Abstract
NGAL is mainly secreted by neutrophils which play the core role in AECOPD. MCP-1 is secreted specifically by monocytes and macrophages. Both biomarkers are involved in the core process of acute inflammatory reaction in COPD. So We analyzed serum NGAL and MCP-1levels to explore their potential clinical values in the chronic obstructive pulmonary disease (COPD) .This study enrolled 97 COPD patients and 50 healthy controls. All participants received blood collection and lung function test and arterial blood gas measurements. The expression levels of serum NGAL and MCP-1 were measured by ELISA. The serum NGAL and MCP-1 levels of COPD with community-acquired pneumonia (COPD-CAP) patients were significantly higher than those of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients and healthy adults. The NGAL levels of the GOLD III and IV groups were significantly higher than those of the GOLD II group. Spearman correlation analysis showed a negative correlation between NGAL and FEV1%pred, FVC% pred. ROC curves indicated that NGAL has a high diagnostic value for both AECOPD and COPD-CAP. NGAL has the value of distinguishing GOLD I and II from GOLD III and IV. MCP-1 have moderate diagnostic value for COPD-CAP and can differentiate COPD-CAP from AECOPD. This study shows NGAL has certain diagnostic value for AECOPD and COPD-CAP, but can not distinguish the two. NGAL is closely related to airway remodeling and can be used as a potential indicator to distinguish the higher GOLD degree. MCP-1 can be used as potential indicator for the diagnosis of COPD-CAP.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common and frequently occurring disease that seriously threats human health, is the fourth-ranking cause of death in the world [Citation1], and is characterized by persistent respiratory symptoms and restricted airflow. Acute exacerbation of chronic obstructive pulmonary disease (AECOPD), a major health issue in COPD patients, is not only an important cause of hospital admission and mortality [Citation2–4] but also a heavy burden on patients’ lung function and economy. Community-acquired pneumonia (CAP) is a common, life-threatening complication in COPD [Citation5]. Patients with pneumonia exacerbation have been found to be admitted to intensive care units more often and stay there longer than those with non-pneumonia exacerbation [Citation6]. Several previous studies found that COPD patients with CAP had higher in-hospital mortality than individuals without CAP [Citation6,Citation7]. Therefore, CAP is reported to be a major contributor to hospitalization for COPD and has a close relationship with poor outcomes [Citation8].
COPD is a complex inflammatory disease in which several types of inflammatory cells and multiple inflammatory mediators are involved. Neutrophil, monocyte and macrophage play a key role in the inflammatory processes of COPD. When the body is infected by bacteria, neutrophils migrate from peripheral blood to infected tissues to control the infection mainly through three strategies: phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NETs) [Citation9]. Neutrophils are the predominant inflammatory cells in COPD, and neutrophile inflammation is associated with both disease progression and the severity of the disease [Citation10].
Neutrophil gelatinase-associated lipocalin (NGAL), also named lipocalin-2, is a 25-kDa protein of the lipocalin superfamily, and its presence was initially observed in activated neutrophils [Citation11]. In addition to secreted by activated neutrophils, NGAL may be released in small quantities by epithelial and kidney tubular cells and during inflammation or injury [Citation11]. NGAL, a key antibacterial protein, is typically very low in various biological fluids [Citation12] but is highly elevated under inflammatory conditions [Citation13,Citation14], especially under acute inflammatory injury. Previous studies have demonstrated that NGAL not only plays a critical role in suppressing local bacterial infection through NETs [Citation9] but also may be a potential target for reversing airway obstruction and remodeling in COPD [Citation15].
Monocyte chemoattractant protein-1 (MCP-1) is a protein consisting of 76 amino acids secreted specifically from monocyte and macrophage [Citation16]. MCP-1 is a pro-inflammatory chemokine that plays a role in the recruitment of monocyte to the sites of injury and infection [Citation17]. MCP-1 can also recruit macrophage, neutrophil, and lymphocyte inflammatory cells to the lungs, an outcome associated with increases in COPD disease severity [Citation18]. Monzon et al. [Citation19] found that sustained MCP-1 levels are involved in the mucin upregulation that is characteristic of airway inflammatory conditions. In addition, a previous study observed that the MCP-1 level in the blood was significantly increased in COPD patients with prevalent emphysema compared with healthy controls [Citation20]. Therefore, we speculate that MCP-1 may play an important role in airway inflammation in COPD patients and is associated with the degree of inflammation in COPD patients.
Materials and methods
This was a cross-sectional, retrospective, single-center study.It was performed at the “Respiratory and Critical Care Medicine Department of Second Affiliated Hospital of Chongqing Medical University” from June 2018 to December 2019. representatives.
