Abstract
Survival in chronic obstructive pulmonary disease (COPD) is enhanced in obese patients, which is called the obesity paradox. Despite some theories, the precise mechanism remains unclear. Since COPD exacerbations play a major role in COPD survival, this study aimed to stratify patients into BMI classes and investigate exacerbation frequency, time to readmission and survival in patients hospitalized with a COPD exacerbation. Therefore, patients hospitalized with an exacerbation of COPD were categorized into BMI groups using cut-offs <18.5, 25, 30 and ≥35 kg/m2 for underweight, normal, overweight, moderately obese and severely obese groups and followed for five years. A total of 604 COPD patients was included. In comparison to normal weight patients, the 5-year exacerbation frequency was significantly decreased by 34–40% in obese patients depending on the BMI group (1.83 ± 1.60 per year in the normal weight group; overweight 1.60 ± 1.41; moderately obese 1.20 ± 1.18; severely obese 1.09 ± 1.13 per year; and 1.59 ± 1.30 in the underweight group). The time to readmission was up to 1.7 times longer for moderately obese patients compared to normal weight patients (954 ± 734 versus 564 ± 660 days). The data were supported by enhanced survival in obese patients and a regression analysis showing that both time to readmission and survival were associated with BMI independent of other possible confounders. In conclusion, this study shows a ‘dose dependent’ reduced exacerbation frequency and an increased time to readmission in obese patients admitted to the hospital with an exacerbation of COPD.
Introduction
Increased weight is associated with increased mortality in the general population [Citation1]. In contrast however, in various chronic conditions obesity correlates inversely with mortality. This ‘obesity paradox’ was first described in overweight and obese patients with heart disease [Citation2,Citation3]. Ever since, numerous other studies showed a favorable influence of obesity on outcomes in other, often chronic conditions including heart failure, hypertension, stroke, pulmonary embolism, diabetes and kidney disease [Citation4,Citation5].
The obesity paradox has also been described in chronic obstructive lung disease (COPD) [Citation6]. Unfortunately, most studies focused upon the lower range BMI groups and hardly differentiated between BMI groups above 30 kg/m2 [Citation7]. Therefore, uncertainty remains about the obesity paradox in the high end BMI groups, whereas a possible clue to the mechanism may be found in the extremes rather than in the middle groups. For example, one study did find an interesting observation in a group of COPD patients with a BMI >35 kg/m2 and a decreased mortality risk: in extreme obesity, the causes of death were mainly associated with comorbidities; whereas comorbidities hardly played a role in underweight patients [Citation8].
In addition to BMI, important risk factors for mortality in COPD patients are age and the degree of airflow limitation [Citation9]. However, these factors can hardly be influenced. Another prominent prognostic factor across all GOLD stages is exacerbation frequency [Citation10]. This factor can actually be modified: a number of strategies have been developed for prevention of exacerbations, including early recognition in the pre-hospital setting, drug interventions, smoking cessation and/or pulmonary rehabilitation strategies [Citation11,Citation12].
For a better understanding of the obesity paradox in COPD, it is mandatory to investigate the relationship between obesity and exacerbation frequency in COPD in more detail [Citation13,Citation14]. Given the relationships between increased survival and higher BMI and between increased mortality and higher exacerbation frequency, we hypothesized that BMI increase could be associated with decreased exacerbation frequency. Therefore, this study aimed to investigate the clinical exacerbation risk, as well as the time to readmission and the mortality risk, in patients with COPD hospitalized for an acute exacerbation who were stratified into BMI groups including a high end group with BMI >35 kg/m2.
Methods
Study design
This retrospective, observational study was performed at the Zuyderland Medical Center (location Heerlen, The Netherlands), a large teaching hospital. All subjects were consecutively admitted to the hospital with a clinical diagnosis of COPD exacerbation between January 2010 and May 2012. COPD was diagnosed by a respiratory physician based upon GOLD definition [Citation15]. An exacerbation was defined as an increase in respiratory symptoms like dyspnea, cough, and/or increased sputum production requiring hospital admission. Exclusion criteria were: no BMI available, predominant asthma symptoms (identified by thorough chart review for each individual), no COPD based on pulmonary function testing and pregnancy. The study was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee - approval: METC-Z 16N219. Adult participant consent was not required because of the retrospective and observational nature of the study.
