014.
- Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi:10.1016/j.rmed.2015.08.002.
- Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015.
- Kardos P, Worsley S, Singh D, et al. Randomized controlled trials and real-world observational studies in evaluating cardiovascular safety of inhaled bronchodilator therapy in COPD. COPD. 2016;11:2885–2895. doi:10.2147/COPD.S118867.
- Pahus L, Burgel PR, Roche N, et al. Randomized controlled trials of pharmacological treatments to prevent COPD exacerbations: applicability to real-life patients. BMC Pulm Med. 2019;19(1):127. doi:10.1186/s12890-019-0882-y.
- Suissa S, Dell'Aniello S, Ernst P. Long-acting bronchodilator initiation in COPD and the risk of adverse cardiopulmonary events: a population-based comparative safety study. Chest. 2017;151(1):60–67. doi:10.1016/j.chest.2016.08.001.
- Shamseer L, Moher D, Clarke M, Ghersi D. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;349(1):g7647–g7647. doi:10.1136/bmj.g7647.
- Scichilone N, Basile M, Battaglia S, et al. What proportion of chronic obstructive pulmonary disease outpatients is eligible for inclusion in randomized clinical trials?. Respiration. 2014;87(1):11–17. doi:10.1159/000355082.
- Travers J, Marsh S, Caldwell B, et al. External validity of randomized controlled trials in COPD. Respir Med. 2007;101(6):1313–1320. doi:10.1016/j.rmed.2006.10.011.
- Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol. 2016;16(1):102. doi:10.1186/s12871-016-0265-3.
- Sylvester RJ, Canfield SE, Lam TBL, et al. Conflict of evidence: resolving discrepancies when findings from randomized controlled trials and meta-analyses disagree. European Urology. 2017;71(5):811–819. doi:10.1016/j.eururo.2016.11.023.
- Cazzola M, Calzetta L, Rogliani P, et al. Triple therapy versus dual bronchodilation and inhaled corticosteroids/long-acting β-agonists in COPD: accumulating evidence from network meta-analyses. Pulm Ther. 2019;5(2):110–117. doi:10.1007/s41030-019-00102-8.

