 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Exertional dyspnea, a key complaint of patients with chronic obstructive pulmonary disease (COPD), ultimately reflects an increased inspiratory neural drive to breathe. In non-hypoxemic patients with largely preserved lung mechanics – as those in the initial stages of the disease – the heightened inspiratory neural drive is strongly associated with an exaggerated ventilatory response to metabolic demand. Several lines of evidence indicate that the so-called excess ventilation (high ventilation-CO2 output relationship) primarily reflects poor gas exchange efficiency, namely increased physiological dead space. Pulmonary function tests estimating the extension of the wasted ventilation and selected cardiopulmonary exercise testing variables can, therefore, shed unique light on the genesis of patients’ out-of-proportion dyspnea. After a succinct overview of the basis of gas exchange efficiency in health and inefficiency in COPD, we discuss how wasted ventilation translates into exertional dyspnea in individual patients. We then outline what is currently known about the structural basis of wasted ventilation in “minor/trivial” COPD vis-à-vis the contribution of emphysema versus a potential impairment in lung perfusion across non-emphysematous lung. After summarizing some unanswered questions on the field, we propose that functional imaging be amalgamated with pulmonary function tests beyond spirometry to improve our understanding of this deeply neglected cause of exertional dyspnea. Advances in the field will depend on our ability to develop robust platforms for deeply phenotyping (structurally and functionally), the dyspneic patients showing unordinary high wasted ventilation despite relatively preserved FEV1.
Introduction
Dyspnea, either induced or worsened by exertion [Citation1], is the primary determinant of disablement and reduced quality-adjusted-life-years in patients facing the devastating consequences of chronic obstructive pulmonary disease (COPD) [Citation2]. Most patients suffering from COPD show a post-bronchodilator forced expiratory volume in one second (FEV1) within normal range or only mildly reduced (herein termed “mild” COPD) [Citation2–4], suggesting only minor disease [Citation5]. However, there is growing recognition that a sizable fraction of these patients experience out-of-proportion dyspnea relative to the severity of the lung-mechanical abnormalities [Citation2–4,Citation6–9]. Extensive work carried out by our group [Citation8,Citation10–27] (reviewed in refs. [Citation9,Citation28]) showed that rather than primarily driven by mechanical constraints, exertional dyspnea is strongly related to high ventilation (V̇E) relative to carbon dioxide output (V̇CO2) (excess ventilation) [Citation16,Citation28], signaling increased wasted ventilation in the physiological dead space (VDphys), i.e. a high VD fraction of the tidal volume (high VD/VT ratio) [Citation18–20,Citation29].
In this concise review, we initially provide a primer on the determinants of gas exchange efficiency in health and inefficiency in COPD. We focus on increased wasted ventilation rather than venous admixture since the latter is not a prominent future in most patients with mild COPD. Based on current neurobiological concepts of exertional dyspnea, we discuss how wasted ventilation can translate into breathlessness in individual patients. The structural underpinnings of wasted ventilation in mild COPD are reviewed: we bring a new perspective (impaired perfusion of non-emphysematous tissue) in addition to emphysema as a cause of VDphys. To further advance the field, we outline some critical unanswered questions on the seeds and consequences of wasted ventilation in these patients. We give special attention to how physiological measurements can be combined with respiratory functional imaging and metrics of emphysema extension and distribution by computed tomography (CT) to improve our understanding of this poorly understood cause of exertional dyspnea.
A primer on gas exchange efficiency in health: rest and exercise
The human lungs are admirably designed to meet their fundamental task – gas exchange – with precision and efficiency. For instance, alveolar ventilation (V̇A),/capillary perfusion (Q̇c) matching is favored by gravitational and non-gravitational mechanisms [Citation30,Citation31]:
Due to the lungs’ weight, both the density of capillaries (high Q̇c) and the compliance of the dependent tissue (high V̇A) increases from apex to base;
The gravity also acts on the weight of the pulmonary blood, increasing the flow across the basal regions;
The effect of gravity on blood flow is greater compared to that exerted on airflow, i.e., V̇A/Q̇c decreases slightly from top to bottom [Citation32]; and,
“Matched” geometries of the airways and arteries, allowing a preferential distribution of V̇ and Q̇ to the same secondary pulmonary lobules.
Young normal subjects show narrow distributions of V̇A/Q̇c ratios. In contrast, the distribution widens with aging [Citation33], largely due to reduced ventilation of dependent lung regions secondary to small airway closure, i.e. the top-to-bottom decrease in V̇A/Q̇c is more pronounced in the elderly. Regardless of age, the aforementioned mechanisms of V̇A/Q̇c matching act to minimize () [Citation35]:
Figure 1. Selected ventilatory and gas exchange responses to incremental cardiopulmonary exercise testing. Proportional decreases in dead space (VD)/tidal volume (VT) ratio (i.e. the physiological dead space) (a) and ventilation (V̇E)/carbon dioxide output (V̇CO2) (b) ratios maintain arterial carbon dioxide partial pressure (PaCO2) close to resting value during mild-to-moderate exercise (c). The V̇E/V̇CO2 response contour is established by both the slope and the intercept of the linear V̇E-V̇CO2 relationship (d). V̇E increases out of proportion to V̇CO2 after the respiratory compensation point (RCP) (b–d) leading to respiratory alkalosis (c) to compensate for progressive lactic acidemia. Note the increases in the lowest V̇E/V̇CO2 when the lactate threshold is reached at a low exercise intensity, i.e. before the stabilization of V̇E/V̇CO2 at the “true” nadir. Given eucapnia during exercise in most dyspneic patients with mild COPD, their high V̇E-V̇CO2 largely reflects a high physiological dead space, i.e. wasted ventilation. Reproduced, with permission of the publisher, from: Neder et al. [Citation34].