Patient characteristics were obtained from medical files at the time of the index admission and included age, sex, smoking status, BMI, COPD Gold stage, Charlson comorbidities index, laboratory values, length of hospital stay, intensive care unit admission and need for noninvasive ventilation. During a follow up of 5 years, additional data were collected including mortality, number of readmissions for COPD exacerbation (expressed as annual exacerbation frequency) and time to readmission (representing the time between the final date of the index admission and the first date of readmission for another COPD exacerbation). According to their BMI, patients were classified as follows: underweight, normal weight, overweight, moderately obese and severely obese using BMI cut-off points < 18.5, 18.5- ≤ 24.9, 25.0- ≤ 29.9, 30.0- ≤ 34.9 and ≥35.0 kg/m2, respectively.
Analysis
Statistical analysis was performed using IBM SPSS version 21.0 and GraphPad Prism version 6.01. The normal weight group was defined as the control group. Group comparisons were performed by Mann-Whitney test, Kruskal Wallis test or Fisher’s exact test as appropriate. Linear regression analyses were performed for exacerbation frequency. Kaplan-Meier analyses were used for the time to readmission and survival. A Breslow test was used for group comparisons. Cox multivariable regression analyses were performed with the Cox proportional hazards regression model. Statistical significance was set at a p-value of <0.05 for all tests. All p-values of 0.10 and above are given as NS (Not statistically Significant).
Results
Patient characteristics
Between January 2010 and May 2012, 630 COPD patients were admitted for an acute exacerbation of COPD. After applying exclusion criteria, 604 patients were included in the study. The baseline characteristics of these patients are presented in . A normal weight was found for 266 (44%) patients, whereas 71 (12%) patients were underweight, 155 (26%) were overweight, 71 (12%) were moderately obese and 41 (7%) were severely obese.
Table 1. Baseline characteristics of patients admitted with a clinical exacerbation of chronic obstructive lung disease (COPD).
COPD exacerbation frequency
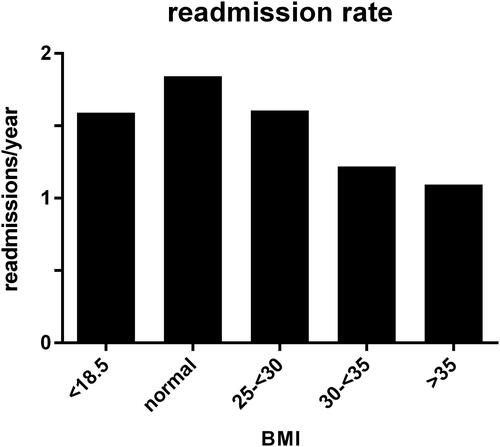
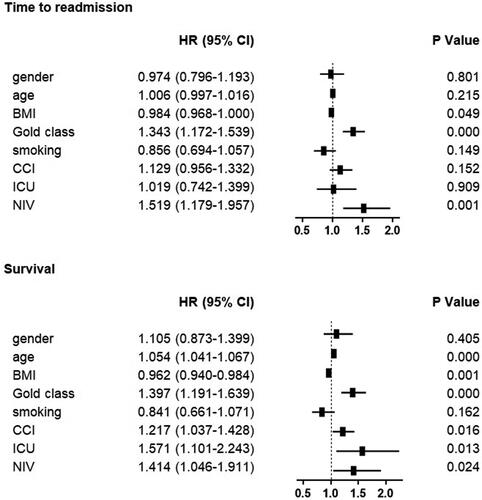
During the five year follow up period, the mean annual frequency of hospital admissions for COPD exacerbation was 1.83 ± 1.60 per year in the normal weight group. With each increasing weight group, the exacerbation frequency decreased (; overweight 1.60 ± 1.41; obese 1.20 ± 1.18; severely obese 1.09 ± 1.13 per year; p = 0.043, <0.000 and <0.000 versus normal weight group, respectively). In the underweight group, exacerbation frequency was not significantly different (1.59 ± 1.30 per year; p = 0.292). The effect of BMI appeared to be independent of other variables including sex, age, GOLD stage, Charlson comorbidities index, admission to the intensive care unit and noninvasive ventilation using linear regression analysis (p = 0.027 for BMI). The 30-day readmission risk was less affected by BMI: 45 patients (17%) were readmitted with a normal weight, 7 underweight (11%; p=NS), 29 overweight (20%; p=NS), 4 moderately obese (6%; p = 0.014) and 5 severely obese (14%; p=NS).
