Abstract
Obesity has been shown to have a paradoxical benefit in a number of conditions, but the long-term effects in obesity after chronic obstructive pulmonary disease (COPD) exacerbation is still unclear. In this study, the effects of obesity on short- and long-term outcomes after a COPD exacerbation were evaluated. This was a secondary analysis of the Rapid Empiric Treatment with Oseltamivir Study (RETOS): a prospective, randomized, unblinded clinical trial. Patients were included in the study if they were hospitalized for acute exacerbation of COPD. Obesity was noted as patients with BMI >30. Clinical outcomes of time to clinical stability, length of stay, and mortality were compared. A total of 301 patients were included in the study, 122 (41%) patients were obese. There was no significant difference in the length of stay and time to clinical stability between patients with and without obesity. Mortality for patients with and without obesity was 3% and 3% at 30 days, 7% and 18% at six months, and 8% and 28% at one year, respectively. After adjusting with multivariable regression analysis, patients with obesity had a significant reduction in odds of dying at one year (adjusted odds ratio (aOR): 0.18; 95% CI: 0.06–0.58; p = .004) and at six months (aOR: 0.28; 95% CI: 0.09–0.89; p = .031). Our study showed that obesity was associated with reduced mortality at one year and six months after a COPD exacerbation. Although patients with obesity had higher rates of comorbidities, they had reduced mortality at one year after multivariable regression analysis.
Introduction
The majority of patients with chronic obstructive pulmonary disease (COPD) have a comorbidity which plays a significant role in the management and progression of the disease [Citation1]. In the last 40 years, there has been a steady increase in obesity around the world. The prevalence of obesity in COPD patients has varied amongst studies but has been estimated between 30% and 40% [Citation2]. The prevalence of obesity within the COPD population has been shown to be higher or similar to the non-COPD population [Citation3,Citation4].
Obesity has been shown to provide a protective effect on multiple diseases like heart failure, coronary artery disease, and chronic kidney disease [Citation5–Citation8]. This has coined the “obesity paradox”. There is controversy in the literature about the effect of obesity in COPD patients. For instance, some studies have shown worse health status, quality of life, dyspnea, and increased health care utilization amongst COPD patients with obesity [Citation1]. On the other hand, more recent studies have established an obesity paradox in the COPD population [Citation9–Citation11].
There have been a few studies evaluating the effects of obesity in hospital mortality after acute exacerbation of COPD [Citation9,Citation10,Citation12]. There has been little data on the long-term effects of obesity on COPD patients. The purpose of this study was to compare short- and long-term outcomes of COPD patients with and without obesity who had an exacerbation requiring hospitalization.
Methods
Study design
This was a secondary analysis of the Rapid Empiric Treatment with Oseltamivir Study (RETOS): a prospective, randomized, unblinded clinical trial [Citation13]. Patients were enrolled in influenza seasons between October 2010 and March 2013. For the primary study, informed consent was obtained from all patients. Additionally, the primary study was approved by the Institutional Review Board (protocol 10.0465).
Criteria for COPD exacerbation included the presence of one respiratory symptom and one sign of acute infection. Respiratory symptoms and signs at the time of admission: new or increased cough, change in sputum production (colour or quantity), evidence for reduced oxygenation (O2 Sat <90%, or for patients on home O2 therapy a 1 L increase in their O2 requirement), new auscultory findings (rales, rhonchi, wheezing), new shortness of breath, rapid respiratory rate (>24 breaths per minute). Signs of acute infection at the time of admission included fever >37.8 °C (100.0 F) or hypothermia <35.6 °C (96.0 F), changes in white blood cell count (leukocytosis, leukopenia, and abnormal differential such as left shift), and altered mental status. Patients without a history of COPD and with an infiltrate on chest X-ray were excluded.
In our secondary analysis, patients were included if they were hospitalized for acute exacerbation of COPD. Patients were considered obese if their calculated body mass index (BMI) was 30 or greater. COPD was established based on documentation in medical record.
Clinical outcomes
Outcomes of time to clinical stability, length of stay, and mortality were assessed. Clinical stability was defined as the first day patient met all of the following four criteria: cough improving, afebrile for at least 8 h, white blood cell count normal or improving, oral intake, and absorption are adequate. Length of stay was defined as date of discharge minus day of admission. Mortality was evaluated at in hospital, 30 days, 6 months, and 1 year.