The Institutional Ethical Committee of the Clinical Hospital Center of Second Affiliated Hospital of Chongqing Medical University approved this study. Written informed consent was obtained from participants or their legal
Patients and diagnostic criteria
The study enrolled COPD patients who need therapy; then we divided them into COPD-CAP and AECOPD groups, all of them were admitted to the hospital.
All of the patients were diagnosed with COPD following GOLD criteria (forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 70% and post-bronchodilator FEV1 < 80%) [Citation2]. AECOPD was defined as an event characterized by acute changes in clinical symptoms beyond normal day-to-day variation according to the GOLD executive summary [Citation21]. Pneumonia was defined as the presence of new infiltrates on chest radiographic images and plus ≥1 of the following manifestations: new cough with or without sputum production; pleuritic chest pain; dyspnea, fever or hypothermia; altered breath sounds on auscultation; and leucocytosis [Citation22,Citation23]. COPD patients with a pneumonic infiltrate (confirmed by chest X-ray or CT scan) were classified as COPD-CAP; otherwise, they were classified as AECOPD. Then, COPD patients were divided into four subgroups according to the GOLD degree.
At the same time, we recruited healthy adult people from the physical examination center as the control group.
Exclusion criteria were as follows: 1) age < 18 years; 2) other respiratory diseases such as asthma, bronchiectasis, pneumoconiosis, tuberculosis and carcinoma of the lungs; 3) a history of chronic kidney disease or end-stage organ disease; 4) chemotherapy and radiotherapy in the past 30 days; 5) immunosuppressant therapy or corticosteroid therapy in the past 2 weeks; 6) major trauma, burns, pregnancy, malignancy, autoimmune disease, cardiogenic or hemorrhagic shock, or parasitic infection; 7) participation in another clinical trial during this hospitalization.
Data collection
Peripheral venous blood sample was obtained from all patients on day 1 of admission before medicine therapy.
Measurements of serum NGAL and MCP-1
Blood samples were centrifuged for 10 min at 3000 rpm to isolate serum samples after we got it. Centrifuged serum samples were then frozen at −80 °C until the date of biochemical analysis for NGAL and MCP-1. NGAL and MCP-1 concentrations were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cloud-clone, Wuhan, China) according to the manufacturer’s instructions.
Measurements of peripheral blood biomarkers, arterial gas parameters and lung function status
We recorded the level of complete and differential blood counts, procalcitonin(PCT), C-reactive protein (CRP), erythrocyte sedimentation rate(ESR), fibrinogen(FB), fibrin degradation products(FDP), lactic dehydrogenase(LDH) and albumin (ALB) from the hospital. Then, we defined the NLR as the ratio of the neutrophil count to the lymphocyte count. Arterial gas analysis, including pH, partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2), oxygenation index (OI), actual bicarbonate concentration (HCO3-), buffer excess (BE) and blood lactic acid (Lac), was performed on day 1 as well.
At the same time, we also reviewed demographic details, when the patient’s condition is stable and can finish the lung function examination, the parameters of lung function are collected: FVC% predicted(FVC% pred), FEV1% predicted(FEV1% pred), the FEV1/FVC ratio(FEV1/FVC%).
Results
The study enrolled 97 COPD patients, among them 57 patients are COPD-CAP, and 40 patients are AECOPD.50 healthy adults were enrolled as control group.
Differences in laboratory and demographic characteristics between AECOPD, COPD-CAP patients and controls
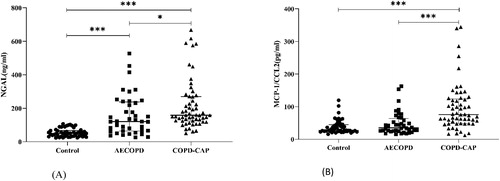
The baseline demographics of AECOPD, COPD-CAP patients and the controls are summarized in . The distribution of age, sex and BMI did not differ among the three groups. As expected, the serum NGAL and MCP-1 levels were significantly higher in the COPD-CAP patients than in the AECOPD and control individuals. However, the MCP-1 levels in AECOPD patients were similar with the controls.