Time to readmission
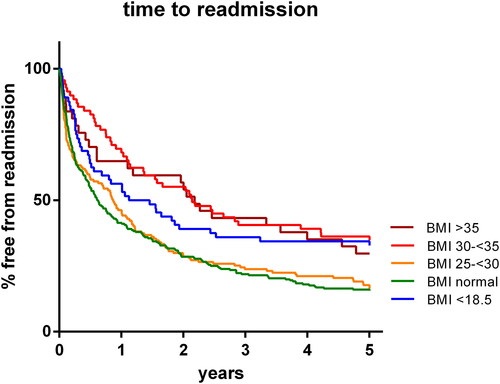
The mean time to readmission was 564 ± 660 days in the normal weight group and 609 ± 689 days in the overweight group (p= NS). In the higher BMI groups, the time to readmission was significantly increased up to 954 ± 734 days in the moderately obese group and 925 ± 752 days in the severely obese group (p < 0.000 and 0.008 versus normal weight group, respectively). In the underweight group, the time to readmission was also increased (815 ± 773 days; p = 0.009). The results appeared consistent over time (). Cox regression analysis showed that the effect was independent of other variables with a decreased hazard ratio of 0.984 (95% CI 0.968-1.000; p = 0.049; data shown in ).
Survival
Five year survival was lowest for underweight patients and normal weight patients (35%; n = 25, and 41%; n = 110, respectively; p=NS). Survival increased with each increasing BMI group (overweight: 47%/n = 73; moderately obese: 51%/n = 36; severely obese 63%/n = 26; p = 0.028, 0.046 and 0.003 versus normal weight patients, respectively). The results appeared consistent over time (). Cox regression analysis showed that the effect was independent of other variables with a decreased hazard ratio of 0.962 (95% CI 0.940–0.984; p = 0.001; data shown in ).
Figure 3. 5-year survival. Survival graph plotting survival over a five year period following a hospitalization for an exacerbation of Chronic Obstructive Lung Disease (COPD). Patients are categorized according to Body Mass Index (BMI) class.
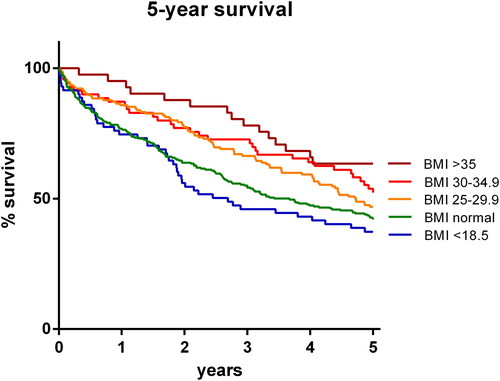
Figure 4. Cox regression analysis of time to readmission and survival. Hazard ratios represent increased chance of readmission or death, respectively. CI=confidence interval. BMI = Body Mass Index. CCI = Charlson comorbidity index. ICU = Intensive Care Unit. NIV = non-invasive ventilation. Yrs = Years. FEV1 = Forced Expiratory Volume in 1 second. FVC = Forced Vital Capacity.