Statistical analysis
Descriptive statistics reported were frequency and percentage for categorical data variables, and medians and interquartile ranges (IQR) for continuous data. Categorical variables were compared using Chi-Squared tests and continuous variables were compared using Mann–Whitney U tests. Log-rank tests were used to compare time to clinical stability and length of stay without adjustment. Cox proportional hazards regression was performed to analyze differences between groups for time to clinical stability and length of stay. Logistic regression was performed to analyze differences in mortality at six months and one year. Variables adjusted for Cox regression and logistic regression were age, sex, history of liver disease, diabetes, congestive heart failure, smoking, home oxygen requirement, peripheral oxygen capillary saturation, stroke, cancer, coronary artery disease, hypertension, hyperlipidemia, altered mental status, hematocrit, blood urea nitrogen, temperature, treatment with and without oseltamivir. p Values of less than .05 were considered statistically significant. A sensitivity analysis with removal of patients with BMI <21 was performed.
Results
Overall characteristics
A total of 301 patients were included in the study. Of these, 122 (41%) patients were obese. Median age of patients with and without obesity were 61 [IQR: 52–68] and 65 [IQR: 56–74], respectively (p < .001). Diabetes mellitus was present in 76 (62%) patients with obesity and 42 (23%) of the patients without obesity (p < .001). Congestive heart failure was present in 49 (40%) patients with obesity and 42 (25%) patients without obesity (p = .006). Coronary artery disease was present in 49 (40%) patients with obesity and 42 (23%) patients without obesity (p = .003). Median BMI in patients with and without obesity were 36 [IQR: 33–41] and 24 [IQR: 21–27], respectively (p < .001). Both the groups had similar rates of severity of illness defined by the need for intensive care unit admission and ventilator support. Further characteristics can be reviewed in .
Table 1. Baseline patient characteristics according to obesity.
Clinical stability and length of stay
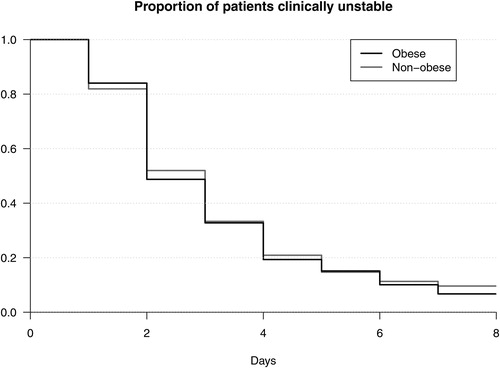
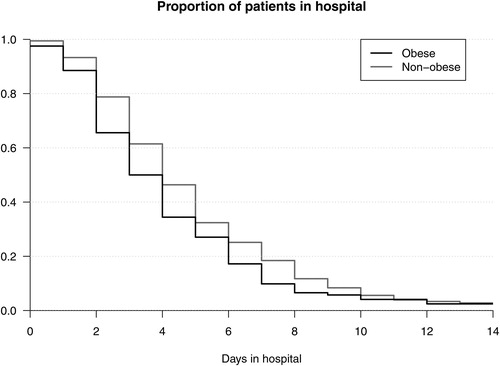
Patients with obesity compared to patients without obesity had a median time to clinical stability of 2 (IQR: 2–4) days vs. 3 (IQR: 2–4) days (see ). In a multivariable regression analysis there was no significant change between the two groups (adjusted hazard ratio (aHR): 1.13; 95% CI: 0.84–1.52; p = .429). Patients with obesity compared to patients without obesity had a median length of stay of 3.5 (IQR: 2–6) days vs. 4 (IQR: 3–6) days (see ). In multivariable regression, stratified on sex, there was no significant change between the two groups (aHR: 1.30; 95% CI: 0.97–1.75; p = .080).
Mortality
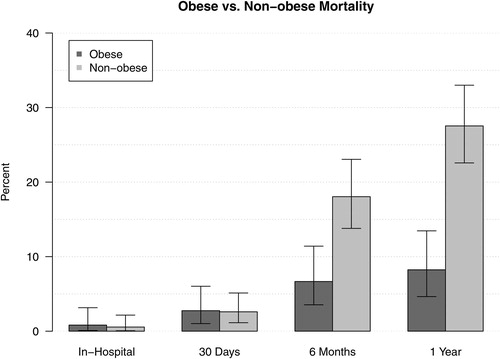
Mortality for patients with obesity was less than 1% at discharge, 3% at 30 days, 7% at six months, and 8% at one year. Mortality for patients without obesity was less than 1% at discharge, 3% at 30 days, 18% at six months, and 28% at one year. See for mortality data. After adjusting for multivariable regression analysis, patients with obesity had a significant reduction in odds of dying at one year (adjusted odds ratio (aOR): 0.18; 95% CI: 0.06–0.58; p = .004) and also at six months (aOR: 0.28; 95% CI: 0.09–0.89; p = .031). In sensitivity analysis, removing patients with BMI <21, one-year mortality remained significant (aOR: 0.24; 95% CI: 0.07–0.87; p = .030; n = 255).