![Figure 1. Selected ventilatory and gas exchange responses to incremental cardiopulmonary exercise testing. Proportional decreases in dead space (VD)/tidal volume (VT) ratio (i.e. the physiological dead space) (a) and ventilation (V̇E)/carbon dioxide output (V̇CO2) (b) ratios maintain arterial carbon dioxide partial pressure (PaCO2) close to resting value during mild-to-moderate exercise (c). The V̇E/V̇CO2 response contour is established by both the slope and the intercept of the linear V̇E-V̇CO2 relationship (d). V̇E increases out of proportion to V̇CO2 after the respiratory compensation point (RCP) (b–d) leading to respiratory alkalosis (c) to compensate for progressive lactic acidemia. Note the increases in the lowest V̇E/V̇CO2 when the lactate threshold is reached at a low exercise intensity, i.e. before the stabilization of V̇E/V̇CO2 at the “true” nadir. Given eucapnia during exercise in most dyspneic patients with mild COPD, their high V̇E-V̇CO2 largely reflects a high physiological dead space, i.e. wasted ventilation. Reproduced, with permission of the publisher, from: Neder et al. [Citation34].](/cms/asset/d59d48f0-67dd-453b-ab0d-792cf95bdacf/icop_a_2301549_f0001_c.jpg)
The amount of end-capillary blood with a low PO2 added to the arterial blood (venous admixture) caused by a “true” shunt (no V̇A, preserved Q̇c) or a shunt “effect” (low V̇A/Q̇c, usually due to areas of low V̇A with normal Q̇c), and
The volume of air not exposed to gas exchanging units which fills the VDphys (wasted ventilation) secondary to anatomical VD, “alveolar” VD (preserved V̇A, no Q̇c), VD “effect” (high V̇A/Q̇c, usually due to normal V̇A but low Q̇c) and any contribution of shunt to increase the arterial partial pressure for CO2 (PaCO2).
Although high-intensity exercise is associated with increasing V̇A/Q̇c inhomogeneity and a progressive widening in alveolar-arterial O2 difference (P(A-a)O2) [Citation36], there is little change in P(A-a)O2 at exercise intensities more relevant to subjects’ functioning in daily life, i.e. mild-moderate [Citation37],. Crucially, VD/VT decreases (↓) markedly on exercise because VT increases (↑) out of proportion to conducting airway VD due to radial traction, and the higher compliance of the alveoli over that of the airways [Citation38]. Since PaCO2 is maintained (↔) within ±3 mmHg from rest, V̇E/V̇CO2 decreases in tandem with VD/VT () [Citation39]:
Equation [1]
Equation [1]
Gas exchange inefficiency and wasted ventilation in mild COPD
As long proposed by Riley and Cournand [Citation40], the term “gas exchange inefficiency” is herein used to characterize all abnormalities that conspire against the ideal lung, i.e. a lung without V̇A/Q̇c inequalities in which an ideal PAO2 can be estimated (assuming no P(A-a)CO2 and a constant respiratory exchange ratio). Specifically, such a definition does not include impaired gas transfer residing at the level of the alveolar-capillary membrane, i.e. “diffusion” limitation. COPD is characterized by a marked heterogeneity in the nature and degree of dysfunction of individual structural components of pulmonary gas exchange (in)efficiency) [Citation41]. Therefore, considerable variability exists in the relative contribution of factors conspiring to increase wasted ventilation as the disease progresses [Citation42] or between individuals showing similar spirometry. In this context, capillary obliteration/dysfunction in areas with alveolar destruction (emphysema) or areas with relatively preserved lung parenchyma may decrease Q̇c relative to V̇A, reducing local PACO2 at a given V̇CO2 (see What is the structural basis of wasted ventilation in the initial stages of COPD?) () [Citation44].
Figure 2. Putative mechanisms by which the structural abnormalities associated with COPD development may increase alveolar ventilation (V̇a)/capillary perfusion (Q̇c) relationship, increasing the wasted ventilation in the physiological dead space. The relative contribution of these abnormalities vary substantially amongst patients with similar spirometric findings justifying the use of more elaborated pulmonary function tests in addition to imaging to improve disease phenotyping. Reproduced, with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Neder [Citation43]. Annals of the American Thoracic Society is an official journal of the American Thoracic Society.
![Figure 2. Putative mechanisms by which the structural abnormalities associated with COPD development may increase alveolar ventilation (V̇a)/capillary perfusion (Q̇c) relationship, increasing the wasted ventilation in the physiological dead space. The relative contribution of these abnormalities vary substantially amongst patients with similar spirometric findings justifying the use of more elaborated pulmonary function tests in addition to imaging to improve disease phenotyping. Reproduced, with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Neder [Citation43]. Annals of the American Thoracic Society is an official journal of the American Thoracic Society.](/cms/asset/3d1e8960-381e-4c31-9572-68de900e5e54/icop_a_2301549_f0002_c.jpg)
Using the multiple inert gas exchange technique (MIGET) (), Wagner et al. showed that a rightward shift of V̇A/Q̇c distribution to high values was more commonly observed in patients with a preponderance of emphysema over airway disease [Citation58]. It remains unclear what the sources of afferent stimuli are that allow the respiratory controller to estimate how much “extra”- V̇E is required to overcome an enlarged VDphys to maintain eucapnia (EquationEquation 1Equation [1]
Equation [1] ) [Citation39]. One potential explanation suggests that intra- or between-breaths fluctuations in PCO2 of the arterial blood leaving the lungs would stimulate the peripheral and/or central chemoreceptors [Citation43]. In several pathological circumstances involving a high VDphys, therefore, PaCO2 is reduced [Citation18,Citation59–61] with the resulting low PCO2 set-point further increasing the ventilatory drive in a vicious circle () [Citation62]. Regardless of the precise determinants, excess ventilation during cardiopulmonary exercise testing (CPET) is anticipated in COPD patients showing the combination of (a) hypocapnia (hyperventilation), (b) increased “alveolar” VD and VD “effect” and (c) a low VT with (b) and (c) jointly conspiring to increase VDphys [Citation63]:
Equation [2]
Equation [2]
Figure 3. A putative explanation by which increased physiological dead space (wasted ventilation) and alveolar hyperventilation may trigger exertional dyspnea. The combination of pulmonary fibrosis and emphysema (CPFE) is depicted for illustrative purposes. In the presence of increased (⇑) airspace (alveolar ventilation (V̇a)) relative to capillary perfusion (Q̇c)) (wasted ventilation), less CO2 from the mixed venous blood is unloaded into the right alveolus. Consequently, the alveolar carbon dioxide tension (PACO2) diminishes (⇓) markedly but PCO2 at the end of the capillary (c’) may rise transitorily, subsequently enriching the PCO2 of the arterial blood sensed by the central and/or peripheral chemoreceptors. This may add to the other sources of afferent stimuli to the pontine–medullary respiratory centers (red circle). The inspiratory neural drive (IND) is heightened; accordingly. Of note, IND is also modulated by cortical (black circle) and limbic (blue circle) influences. Excessive ventilation of the left alveolus with preserved V̇a/Q̇c relationship, triggered by increased chemostimulation or other source(s) of additional ventilatory stimuli, leads to alveolar hyperventilation and hypocapnia. A low arterial Pco2 may downward shift the CO2 set-point, impair the cerebrovascular reactivity (CVR) to CO2, and increase the central and peripheral chemoreceptor gains. Hypoxemia (arterial partial pressure for O2 (PaO2)), if present, sums up with these excitatory inputs to further increase the IND in a vicious circle that fuels breathlessness. In mild COPD, the evidence accrued to date does not support a major contribution of hyperventilation and/or low PaO2 to the patient high IND, suggesting a dominant role for the wasted ventilation.