Table 1. Baseline demographic of AECOPD, COPD-CAP patients and the control group.
presents the baseline laboratory parameters and clinical characteristics of the COPD-CAP and AECOPD patients. There was no difference between the two groups in pack-years, pH, PaO2, PaCO2, OI, Lac, BE, HCO3-, RF, FVC% pred, FEV1% pred, FEV1/FVC%, red blood cell(RBC), hemoglobin(Hb), haematocrit(HCT), white blood cell(WBC), percentage of eosnophil (EOS%), eosnophil(EOS), percentage of neutrophil(NS%), neutrophil(NS), percentage of lymphocyte(Lym%), lymphocyte(Lym), percentage of mononuclear macrophage(Micro%), mononuclear macrophage(Micro), PCT, NLR, LOS and comorbidities. Compared with the NGAL and MCP-1 levels in AECOPD patients, the levels were significantly elevated in COPD-CAP patients. Similarly, the COPD-CAP patients had significantly higher levels of CRP, ESR, FB, FDP, and LDH than the AECOPD patients. In addition, COPD stages were not distributed equally in the two groups. COPD-CAP patients tended to have a higher GOLD degree.
Table 2. Presents baseline laboratory parameters and clinical characteristics in the COPD-CAP and AECOPD subgroup patients.
The NGAL and MCP-1 concentrations of COPD-CAP patients and AECOPD patients compared to those of controls are shown in . From the scatter plot, we can see that NGAL levels in the subgroups with or without pneumonia were both significantly higher than those in the controls (). However, there was no difference between the AECOPD subgroup and the controls in serum MCP-1 levels ().
Figure 1. NGAL and MCP-1 concentration of AECOPD patients and COPD-CAP patients compared to controls.The horizontal line with error bar denotes mean values (±SE). *p < 0.05; ***p < 0.001.
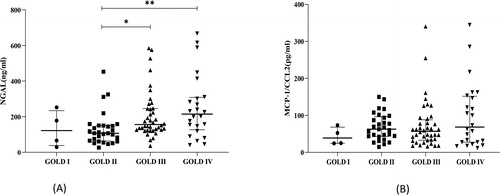
The NGAL and MCP-1 concentrations comparison of GOLD I, II, III, IV subgroups are shown in . We can see that serum NGAL concentrations in the GOLD III and IV groups were greater than those in the GOLD II group (), but MCP-1 was similar among all the groups ().
Correlations of NGAL and MCP-1 levels with parameters related to lung function and inflammatory status
From , we can see that the level of NGAL was negatively correlated with FEV1%pred and FVC% pred but positively correlated with NS, NLR. There was no correlation between MCP-1 and lung function or other inflammatory indices.
Table 3. Correlations of NGAL and MCP-1 levels with parameters related to the lung function and inflammatory status of COPD patients.
ROC curve analysis for NGAL, MCP-1 and NS% in COPD patients
To assess the diagnostic values of NGAL, MCP-1 and NS% in AECOPD, COPD-CAP and GOLD degree, 6 different ROC were generated, as shown in . The ROC of NGAL and MCP-1 were also generated as a reference in each panel. Panel (A) shows that NGAL could distinguish COPD-CAP from controls, and its AUC was obviously greater than that of MCP-1.
Figure 3. ROCs for NGAL, MCP-1, NS% and their combination in the verification group. (A) One group includes controls, and the other group includes COPD-CAP patients. (B) One group includes controls, and the other group includes AECOPD patients. (C) One group includes COPD-CAP pateints, and the other group includes AECOPD patients. (D) One group includes COPD patients with GOLD I, and the other group includes COPD patients with GOLD II, III and IV. (E) One group includes COPD patients with GOLD I and II, the other group includes COPD patients with GOLD III and IV. (F) One group includes COPD patients with GOLD I, II and III, the other group includes COPD patients with GOLD IV.
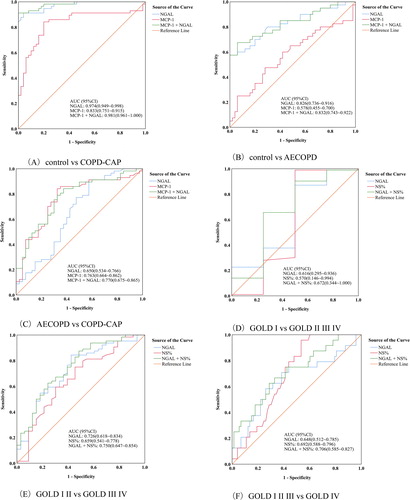
Panel (B) shows that NGAL has moderate diagnosis value to distinguish AECOPD patients from controls, but the MCP-1.
Panel (C) shows that NGAL could not distinguish COPD-CAP patients from AECOPD patients, but MCP-1 show moderate diagnosis value to distinguish the two groups.
Panel (D) shows that NGAL, NS% and their combination could not distinguish GOLD I from GOLD II, III and IV for a COPD patient.
Panel (E) shows that NGAL could distinguish GOLD I and II from GOLD III and IV for a COPD patient. NS% did not distinguish them alone, but the combination of NGAL and NS% performed slightly better than NGAL alone.