Discussion
Our study showed that obesity was associated with reduced mortality at one year and six months after a COPD exacerbation. Patients with obesity had higher rates of comorbidities including hypertension, hyperlipidemia, diabetes mellitus, congestive heart failure, and coronary artery disease. Despite the higher rates of comorbidities, patients with obesity still had lower mortality at one year.
Adjustment analysis did not show a change in time to clinical stability nor length of stay between patients with and without obesity. Mortality benefit was evident at one year and six months, but was not demonstrated on short-term follow up. Previous studies have also noted no mortality benefit in short-term follow up [Citation9,Citation10]. The reduced mortality may not be evident on the short-term follow up due to the small number of deaths during that period. Patients with obesity also tended to have younger age. Both the groups had similar severity of illness with less than 10% of patients in each group requiring admission to the intensive care unit.
Similarly, Lainscak et al. demonstrated in a retrospective study that higher BMI was independently predictive of better long-term survival up to 3 years after a COPD exacerbation. Patients in their overweight group, BMI 25–29, had the best long-term survival followed closely by patients with BMI >30. Similar to our analysis, normal weight and underweight patients had the worst long-term survival. In their study, they were able to accurately identify COPD patients with spirometry [Citation10].
Obesity has been established as a risk factor for cardiovascular disease, diabetes, hypertension, and other diseases that often lead to worse outcomes. Therefore, one would expect that obesity would worsen outcomes in COPD patients. The BMI, obstruction, dyspnea, exercise capacity index (BODE) were developed to predict prognosis in COPD patients. Based on this scoring system those with lower BMI have worse prognosis, though it is mostly limited to outpatient use [Citation14]. This study alternatively supports the notion that lower BMI patients tend to have a worse prognosis. Low BMI COPD patients have consistently shown worse outcomes [Citation14]. In our study, even when patients with BMI <21 were removed from the analysis, we demonstrated that patients with obesity had improved survival compared to normal and overweight patients.
Obesity has been associated with decreased lung volumes, hyperinflation, and functional residual capacity [Citation1]. Despite these findings, patients with obesity and COPD are shown to do better at one year, according to our analysis. There have been multiple theories proposed to argue this epidemiological phenomenon, identified as the obesity paradox. A few of the main arguments supporting the obesity paradox are regarding muscle mass and the effects of adipose tissue on inflammation.
In regard to muscle mass, the theory argues that higher BMIs are naturally associated with larger muscle mass. Muscle mass becomes a large component of patient’s ability to breathe, especially during COPD exacerbations. Muscle fatigue is a common cause of respiratory failure in the setting of an exacerbation. During an exacerbation, an obese patient would have more respiratory reserve due to increased muscle mass [Citation15].
The obesity paradox has also been demonstrated in congestive heart failure, coronary artery disease, pulmonary embolism, and end stage renal disease (ESRD) [Citation1,Citation16]. The theory of loss of muscle mass may also explain the obesity paradox in cardiovascular and renal disease as well. It has been established in the literature that loss of muscle mass is associated with worse outcomes in cancer, COPD, heart failure, and ESRD populations [Citation17]. Therefore, patients resistant to loss of muscle mass have improved survival. It is plausible that although patients have normal weight, they may have a significant loss of healthy fat and muscle not being appropriately measured. BMI does not properly account for lean mass. In the cardiovascular population, increased muscle mass results in increased blood volume, stroke volume, cardiac output, and improved cardiorespiratory fitness. All of these features combined are associated with improved outcomes in heart failure and cardiovascular disease [Citation18,Citation19]. In the cardiovascular population, it has been shown that cardiorespiratory fitness is a better predictor of outcomes than levels of obesity and likely negates the negative effects of obesity [Citation20]. This is further supported by a study that found midthigh muscle mass to be a better predictor of mortality than BMI [Citation15].
The adipose tissue’s effects on inflammation theory argues that based on the type of adipose tissue it may have a beneficial effect. Obesity, ESRD, congestive heart failure, COPD are all associated with increased pro-inflammatory cytokines. Obesity is thought to cause a chronic low-level inflammation. Paradoxically there is some evidence that adipose tissue, based on the type and location, may negate the effects of inflammation by eliminating pro-inflammatory cytokines [Citation21]. There is white and brown adipose tissue in our body. Brown adipose tissue is more similar to muscle than to fat. Brown fat can secrete lipoproteins, which can break down lipopolysaccharides that otherwise would have propagated the inflammatory cascade [Citation19]. In certain obese patients, there is conversion of white fat cells to brown, thereby decreasing the pro-inflammatory effects of white fat and substantiating the anti-inflammatory effects of brown fat [Citation22].