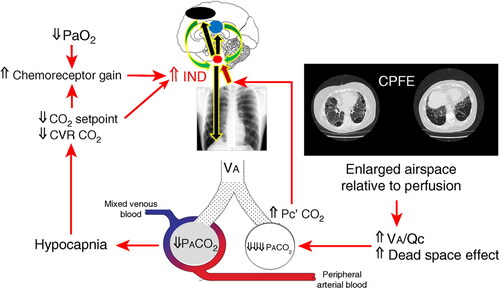
Figure 4. Schematic representation of the key mechanisms by which alveolar ventilation (V̇a)/capillary perfusion (Q̇c) mismatching may conspire to increase the inspiratory neural drive (IND), eventually leading to exertional dyspnea and exercise intolerance in patients with mild COPD. The putative mechanisms that have been ruled out in previous studies are highlighted (reviewed in refs. [Citation9,Citation28]). Emphasis is given to the unknown contribution of impaired perfusion of non-emphysematous lung tissue (in addition to emphysema) to increased (↑) wasted ventilation in the physiological dead space (high dead space (VD)/tidal volume (VT) ratio), heightened dyspnea at given work rate (WR) in these patients was largely commensurate to preserved dyspnea-ventilation (V̇E) relationship, in keeping with the pattern of “excessive breathing” (). See text for further elaboration. Definition of abbreviations: HPV: hypoxic pulmonary vasoconstriction; PAP = pulmonary arterial pressure. Modified, with permission of the publisher, from: Neder et al. [Citation111].
![Figure 4. Schematic representation of the key mechanisms by which alveolar ventilation (V̇a)/capillary perfusion (Q̇c) mismatching may conspire to increase the inspiratory neural drive (IND), eventually leading to exertional dyspnea and exercise intolerance in patients with mild COPD. The putative mechanisms that have been ruled out in previous studies are highlighted (reviewed in refs. [Citation9,Citation28]). Emphasis is given to the unknown contribution of impaired perfusion of non-emphysematous lung tissue (in addition to emphysema) to increased (↑) wasted ventilation in the physiological dead space (high dead space (VD)/tidal volume (VT) ratio), heightened dyspnea at given work rate (WR) in these patients was largely commensurate to preserved dyspnea-ventilation (V̇E) relationship, in keeping with the pattern of “excessive breathing” (Figure 5). See text for further elaboration. Definition of abbreviations: HPV: hypoxic pulmonary vasoconstriction; PAP = pulmonary arterial pressure. Modified, with permission of the publisher, from: Neder et al. [Citation111].](/cms/asset/8cf4823a-83f8-4ed4-9ac7-ee438bcd5f28/icop_a_2301549_f0004_c.jpg)
Figure 5. A simplified framework to expose the major “pulmonary” determinants of exertional dyspnea during incremental cardiopulmonary exercise testing as related to the pathophysiology of COPD. The key physiological abnormalities are highlighted: excess ventilation (high ventilation (V̇E)-(carbon dioxide output (V̇CO2) relationship) and the attainment of critical inspiratory constraints (CIC). In most dyspneic patients with mild COPD, increased wasted ventilation in the physiological dead space (VDphys) prompts a high, V̇E/V̇CO2 ratio leading to a pattern of “excessive breathing” rather than “restrained” (or “constrained”) breathing, i.e. increased dyspnea at given work rate (WR) but within the expected range when expressed relative to the heightened V̇E. Definition of abbreviations and symbols: ⇑: high; ⇓: low’; V̇A: alveolar ventilation; Q̇c: capillary perfusion; Pa: arterial partial pressure; PET: end-tidal partial pressure; Hypervent: alveolar hyperventilation; CIC: end-inspiratory lung volume/total lung capacity≥ 0.9, tidal volume (VT)/inspiratory capacity ≥ 0.7, VT plateau, all reached at an abnormally-low work rate. Ranges of dyspnea severity based on percentiles distribution of scores at a given ventilation as a function of age and sex: 5th–25th: “mild”; 25th–50th: “moderate”; 50th 75th: “severe”; 75th 95th: “very severe” [Citation14]. Reproduced, with permission of the publisher, from: Neder [Citation66].