Discussion
In this study, the exacerbation frequency requiring hospitalization and the time to readmission were reduced significantly in obese patients with COPD. The study demonstrated that the number of readmissions over a period of 5 years was significantly decreased by 34–40% in obese patients depending on the BMI group in comparison to the normal weight group. In addition, the time to readmission for moderately obese patients was 1.7 times longer compared to normal weight patients. The results were supported by decreased mortality in obese patients. Moreover, regression analysis showed that the relationships between BMI and time to readmission and between BMI and survival were independent of other possible confounding factors. Of note, since the effects were generally more pronounced in higher BMI classes including the high end group ≥35 kg/m2, these findings suggest a clinically meaningful and ‘dose-dependent’ relationship.
Previous studies suggested that harm by BMI was typically ‘U’ shaped, indicating more harm at both ends of the spectrum from underweight to extreme obesity [Citation8]. The ideal body weight in these studies was a BMI between 25 and 30 kg/m2, which is ironically still being addressed as ‘overweight’ despite the overwhelming evidence that survival is optimal in this group also in the general population [Citation1]. Though our data support the concept of increased harm at the underweight end, no increased harm was observed in the BMI ≥35 kg/m2 group in our patients with COPD. Moreover, this severe obesity group showed the best outcomes in terms of exacerbation frequency and survival. Although our study did not discriminate further in higher BMI groups, our study challenges the ‘U’ shaped concept in COPD with an optimal BMI between 25 and 30 kg/m2 and suggests that the ‘U’ turn is possibly taken in a much higher BMI group.
The association found in this study between a higher BMI and a lower exacerbation frequency may to some extent help to explain the obesity paradox observed in survival in COPD. Clinical exacerbations have been proven key events in the course of COPD [Citation16,Citation17]. Clinical exacerbations are strongly associated with increased mortality [Citation10,Citation18]. After clinical exacerbations, persisting decreases in performance and lung function have been described [Citation19–21]. One explanation is the release of inflammatory mediators during an exacerbation, which is associated with increased structural lung parenchymal damage [Citation22,Citation23]. This damage impairs functioning of airway clearance mechanisms, thereby increasing the pathogen burden and chances of recurrent infections, thus leading to a vicious circle of events [Citation24]. Another explanation is the loss of cardiorespiratory fitness after hospital stay [Citation25]. This loss of fitness disrupts ongoing physiotherapy and/or rehabilitation programs not only temporarily but may also discourage patients on a longer term. A loss of cardiorespiratory fitness was associated with increased mortality in a number of studies [Citation26,Citation27].
The obesity paradox in COPD has also been explained by a number of theories unrelated to exacerbation frequency. One theory assumed that it is obesity itself that contributes to a lower FEV1, resulting in an overestimation of the incidence and severity of COPD [Citation28–30]. Another factor might be a tendency of obese patients to experience more symptoms of dyspnea, resulting in receiving medical attention earlier than normal weight patients [Citation31–33]. Other authors noted a beneficial effect of obesity upon the annual decline of FEV1 [Citation34,Citation35]. Also, less hyperinflation in COPD has been described in obese patients [Citation36,Citation37]. Another hypothesis is that cardiovascular comorbidities, especially extensive atherosclerosis, cause a higher mortality rate among leaner patients with COPD [Citation38]. Furthermore, an interesting physiologic explanation might be that in patients with a higher BMI, a protective increase in lung recoil has been found [Citation39], whereas loss of lung recoil is generally associated with lung function decline and progression of COPD [Citation40]
In addition to these proposed explanations about the obesity paradox in COPD, the observation that the obesity paradox is also noted in other chronic diseases suggests a common mechanism. Possibly, the higher weight does not only reflect a higher fat mass but also a higher lean muscle mass, associated with a better cardiorespiratory fitness [Citation41]. Studies showed that subjects with increased BMI may not necessarily have increased fat mass and a normal BMI does not preclude an individual from having increased fat mass [Citation42,Citation43]. Another possibility may be that the fat reserve offers a protective source of energy during hospitalization with a critical illness. Indeed, critically ill patients with a high BMI were shown to have better survival at the intensive care unit [Citation44,Citation45]. Furthermore, obese individuals with preserved muscle mass may have a better prognosis due to increased stroke volume and thus cardiac output [Citation41]. Finally, obese patients tend to have lower systemic vascular resistance, especially patients who are normotensive [Citation46]. This reduction in the afterload could improve forward flow and cardiac output.