One strength of the study was that the data were prospectively collected and followed-up long-term. Additionally, we obtained clinical data as opposed to administrative data. One limitation was that the diagnosis of COPD was not based on spirometry. It has been reported in prior studies that overdiagnosis of COPD in obese patients occurred using just symptoms and patient self diagnosis [Citation23,Citation24]. To counter that point, it has been noted that ER administrative coding accurately identifies the presence of COPD and obesity [Citation25]. Another limitation is the relative small sample size that does not allow us to further characterize BMI subgroups into underweight or morbidly obese groups. In this study, no direct measurements of muscle mass or body composition nor assessment of current physical activity were performed.
Based on our current understanding, future studies of COPD patients may need to evaluate a better characterization of muscle mass in obesity and the role of types of adipose tissue on inflammation. Future studies may need to characterize muscle with ultrasound, characterize type of adipose tissue via biopsy, and ratio of proinflammatory and anti-inflammatory cytokines.
In summary, our study showed that obesity was associated with decreased long-term mortality. A better understanding of why obesity is protective may help us to generate new approaches to manage COPD patients.
Acknowledgments
DAD had full access of the data and the accuracy of the data analysis. DAD and RC contributed to the data interpretation and writing of manuscript. JAR contributed to the study design and writing of the manuscript. CG and SF contributed to data analysis and writing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Putcha N, Drummond MB, Wise RA, et al. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(4):575–591. doi:10.1055/s-0035-1556063.
- Rodriguez DA, Garcia-Aymerich J, Valera JL, et al. Determinants of exercise capacity in obese and non-obese COPD patients. Respir Med. 2014;108(5):745–751. doi:10.1016/j.rmed.2014.02.004.
- Garcia-Rio F, Soriano JB, Miravitlles M, et al. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e105220. doi:10.1371/journal.pone.0105220.
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi:10.1001/jama.2014.732.
- Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9(1):29–35. doi:10.1054/jcaf.2003.4.
- Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. doi:10.1093/ajcn/81.3.543.
- Dimov D, Tacheva T, Koychev A, et al. Obesity in Bulgarian patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(4):215–222. doi:10.1177/1479972313504940.
- Naderi N, Kleine CE, Park C, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61(2):168–181. doi:10.1016/j.pcad.2018.07.001.
- Goto T, Hirayama A, Faridi MK, et al. Obesity and severity of acute exacerbation of chronic obstructive pulmonary disease. Ann ATS. 2018;15(2):184–191. doi:10.1513/AnnalsATS.201706-485OC.
- Lainscak M, von Haehling S, Doehner W, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86. doi:10.1007/s13539-011-0023-9.
- Chittal P, Babu AS, Lavie CJ. Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease? COPD. 2015;12(1):14–18. doi:10.3109/15412555.2014.915934.
- Yamauchi Y, Hasegawa W, Yasunaga H, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2014;9:1337–1346.
- Ramirez J, Peyrani P, Wiemken T, et al. A randomized study evaluating the effectiveness of oseltamivir initiated at the time of hospital admission in adults hospitalized with influenza-associated lower respiratory tract infections. Clin Infect Dis. 2018;67(5):736–742. doi:10.1093/cid/ciy163.
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322.
- Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi:10.1164/rccm.2107031.
- Keller K, Hobohm L, Munzel T, et al. Survival benefit of obese patients with pulmonary embolism. Mayo Clin Proc. 2019;94(10):1960–1973. doi:10.1016/j.mayocp.2019.04.035.
- von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–509. doi:10.1002/jcsm.12167.
- Carbone S, Canada JM, Billingsley HE, et al. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. doi:10.2147/VHRM.S168946.
- Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142–150. doi:10.1016/j.pcad.2018.07.003.
- Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345–1354. doi:10.1016/j.jacc.2014.01.022.
- Delgado C, Chertow GM, Kaysen GA, et al. Associations of body mass index and body fat with markers of inflammation and nutrition among patients receiving hemodialysis. Am J Kidney Dis. 2017;70(6):817–825. doi:10.1053/j.ajkd.2017.06.028.
- Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res. 2017;113(9):1074–1086. doi:10.1093/cvr/cvx106.
- Barbarito N, De Mattia E. Grading the severity of obstruction in patients with chronic obstructive pulmonary disease and morbid obesity. Monaldi Arch Chest Dis. 2013;79(3–4):121–127. doi:10.4081/monaldi.2013.5210.
- Collins BF, Feemster LC, Rinne ST, et al. Factors predictive of airflow obstruction among veterans with presumed empirical diagnosis and treatment of COPD. Chest. 2015;147(2):369–376. doi:10.1378/chest.14-0672.
- Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. doi:10.1111/j.1475-6773.2007.00822.x.