![Figure 5. A simplified framework to expose the major “pulmonary” determinants of exertional dyspnea during incremental cardiopulmonary exercise testing as related to the pathophysiology of COPD. The key physiological abnormalities are highlighted: excess ventilation (high ventilation (V̇E)-(carbon dioxide output (V̇CO2) relationship) and the attainment of critical inspiratory constraints (CIC). In most dyspneic patients with mild COPD, increased wasted ventilation in the physiological dead space (VDphys) prompts a high, V̇E/V̇CO2 ratio leading to a pattern of “excessive breathing” rather than “restrained” (or “constrained”) breathing, i.e. increased dyspnea at given work rate (WR) but within the expected range when expressed relative to the heightened V̇E. Definition of abbreviations and symbols: ⇑: high; ⇓: low’; V̇A: alveolar ventilation; Q̇c: capillary perfusion; Pa: arterial partial pressure; PET: end-tidal partial pressure; Hypervent: alveolar hyperventilation; CIC: end-inspiratory lung volume/total lung capacity≥ 0.9, tidal volume (VT)/inspiratory capacity ≥ 0.7, VT plateau, all reached at an abnormally-low work rate. Ranges of dyspnea severity based on percentiles distribution of scores at a given ventilation as a function of age and sex: 5th–25th: “mild”; 25th–50th: “moderate”; 50th 75th: “severe”; 75th 95th: “very severe” [Citation14]. Reproduced, with permission of the publisher, from: Neder [Citation66].](/cms/asset/499ebab6-5d89-4667-8781-12931df46ad8/icop_a_2301549_f0005_c.jpg)
Figure 6. An illustrative example of how respiratory functional imaging (phase-resolved functional lung (PREFUL) 1H magnetic resonance imaging for ventilation and perfusion [Citation109–113] can be combined with computed tomography-based emphysema extension and distribution (parametric response mapping) to provide relevant insights into the genesis of out-of-proportion dyspnea in patients with mild COPD. This 54 years old woman with preserved FEV1 and out-of-proportion decrease in lung diffusing capacity for carbon monoxide showed high ventilation-CO2 output and an increased physiological dead space (VDphys) on cardiopulmonary exercise testing. Despite trivial emphysema (red and pink), note extensive areas of perfusion deficit percent (QDP) in non-emphysematous regions of the lung which were well ventilated (low ventilation deficit percent (VDP)) at rest and during exercise (light green). These findings are in keeping with the notion that impaired pulmonary perfusion beyond expected by emphysema, may increase the wasted ventilation in these patients, contributing to exertional dyspnea.
![Figure 6. An illustrative example of how respiratory functional imaging (phase-resolved functional lung (PREFUL) 1H magnetic resonance imaging for ventilation and perfusion [Citation109–113] can be combined with computed tomography-based emphysema extension and distribution (parametric response mapping) to provide relevant insights into the genesis of out-of-proportion dyspnea in patients with mild COPD. This 54 years old woman with preserved FEV1 and out-of-proportion decrease in lung diffusing capacity for carbon monoxide showed high ventilation-CO2 output and an increased physiological dead space (VDphys) on cardiopulmonary exercise testing. Despite trivial emphysema (red and pink), note extensive areas of perfusion deficit percent (QDP) in non-emphysematous regions of the lung which were well ventilated (low ventilation deficit percent (VDP)) at rest and during exercise (light green). These findings are in keeping with the notion that impaired pulmonary perfusion beyond expected by emphysema, may increase the wasted ventilation in these patients, contributing to exertional dyspnea.](/cms/asset/0300abbc-25a9-425d-9d69-e129e213045b/icop_a_2301549_f0006_c.jpg)
Table 1. A simplified overview of main invasive and noninvasive physiologic measurements potentially sensitive to disturbances in pulmonary gas exchange efficiency in patients with mild COPD.
The following potential explanations for increased afferent stimuli and excess ventilation have been carefully excluded in our investigations involving patients with mild COPD: (a) high pulmonary arterial pressures; (b) metabolic acidotic drive [Citation64], since excess ventilation emerged well before the so-called lactate threshold (in fact, it was frequently seen at rest)[Citation65]; (c) hypoxemia[Citation66]; (d) reduced CO2 set-point as overt respiratory alkalosis was an exception rather than a rule[Citation18]; (e) low VT leading to a high VD/VT as patients dynamically hyperinflate only at near maximum exercise; and, (f) heightened CO2 chemosensitivity[Citation26]. Thus, the only extant explanation for excess ventilation is increased areas of high V̇A/Q̇c; in fact, enlarged VDphys was the closest correlate of excess ventilation in our studies () [Citation10,Citation14,Citation18,Citation67].
It should be acknowledged, however, that in practice, VD/VT is calculated by Enghoff’s modification of the seminal Bohr’s formulation. Due to the complexities in measuring a representative PACO2, Enghoff proposed using PaCO2 instead. However, the higher the CO2 volume added to the arterial blood relative to PACO2, the greater the overestimation of VDphys by the Enghoff formulation. The bottom line is that spuriously high Enghoff’s VD/VT might be seen in patients showing a sizeable shunt and those with more extensive V̇A/Q heterogeneity [Citation68]. Although it might be argued that this is likely more relevant in patients with advanced COPD, it may have contributed to enlarged VDphys in our studies with milder disease [Citation10,Citation14,Citation18,Citation67].
Translating wasted ventilation into exertional dyspnea in mild COPD
Dynamic exercise is associated with a manyfold increase in V̇E. Remarkably, the sense of breathing effort/work does not increase substantially in healthy individuals. For instance, most non-trained men and women report only mild-moderate respiratory discomfort up to near-maximum CPET [Citation69]. A downward shift in the end-expiratory lung volume to encroach on the expiratory reserve volume allows VT to be positioned on the most compliant portion of the sigmoid pressure-volume relationship of the respiratory system [Citation70]. Optimization of the lungs’ dynamic compliance causes a harmonious coupling of the inspiratory neural drive (IND) from bulbo-pontine and cortical respiratory control centers and VT expansion, mitigating the breathing discomfort [Citation9].