Panel (F) shows that NGAL, NS% and the combination could not distinguish the GOLD I, II, III and IV groups.
Discussion
Our study found that the NGAL, MCP-1, CRP values of COPD-CAP patients were all significantly higher than those of AECOPD patients, but there was no difference between the two groups in PCT concentrations.
Several studies suggest different inflammatory responses in patients with COPD-CAP and AECOPD [Citation23–25]. Almirall et al. [Citation26] found that CRP values were significantly higher in definite CAP patients than in healthy controls and suspected CAP patients. Huerta et al. and Alex Pizzini et al. demonstrated higher concentrations of CRP and PCT in COPD-CAP patients than in AECOPD patients [Citation25,Citation27]. The same study also showed that CRP levels enabled discrimination between COPD-CAP and AECOPD [Citation28]. Boussekey et al. [Citation29] and Polzin et al. [Citation30] failed to demonstrate that PCT can have a role in predicting the presence of CAP. According to these studies, we can see that the ability of CRP and PCT to identify patients with pneumonia is still not clear. Further studies are required to identify novel, sensitive and specific biological markers. Thus, it would be valuable to explore the potential diagnosis value of NGAL and MCP-1 in COPD.
From the correlation analysis in our study, we found that NGAL was negatively correlated with FEV1%pred and FVC% pred, but positively correlated with NS, NLR.
NGAL levels were significantly elevated in GOLD III and IV compared with GOLD II.
As we know, NGAL is mainly secreted by activated neutrophils. So, the higher NS and NLR values, the higher NGAL values. Small-airway-wall remodeling is proposed as the reason for airflow limitation, a decline in lung function, and poor responses to available therapies in COPD [Citation31]. Epithelial cells, macrophages and airway smooth muscle cells are associated with the airway remodeling process of COPD. A previous study identified that elevated NGAL promotes COPD airway remodeling possibly through altered epithelial-mesenchymal transition (EMT), and NGAL promoted the proliferation and migration of human bronchial smooth muscle cells (HASMCs) [Citation15]. NGAL can reflect the positive correlation between inflammation and the severity of lung dysfunction to some extent [Citation28]. Similary, in our study the higher GOLD degree, the lower FEV1%pred and FVC% pred, the higher NGAL. So it indicates that NGAL can reflect the severity of lung dysfunction or airway limitation.
MCP-1 could distinguish COPD-CAP patients from AECOPD patients, but NGAL could not distinguish the two. Meantime, ROC curve analysis showed that only NGAL could distinguish GOLD I and II from GOLD III and IV. These result remind us maybe we can use MCP-1 as a suitable biomarker to distinguish COPD-CAP from AECOPD. In addition, it shows that NGAL can play a role in identifying more severe COPD and might assist in monitoring the progression of patients with GOLD II. Therefore, we can see that NGAL may be a good predictor of the higher GOLD degree.
Limitation
First, the sample size of the study included is small, reducing the statistical power of evaluate the effect, so the result may not be used in other hospitals. Second, the present study is performed in a single institution, selection bias may have influenced the significance of the present findings and the possibility of recording errors related to baseline clinical data cannot be excluded, thus a multi-center study is required to validate the results. Therefore, in the next step, we need more rigorous experimental design and experimental methods to verify this conclusion.
In conclusion, this study suggests that NGAL may be useful biomarker for the diagnosis of AECOPD and COPD-CAP patients, MCP-1 has certain value for the diagnosis of COPD-CAP. It indicates that both NGAL and MCP-1 are involved in the inflammatory response of COPD. In addition, NGAL has certain value for predicting more higher GOLD degree and monitoring disease progress in GOLD II patients. This suggests that NGAL plays an important role in airway remodeling in COPD patients. However, more research on the value of MCP-1 and NGAL in COPD prognosis needs to be conducted to confirm these results.
Author contributions
Xing-Ru Chen designed this investigation, collected and analyzed the data and wrote the manuscript. Dao-Xing Wang conducted statistical analysis and revision of the manuscript.
Acknowledgments
We are thankful to the patients and controls for taking part in this study.
Conflict of interest
The authors declare no conflict of interest.