Underweight patients in our study showed increased mortality. These results have been confirmed by many others previously and support dietary measures in this group [Citation6,Citation7,Citation33,Citation47]. The increased mortality has been attributed to less muscle mass, decreased cardiorespiratory fitness, limited energy storage and/or decreased cardiac output in these patients [Citation44,Citation45]. Interestingly, the underweight patients showed an increased time to readmission in our study. When taking into consideration that hospital admissions greatly enhance medical attention, one could argue that another factor contributing to increased mortality in underweight patients may be a relative lack of medical attention preceding the exacerbation.
In comparison to the 5-year exacerbation risk, the 30-day readmission risk was little affected by BMI in the current study, showing only a significant reduction in the moderately obese group. Probably, reasons for 30-day readmission are more complex and more easily influenced by temporarily occurring factors [Citation48]. Of note, body composition changes are not easily achieved within this short period.
This study was performed in a very specific patient group of COPD patients admitted to the hospital with an exacerbation. Please note that these patients, as well as other patient groups associated with an obesity paradox, already are suffering from disease. Therefore, the results of our study do not apply to the population level, especially not for disease prevention. Despite the results of our study, we wish to emphasize that obesity in metabolically healthy persons is associated with increased cardiovascular risk [Citation49]; therefore we support every measure taken to reduce obesity in the general population [Citation50,Citation51] and we do not advocate for any method aiming to just increase body weight above normal weight in COPD patients. However, because the obesity paradox does exist in COPD patients, it is very important to determine the factor most contributing to this paradox, in order to target that specific factor for future intervention.
The study was designed retrospectively; as such, some information could not be collected systematically including more detailed body composition measurements, like fat free mass index and/or lean mass, both known to provide more information than BMI alone [Citation52]. However, BMI was available for all patients in our study since both body weight and length were systematically determined by nurse at admission. Moreover, the ‘dose-response’ data from the current study and others support the concept of a clinically meaningful relationship [Citation53,Citation54]. Another limitation of the study is a lack of knowledge about exacerbations not requiring hospital admission. However, the influence of these relatively mild exacerbations upon mortality is probably limited. Finally, one inherent limitation of research studying prognostic factors in COPD, is that the list of possibly involved factors is long and dynamic [Citation55–57] and no consensus has been reached about which to include.
In conclusion, this study shows a large reduction in exacerbation frequency and an increased time to readmission in obese patients admitted to the hospital with an exacerbation of COPD. These results warrant further prospective research since the data may help to elucidate the mechanism and impact of the obesity paradox in COPD.
| Abbreviations | ||
| BMI | = | Body Mass Index |
| COPD | = | Chronic Obstructive L Disease |
| NS | = | Not Significant |
| CCI | = | Charlson Comorbidity Index |
| ICU | = | Intensive Care Unit |
| FEV1 | = | Forced Expiratory Volume 1st second |
| FVC | = | Forced Vital Capacity |
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, MDK.
Disclosure Statement
The authors report no conflict of interest.