Dyspnea arises in patients with cardiopulmonary diseases due to a disparity between the heightened IND and the capacity of the respiratory system to respond appropriately [Citation71]. Such an imbalance has been reflected in a consistent increase in several physiologic ratios that ultimately relate ventilatory demand to capacity [Citation72], such as:
Instantaneous V̇E/maximal ventilatory capacity (measured or estimated from FEV1), recently termed dynamic ventilatory reserve [Citation73];
Diaphragm electromyography and esophageal pressure, both related to values at maximal inspiration [Citation67]; and,
VT/inspiratory capacity or vital capacity, i.e., the volumes theoretically available for tidal expansion [Citation74].
The relationship of these ratios to dyspnea intensity is modulated by the presence or absence of mechanical constraints that are sufficiently high to hinder the translation of the IND into the mechanical act of breathing (VT and V̇E) ():
If the heightened IND can be fully converted to VT displacement and a higher V̇E, patients are more likely to use the words “increased work/effort” to describe their uncomfortable respiratory sensations [Citation27]. Since the increased dyspnea ratings are rather commensurate to the heightened ventilatory demands, we descriptively describe this source of exertional dyspnea as excessive breathing; or
If the heightened IND cannot be appropriately converted into VT displacement and a higher V̇E due to significant restrictive mechanical constraints, patients frequently report “unsatisfied inspiration” [Citation27]. Thus, dyspnea ratings increase at any given work rate and V̇E compared with healthy subjects. (constrained (or restrained) breathing).
The corollary is that wasted ventilation contributes to a high IND, a high V̇E/V̇CO2, and the CPET pattern of excessive breathing ( and ) in dyspneic patients with largely preserved FEV1 [Citation8,Citation75].
What are the structural bases of wasted ventilation in mild COPD?
Emphysema as the sole determinant of wasted ventilation
Emphysema epitomizes a structural abnormality associated with increased VDphys since it reduces the surface for gas exchange, and alveolar coalescence increases distal airspaces that are not perfused (“true” VD) [Citation68,Citation76,Citation77]. Classical studies found that panlobular and centrilobular emphysema may lead to increased VDphys since the earlier stages of COPD [Citation81–83]. In fact, we [Citation66] and others [Citation78,Citation79] found that wasted ventilation in emphysematous areas is associated with excess ventilation in mild-moderate COPD. Disproportionally impaired lung diffusing capacity for carbon monoxide (DLCO) relative to FEV1 has been associated with emphysema burden in several studies with mild COPD [Citation2,Citation80,Citation81]. Moreover, at least part of the low DLCO depicted by patients with incipient/minor emphysema has been ascribed to the “membrane” component [Citation82]. Emphysema extension was related to resting V̇A/Q̇c heterogeneity measured by MIGET in mild COPD[Citation83]. but this was not the case during exercise [Citation83]. Importantly, however, excess ventilation was found in patients with only trace emphysema [Citation24,Citation66,Citation84]. Since no study to date has assessed the topographic/regional distribution of V̇A/Q̇c under the modulating influence of emphysema, it remains unclear whether capillary destruction due to emphysema fully explains their increased wasted ventilation (Figure 2) (see Perspectives: amalgamating functional imaging and clinical physiology to advance the field) [Citation68,Citation77,Citation85,Citation86].
Reduced capillary blood volume in non-emphysematous lung
Tobacco-related injury/dysfunction of the lung microvasculature[Citation87] and vascular compression/distortion by areas of gas trapping secondary to small airway disease[Citation88] may increase Q̇c, leading to areas of high V̇A/Q̇c (VD “effect”)[Citation68,Citation77]. Impaired release of endothelium-derived relaxing factors, reduced endothelial nitric oxide synthase, and increased expression of endothelin-1 have been described in the pulmonary arterioles of patients with mild-moderate COPD [Citation89]. A recent review identified sixteen pathways associated with pulmonary vascular remodeling in COPD, most related to endothelial cell dysfunction and damage and only remotely linked to emphysema development [Citation90]. Pulmonary vascular tone had a central role in regulating V̇A/Q̇c in non-hypoxemic patients with largely preserved FEV1 [Citation83]. In fact, impaired microvascular blood flow out-of-proportion to emphysema burden has been reported in these patients[Citation91,Citation92]; moreover, manipulations of Q̇c via increased venous return [Citation93] and inhaled nitric oxide[Citation94] variably lessened excess ventilation. We [Citation66] and others [Citation95] found impaired global pulmonary perfusion during exercise in patients with mild COPD showing excess ventilation. Our group [Citation67] and other investigators [Citation81,Citation96] also described a strong association between a low DLCO, a “window to pulmonary microcirculation” [Citation97], and VDphys in mild COPD. Recently, we reported that reduced pulmonary vascular volume adjusted by emphysema extent was associated with low DLCO and heightened exertional ventilation in dyspneic smokers with minor emphysema [Citation98]. Moreover, gadolinium-enhanced magnetic resonance imaging (MRI) studies found indirect evidence of reduced perfusion in non-emphysematous lung in smokers and patients with minor COPD [Citation92,Citation99].
Areas of air trapping – a common finding in mild COPD – [Citation100] are thought to be poorly ventilated, decreasing (instead of increasing) V̇A/Q̇c [Citation101]. However, patchy pockets of air trapping may theoretically compress adjacent arterioles/capillaries, increasing V̇A/Q̇c in non-emphysematous tissue () [Citation88] This scenario, however, remains highly speculative since no study to date has shown impaired perfusion adjacent to scattered regions of air trapping, and inhaled bronchodilators typically fail to improve VDphys in these patients [Citation102].