References
- Gardiner C, Gott M, Small N, et al. Living with advanced chronic obstructive pulmonary disease: patients concerns regarding death and dying. Palliat Med. 2009;23(8):691–697. DOI:10.1177/0269216309107003
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. DOI:10.1164/rccm.201204-0596PP
- Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. DOI:10.1016/S0140-6736(07)61382-8
- Wedzicha JA, Brill SE, Allinson JP, et al. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. DOI:10.1186/1741-7015-11-181
- Mullerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012; 106(8):1124–1133. DOI:10.1016/j.rmed.2012.04.008
- Cheng Y, Lu Z, Tu X, et al. Community-acquired pneumonia and survival of critically ill acute exacerbation of COPD patients in respiratory intensive care units. COPD. 2016;11:1867–1872. DOI:10.2147/COPD.S113510
- Takir HB, Karakurt Z, Salturk C, et al. Reasons for ICU demand and long-term follow-up of a chronic obstructive pulmonary disease cohort. COPD. 2014;11(6):627–638. DOI:10.3109/15412555.2014.898041
- Park S, Lee SJ, Shin B, et al. The association of delta neutrophil index with the prognosis of acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med. 2020;20(1):47 DOI:10.1186/s12890-020-1083-4
- Li H, Feng D, Cai Y, et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology. 2018;68(4):1604–1620. DOI:10.1002/hep.29919
- Pain M, Bermudez O, Lacoste P, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014;23(131):118–130. DOI:10.1183/09059180.00004413
- Xiao R, Chen R. Neutrophil gelatinase‑associated lipocalin as a potential novel biomarker for ventilator‑associated lung injury. Mol Med Rep. 2017;15(6):3535–3540. DOI:10.3892/mmr.2017.6442
- Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75(3):285–294. DOI:10.1038/ki.2008.499
- Xu MJ, Feng D, Wu H, et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: a critical role for IL-6/STAT3. Hepatology. 2015;61(2):692–702. DOI:10.1002/hep.27447
- Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr. 2017;37:103–130. DOI:10.1146/annurev-nutr-071816-064559
- Wang Y, Jia M, Yan X, et al. Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin Sci. 2017;131(11):1147–1159. DOI:10.1042/CS20170096
- Song Y-J, Zhou Z-H, Liu Y-K, et al. Prothrombotic state in senile patients with acute exacerbations of chronic obstructive pulmonary disease combined with respiratory failure. Exp Ther Med. 2013;5(4):1184–1188. DOI:10.3892/etm.2013.919
- Shinke H, Masuda S, Togashi Y, et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol. 2015;76(5):989–996. DOI:10.1007/s00280-015-2880-y
- Starrett W, Blake DJ. Sulforaphane inhibits de novo synthesis of IL-8 and MCP-1 in human epithelial cells generated by cigarette smoke extract. J Immunotoxicol. 2011;8(2):150–158. DOI:10.3109/1547691X.2011.558529
- Monzon ME, Forteza RM, Casalino-Matsuda SM. MCP-1/CCR2B-dependent loop upregulates MUC5AC and MUC5B in human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2011;300(2):L204–215. DOI:10.1152/ajplung.00292.2010
- Di Stefano A, Coccini T, Roda E, et al. Blood MCP-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int J Chron Obstruct Pulmon Dis. 2018;13:1691–1700. DOI:10.2147/COPD.S159915
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. 39930 03.00214-2017. DOI:10.1183/1
- Gomez-Junyent J, Garcia-Vidal C, Viasus D, et al. Clinical features, etiology and outcomes of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e105854. DOI:10.1371/journal.pone.0105854
- Crisafulli E, Menendez R, Huerta A, et al. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest. 2013;143(4):1009–1017. DOI:10.1378/chest.12-1684
- Gutierrez P, Closa D, Piner R, et al. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. Eur Respir J. 2010;36(2):285–291. DOI:10.1183/09031936.00118909
- Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and Nonpneumonic Exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. DOI:10.1378/chest.13-0488
- Almirall J, Bolibar I, Toran P, et al. Contribution of C-reactive nprotein to the diagnosis and assessment of severity of community- acquired pneumonia. Chest. 2004;125(4):1335–1342. DOI:10.1378/chest.125.4.1335
- Yamauchi Y, Yasunaga H, Matsui H, et al. Comparison of clinical characteristics and outcomes between aspiration pneumonia and community-acquired pneumonia in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:69. DOI:10.1186/s12890-015-0064-5
- Aghasafari P, George U, Pidaparti R. A review of inflammatory mechanism in airway diseases. Inflamm Res. 2019;68(1):59–74. DOI:10.1007/s00011-018-1191-2
- Boussekey N, Leroy O, Georges H, et al. Diagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infect. 2005;33(4):257–263. DOI:10.1007/s15010-005-4096-2
- Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J. 2003;21(6):939–943. DOI:10.1183/09031936.03.00055103
- Chung KF. The Role of Airway Smooth Muscle in the Pathogenesis of Airway Wall Remodeling in Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2005;2(4):347–354. DOI:10.1513/pats.200504-028SR