References
- Flegal KM , Kit BK , Orpana H , et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. DOI:10.1001/jama.2012.113905
- Gruberg L , Weissman NJ , Waksman R , et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. DOI:10.1016/S0735-1097(01)01802-2
- Horwich TB , Fonarow GC , Hamilton MA , et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. DOI:10.1016/S0735-1097(01)01448-6
- Chittal P , Babu AS , Lavie CJ . Obesity paradox: does fat alter outcomes in chronic obstructive pulmonary disease?. COPD. 2015;12(1):14–18. DOI:10.3109/15412555.2014.915934
- Keller K , Hobohm L , Munzel T , et al. Survival benefit of obese patients with pulmonary embolism. Mayo Clin Proc. 2019;94(10):1960–1973. DOI:10.1016/j.mayocp.2019.04.035
- Cao C , Wang R , Wang J , et al. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012;7(8):e43892. DOI:10.1371/journal.pone.0043892
- Guo Y , Zhang T , Wang Z , et al. Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine (Baltimore). 2016;95(28):e4225. DOI:10.1097/MD.0000000000004225
- Divo MJ , Cabrera C , Casanova C , et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1(2):229–238. DOI:10.15326/jcopdf.1.2.2014.0117
- Puhan MA , Hansel NN , Sobradillo P , et al. Large-scale international validation of the ADO index in subjects with COPD: an individual subject data analysis of 10 cohorts. BMJ Open. 2012;2(6):e002152. doi: bmjopen-2012-002152 [pii] DOI:10.1136/bmjopen-2012-002152
- van Hirtum PV , Sprooten RTM , van Noord JA , et al. Long term survival after admission for COPD exacerbation: a comparison with the general population. Respir Med. 2018;137:77–82. DOI:10.1016/j.rmed.2018.02.015
- Anzueto A , Miravitlles M . Chronic obstructive pulmonary disease exacerbations: a need for action. Am J Med. 2018;131(9S):15–22. DOI:10.1016/j.amjmed.2018.05.003
- Burchette JE , Campbell GD , Geraci SA . Preventing hospitalizations from acute exacerbations of chronic obstructive pulmonary disease. Am J Med Sci. 2017;353(1):31–40. DOI:10.1016/j.amjms.2016.06.006
- Wei YF , Tsai YH , Wang CC , et al. Impact of overweight and obesity on acute exacerbations of COPD - subgroup analysis of the Taiwan Obstructive Lung Disease cohort. Int J Chron Obstruct Pulmon Dis. 2017;12:2723–2729. DOI:10.2147/COPD.S138571
- Wu Z , Yang D , Ge Z , et al. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10(8):5086–5099. DOI:10.21037/jtd.2018.08.67
- Singh D , Agusti A , Anzueto A , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. DOI:10.1183/13993003.00164-2019
- Slenter RH , Sprooten RT , Kotz D , et al. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2013;85(1):15–26. DOI:10.1159/000342036
- Soler-Cataluna JJ , Martinez GM , Roman SP , et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. DOI:10.1136/thx.2005.040527
- Sprooten RTM , Rohde GGU , Lawyer G , et al. Risk stratification for short-term mortality at hospital admission for acute exacerbations of COPD. Respirology. 2019;24(8):765–776. DOI:10.1111/resp.13538
- Donaldson GC , Seemungal TA , Bhowmik A , et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. DOI:10.1136/thorax.57.10.847
- Donaldson GC , Wilkinson TM , Hurst JR , et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(5):446–452. DOI:10.1164/rccm.200408-1054OC
- Seemungal TA , Donaldson GC , Bhowmik A , et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. DOI:10.1164/ajrccm.161.5.9908022
- Franssen FM , O'Donnell DE , Goossens GH , et al. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63(12):1110–1117. DOI:10.1136/thx.2007.086827
- Wedzicha JA . Exacerbations: etiology and pathophysiologic mechanisms. Chest. 2002;121(5 Suppl):136S–141S. DOI:10.1378/chest.121.5_suppl.136s
- Beeh KM , Burgel PR , Franssen FME , et al. How do dual long-acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2017;196(2):139–149. DOI:10.1164/rccm.201609-1794CI
- Abdulai RM , Jensen TJ , Patel NR , et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(4):433–449. DOI:10.1164/rccm.201703-0615CI
- Blondeel A , Demeyer H , Janssens W , et al. The role of physical activity in the context of pulmonary rehabilitation. COPD. 2018;15(6):632–639. DOI:10.1080/15412555.2018.1563060
- Hansen GM , Marott JL , Holtermann A , et al. Midlife cardiorespiratory fitness and the long-term risk of chronic obstructive pulmonary disease. Thorax. 2019;74(9):843–848. DOI:10.1136/thoraxjnl-2018-212821