Perspectives: amalgamating functional imaging and clinical physiology to advance the field
Despite the marked advances in our understanding of the decisive role of wasted ventilation in eliciting out-of-proportion exertional dyspnea in patients with apparently minor COPD [Citation8,Citation10–27], several questions remain unanswered:
Does high V̇A/Q̇c due to relative oligemia in areas with still preserved lung parenchyma (non-emphysematous) contribute substantially to increased VDphys?
If this proves true, are areas of high V̇A/Q̇c due to low Q̇c in non-emphysematous lung at least partially reversible with pulmonary vasodilators or, in fact, do they reflect largely irreversible vascular damage (or, alternatively, the compressive effects of patchy areas of air trapping ()?
If these abnormalities are reversible, is the expected improvement in VDphys large enough to decrease excess ventilation and exertional dyspnea, i.e., complex endpoints that are characteristically multifactorial in COPD?[Citation34]
How are these responses modulated by comorbidities that are known to increase exertional ventilation, such as pulmonary hypertension, lung fibrosis, and diastolic dysfunction?
Does the burden of high V̇A/Q̇c due to low Q̇c in non-emphysematous lungs hold any mechanistic influence in out-of-proportion exertional pulmonary hypertension in COPD? and,
Do these abnormalities precede the development of emphysema in longitudinal studies – as long proposed by Liebow [Citation103]?
A crucial limitation of pulmonary function tests aiming at interrogating lungs’ efficiency in gas transfer () is their inherent lack of information on the regional distribution of V̇A and Q̇c as modulated by emphysema [Citation104,Citation105]. To fill this gap, there is a growing role for respiratory functional imaging approaches co-registered to emphysema distribution by CT [Citation106–108], An illustrative approach may combine free-breathing phase-resolved functional lung (PREFUL) 1H MRI [Citation109–113] with computed tomography to provide quantitative, spatially-resolved ventilation, perfusion, and ventilation/perfusion deficits, taking into consideration the modulating influence of emphysema in patients showing or not increased VDphys ().
Conclusions
Restraining the functional assessment of dyspneic patients with COPD to spirometry is prone to provide an incomplete and potentially misleading picture of the actual extension of the disease. Such a caveat is particularly true in patients in the initial stages of when FEV1 is largely preserved. In this concise review, we outlined how increased wasted ventilation – frequently well beyond expected from the severity of FEV1 impairment and the emphysema burden – is relevant to patients’ breathlessness. In fact, DLCO, a physiological biomarker that is known to be negatively influenced by VDphys [Citation114], remains the closest correlate of exertional dyspnea in these patients [Citation67]. Moreover, bronchodilators, the cornerstone for treatment of the consequences of expiratory flow limitation (air trapping and high lung volumes) [Citation115] with negligible effects on VDphys [Citation102] are poorly effective in improving exertional dyspnea in mild disease [Citation116]. Advances in the field will therefore depend on our ability to develop robust platforms for comprehensively phenotyping (structurally and functionally), the dyspneic patients showing unexpectedly high VDphys [Citation57]. This will create the bases for testing existing (e.g. oral and inhaled vasodilators) and newer therapeutic approaches geared to improve V̇A/Q̇c in carefully selected patients showing the deleterious respiratory sensory consequences of increased wasted ventilation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- O’Donnell DE, Milne KM, James MD, et al. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. 2020;37(1):1–11. doi:10.1007/s12325-019-01128-9.
- Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822–829. doi:10.1136/thoraxjnl-2015-206938.
- Tan WC, Hague CJ, Leipsic J, et al. Findings on thoracic computed tomography scans and respiratory outcomes in persons with and without chronic obstructive pulmonary disease: a Population-Based cohort study. PLoS ONE. 2016;11(11):e0166745. doi:10.1371/journal.pone.0166745.
- Cherian M, Jensen D, Tan WC, et al. Dyspnoea and symptom burden in mild-moderate COPD: the Canadian cohort obstructive lung disease study. ERJ Open Res. 2021;7(2):00960–2020. doi:10.1183/23120541.00960-2020.
- GOLD Executive Committee. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2019. [Internet]. Available from: www.goldcopd.org.
- O’Donnell DE, Gebke KB. Examining the role of activity, exercise, and pharmacology in mild COPD. Postgrad Med. 2014;126(5):135–145. doi:10.3810/pgm.2014.09.2808.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577–588. doi:10.2147/COPD.S62766.
- James MD, Milne KM, Phillips DB, et al. Dyspnea and exercise limitation in mild COPD: the value of CPET. Front Med (Lausanne). 2020;7:442. doi:10.3389/fmed.2020.00442.
- Neder JA, de Torres JP, O’Donnell DE. Recent advances in the physiological assessment of dyspneic patients with mild COPD. COPD. 2021;18(3):374–384. doi:10.1080/15412555.2021.1913110.
- Ofir D, Laveneziana P, Webb KA, et al. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(6):622–629. doi:10.1164/rccm.200707-1064OC.
- Deesomchok A, Webb KA, Forkert L, et al. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD. 2010;7(6):428–437. doi:10.3109/15412555.2010.528087.
- Guenette JA, Raghavan N, Harris-McAllister V, et al. Effect of adjunct fluticasone propionate on airway physiology during rest and exercise in COPD. Respir Med. 2011;105(12):1836–1845. doi:10.1016/j.rmed.2011.08.021.
- Guenette JA, Webb KA, O’Donnell DE. Effect of fluticasone/salmeterol combination on dyspnea and respiratory mechanics in mild-to-moderate COPD. Respir Med. 2013;107(5):708–716. doi:10.1016/j.rmed.2013.01.009.
- Chin RC, Guenette JA, Cheng S, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187(12):1315–1323. doi:10.1164/rccm.201211-1970OC.
- Guenette JA, Chin RC, Cheng S, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J. 2014;44(5):1177–1187. doi:10.1183/09031936.00034714.
- Neder JA, Arbex FF, Alencar MCN, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J. 2015;45(2):377–387. doi:10.1183/09031936.00135514.
- Neder JA, O’Donnell CDJ, Cory J, et al. Ventilation distribution heterogeneity at rest as a marker of exercise impairment in mild-to-Advanced COPD. COPD. 2015;12(3):249–256. doi:10.3109/15412555.2014.948997.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384–1394. doi:10.1164/rccm.201501-0157OC.
- Elbehairy AF, Raghavan N, Cheng S, et al. Physiologic characterization of the chronic bronchitis phenotype in GOLD grade IB COPD. Chest. 2015;147(5):1235–1245. doi:10.1378/chest.14-1491.
- Elbehairy AF, Guenette JA, Faisal A, et al. Mechanisms of exertional dyspnoea in symptomatic smokers without COPD. Eur Respir J. 2016;48(3):694–705. doi:10.1183/13993003.00077-2016.
- Faisal A, Alghamdi BJ, Ciavaglia CE, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med. 2016;193(3):299–309. doi:10.1164/rccm.201504-0841OC.
- Elbehairy AF, Faisal A, Guenette JA, et al. Resting physiological correlates of reduced exercise capacity in smokers with mild airway obstruction. COPD. 2017;14(3):267–275. doi:10.1080/15412555.2017.1281901.
- Elbehairy AF, Parraga G, Webb KA, et al. Mild chronic obstructive pulmonary disease: why spirometry is not sufficient!. Expert Rev Respir Med. 2017;11(7):549–563. doi:10.1080/17476348.2017.1334553.
- Zelt JT, Jones JH, Hirai DM, et al. Systemic vascular dysfunction is associated with emphysema burden in mild COPD. Respir Med. 2018;136:29–36. doi:10.1016/j.rmed.2018.01.007.
- Phillips DB, James MD, Elbehairy AF, et al. Reduced exercise tolerance in mild chronic obstructive pulmonary disease: the contribution of combined abnormalities of diffusing capacity for carbon monoxide and ventilatory efficiency. Respirology. 2021;26(8):786–795. doi:10.1111/resp.14045.
- Phillips DB, Domnik NJ, Elbehairy AF, et al. Elevated exercise ventilation in mild COPD is not linked to enhanced central chemosensitivity. Respir Physiol Neurobiol. 2021;284:103571. doi:10.1016/j.resp.2020.103571.
- Phillips DB, Neder JA, Elbehairy AF, et al. Qualitative components of dyspnea during incremental exercise across the COPD continuum. Med Sci Sports Exerc. 2021;53(12):2467–2476. doi:10.1249/MSS.0000000000002741.
- Neder JA, Berton DC, Phillips DB, et al. Exertional ventilation/carbon dioxide output relationship in COPD: from physiological mechanisms to clinical applications. Eur Respir Rev. 2021;30(161):200190. doi:10.1183/16000617.0190-2020.
- Elbehairy AF, O’Donnell CD, Abd Elhameed A, et al. Low resting diffusion capacity, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. J Appl Physiol (1985). 2019;127(4):1107–1116., doi:10.1152/japplphysiol.00341.2019.
- Glenny RW, Robertson HT. Spatial distribution of ventilation and perfusion: mechanisms and regulation. Compr Physiol. 2011;1(1):375–395. doi:10.1002/cphy.c100002.
- West JB. State of the art: ventilation-perfusion relationships. Am Rev Respir Dis. 1977;116(5):919–943. doi:10.1164/arrd.1977.116.5.919.
- West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol. 1962;17(6):893–898. doi:10.1152/jappl.1962.17.6.893.
- Cardús J, Burgos F, Diaz O, et al. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156(2 Pt 1):648–653. doi:10.1164/ajrccm.156.2.9606016.
- Neder JA, Berton DC, Arbex FF, et al. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J. 2017;49(3):1602036. doi:10.1183/13993003.02036-2016.
- West JB. Understanding pulmonary gas exchange: ventilation-perfusion relationships. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1071–1072. doi:10.1152/classicessays.00024.2004.
- Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol (1985). 1999;87(6):1997–2006. doi:10.1152/jappl.1999.87.6.1997.
- Wagner PD. Ventilation-perfusion matching during exercise. Chest. 1992;101(5 Suppl):192S–198S. doi:10.1378/chest.101.5_supplement.192s.
- Whipp BJ, Ward SA. Cardiopulmonary coupling during exercise. J Exp Biol. 1982;100(1):175–193. doi:10.1242/jeb.100.1.175.
- Whipp BJ. Control of the exercise hyperpnea: the unanswered question. Adv Exp Med Biol. 2008;605:16–21. doi:10.1007/978-0-387-73693-8_3.
- Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol. 1949;1(12):825–847. doi:10.1152/jappl.1949.1.12.825.
- Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi:10.1056/NEJMra1900475.
- Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol (1985). 2009;106(6):1902–1908. doi:10.1152/japplphysiol.00085.2009.
- Neder JA. Residual exertional dyspnea in cardiopulmonary disease. Ann Am Thorac Soc. 2020;17(12):1516–1525. doi:10.1513/AnnalsATS.202004-398FR.
- Pain CF, Glazier JB, Simon H, et al. Regional and overall inequality of ventilation and blood flow in patients with chronic airflow obstruction. Thorax. 1967;22(5):453–461. doi:10.1136/thx.22.5.453.
- West JB, Crouch DR, Fine JM, et al. A new, noninvasive method of measuring impaired pulmonary gas exchange in lung disease: an outpatient study. Chest. 2018;154(2):363–369. doi:10.1016/j.chest.2018.02.001.
- Filley GF, Gregoire F, Wright GW. Alveolar and arterial oxygen tensions and the significance of the alveolar-arterial oxygen tension difference in normal men. J Clin Invest. 1954;33(4):517–529. doi:10.1172/JCI102922.
- Kreit JW. Volume capnography in the intensive care unit: physiological principles, measurements, and calculations. Ann Am Thorac Soc. 2019;16(3):291–300. doi:10.1513/AnnalsATS.201807-501CME.