- Barbarito N , De Mattia E . Obesity paradox in chronic obstructive pulmonary disease: A result of airflow obstruction over-grading? Respir Med. 2017;126:133DOI:10.1016/j.rmed.2016.08.012
- Landbo C , Prescott E , Lange P , et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. DOI:10.1164/ajrccm.160.6.9902115
- Ray CS , Sue DY , Bray G , et al. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128(3):501–506. DOI:10.1164/arrd.1983.128.3.501
- Cecere LM , Littman AJ , Slatore CG , et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD. 2011;8(4):275–284. DOI:10.3109/15412555.2011.586660
- Parameswaran K , Todd DC , Soth M . Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210. DOI:10.1155/2006/834786
- Rutten EP , Calverley PM , Casaburi R , et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes? Ann Nutr Metab. 2013;63(3):239–247. DOI:10.1159/000353211
- Sun Y , Milne S , Jaw JE , et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. DOI:10.1186/s12931-019-1209-5
- Watson L , Vonk JM , Lofdahl CG , et al. Predictors of lung function and its decline in mild to moderate COPD in association with gender: results from the Euroscop study. Respir Med. 2006;100(4):746–753. DOI:10.1016/j.rmed.2005.08.004
- Ramachandran K , McCusker C , Connors M , et al. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5(4):205–209. DOI:10.1177/1479972308096711
- Sava F , Laviolette L , Bernard S , et al. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10(1):55. DOI:10.1186/1471-2466-10-55
- Blum A , Simsolo C , Sirchan R , et al. Obesity paradox" in chronic obstructive pulmonary disease. Isr Med Assoc J. 2011;13(11):672–675.
- Pellegrino R , Gobbi A , Antonelli A , et al. Ventilation heterogeneity in obesity. J Appl Physiol. (1985). 2014;116(9):1175–1181. DOI:10.1152/japplphysiol.01339.2013
- O'Donnell DE , Laveneziana P . The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–232. DOI:10.1080/15412550600977478
- Carbone S , Lavie CJ , Arena R . Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92(2):266–279. DOI:10.1016/j.mayocp.2016.11.001
- Gomez-Ambrosi J , Silva C , Galofre JC , et al. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring). 2011;19(7):1439–1444. DOI:10.1038/oby.2011.36
- Kraemer WJ , Torine JC , Silvestre R , et al. Body size and composition of National Football League players. J Strength Cond Res. 2005;19(3):485–489. DOI:10.1519/18175.1
- Pepper DJ , Sun J , Welsh J , et al. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2016;20(1):181. DOI:10.1186/s13054-016-1360-z
- Zhao Y , Li Z , Yang T , et al. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS One. 2018;13(6):e0198669. DOI:10.1371/journal.pone.0198669
- Lavie CJ , Mehra MR , Milani RV . Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J. 2005;26(1):5–7. DOI:10.1093/eurheartj/ehi055
- McDonald MN , Wouters EFM , Rutten E , et al. It's more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res. 2019;20(1):100. DOI:10.1186/s12931-019-1073-3
- Shah T , Press VG , Huisingh-Scheetz M , et al. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. DOI:10.1016/j.chest.2016.05.002
- Caleyachetty R , Thomas GN , Toulis KA , et al . Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70(12):1429–1437. DOI:10.1016/j.jacc.2017.07.763
- August GP , Caprio S , Fennoy I , et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93(12):4576–4599. DOI:10.1210/jc.2007-2458
- Kotsis V , Jordan J , Micic D , et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. 2018;36(7):1427–1440. DOI:10.1097/HJH.0000000000001730
- Pischon T , Boeing H , Hoffmann K , et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–2120. DOI:10.1056/NEJMoa0801891
- Vestbo J , Prescott E , Almdal T , et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83. DOI:10.1164/rccm.200506-969OC
- Lainscak M , von Haehling S , Doehner W , et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–86. DOI:10.1007/s13539-011-0023-9
- Houchen-Wolloff L , Williams JE , Green RH , et al. Survival following pulmonary rehabilitation in patients with COPD: the effect of program completion and change in incremental shuttle walking test distance. COPD. 2017;13:37–44. DOI:10.2147/COPD.S143101
- Putman RK , Hatabu H , Araki T , et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315(7):672–681. DOI:10.1001/jama.2016.0518
- Stanchina ML , Welicky LM , Donat W , et al. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;09(08):767–772. DOI:10.5664/jcsm.2916