- Krogh M. The diffusion of gases through the lungs of man. J Physiol. 1915;49(4):271–300. doi:10.1113/jphysiol.1915.sp001710.
- Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290–302. doi:10.1152/jappl.1957.11.2.290.
- Hughes JM, Pride NB. In defence of the carbon monoxide transfer coefficient kco (TL/VA). Eur Respir J. 2001;17(2):168–174. doi:10.1183/09031936.01.17201680.
- Stam H, Versprille A, Bogaard JM. The components of the carbon monoxide diffusing capacity in man dependent on alveolar volume. Bull Eur Physiopathol Respir. 1983;19(1):17–22.
- Guenard H, Varene N, Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol. 1987;70(1):113–120. doi:10.1016/s0034-5687(87)80036-1.
- Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979;47(5):954–960. doi:10.1152/jappl.1979.47.5.954.
- Bohr C. [Ueber die Lungenathmung]. Skand Arch Physiol. 1891;2(1):236–268. doi:10.1111/j.1748-1716.1891.tb00581.x.
- Enghoff H[, I, Volumen. Bermekungen zur Frage des shadlichen Raumes. Upsala Laekarefoeren]. Foerh. 1938;14:191–218.
- Ming DKY, Patel MS, Hopkinson NS, et al. The ‘anatomic shunt test’ in clinical practice; contemporary description of test and in-service evaluation. Thorax. 2014;69(8):773–775. doi:10.1136/thoraxjnl-2013-204103.
- Neder JA, Kirby M, Santyr G, et al. V̇/Q̇ mismatch: a novel target for COPD treatment. Chest. 2022;162(5):1030–1047. doi:10.1016/j.chest.2022.03.033.
- Wagner PD, Dantzker DR, Dueck R, et al. Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest. 1977;59(2):203–216. doi:10.1172/JCI108630.
- Rocha A, Arbex FF, Sperandio PA, et al. Excess ventilation in chronic obstructive pulmonary disease-Heart failure overlap. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2017;196(10):1264–1274. doi:10.1164/rccm.201704-0675OC.
- Costa CM, Neder JA, Verrastro CG, et al. Uncovering the mechanisms of exertional dyspnoea in combined pulmonary fibrosis and emphysema. Eur Respir J. 2020;55(1):1901319. doi:10.1183/13993003.01319-2019.
- Neder JA, Rocha A, Arbex FF, et al. Exertional oscillatory ventilation in subjects without heart failure reporting chronic dyspnoea. ERJ Open Res. 2023;9(1):00324–2022. doi:10.1183/23120541.00324-2022.
- Dempsey JA, Smith CA. Pathophysiology of human ventilatory control. Eur Respir J. 2014;44(2):495–512. doi:10.1183/09031936.00048514.
- O’Donnell DE, Elbehairy AF, Berton DC, et al. Advances in the evaluation of respiratory pathophysiology during exercise in chronic lung diseases. Front Physiol. 2017;8:82. doi:10.3389/fphys.2017.00082.
- Wasserman K, Whipp BJ, Koyl SN, et al. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35(2):236–243. doi:10.1152/jappl.1973.35.2.236.
- Neder JA, Berton DC, Marillier M, et al. Resting V’E/V’CO2 adds to inspiratory capacity to predict the burden of exertional dyspnoea in COPD. Eur Respir J. 2020;56(1):1902434. doi:10.1183/13993003.02434-2019.
- Jones JH, Zelt JT, Hirai DM, et al. Emphysema on thoracic CT and exercise ventilatory inefficiency in mild-to-moderate COPD. COPD. 2017;14(2):210–218. doi:10.1080/15412555.2016.1253670.
- James MD, Phillips DB, Elbehairy AF, et al. Mechanisms of exertional dyspnea in patients with mild COPD and a low resting DLCO. COPD. 2021;18(5):501–510. doi:10.1080/15412555.2021.1932782.
- Robertson HT. Dead space: the physiology of wasted ventilation. Eur Respir J. 2015;45(6):1704–1716. doi:10.1183/09031936.00137614.
- Neder JA, Berton DC, Nery LE, et al. A frame of reference for assessing the intensity of exertional dyspnoea during incremental cycle ergometry. Eur Respir J. 2020;56(4):2000191. doi:10.1183/13993003.00191-2020.
- Dempsey JA, Vidruk EH, Mastenbrook SM. Pulmonary control systems in exercise. Fed Proc. 1980;39(5):1498–1505.
- Mahler DA, O’Donnell DE. Recent advances in dyspnea. Chest. 2015;147(1):232–241. doi:10.1378/chest.14-0800.
- Laviolette L, Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. 2014;43(6):1750–1762. doi:10.1183/09031936.00092613.
- Berton DC, Plachi F, James MD, et al. Dynamic ventilatory reserve during incremental exercise: reference values and clinical validation in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2023;20(10):1425–1434. doi:10.1513/AnnalsATS.202304-303OC.
- O’Donnell DE, Milne KM, Vincent SG, et al. Unraveling the causes of unexplained dyspnea: the value of exercise testing. Clin Chest Med. 2019;40(2):471–499. doi:10.1016/j.ccm.2019.02.014.
- Neder JA. Cardiopulmonary exercise testing applied to respiratory medicine: myths and facts. Respir Med. 2023;214:107249. doi:10.1016/j.rmed.2023.107249.
- Macklem PT. The pathophysiology of chronic bronchitis and emphysema. Med Clin North Am. 1973;57(3):669–670. doi:10.1016/s0025-7125(16)32266-0.
- Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014;44(4):1023–1041. doi:10.1183/09031936.00037014.
- Crisafulli E, Alfieri V, Silva M, et al. Relationships between emphysema and airways metrics at High-Resolution Computed Tomography (HRCT) and ventilatory response to exercise in mild to moderate COPD patients. Respir Med. 2016;117:207–214. doi:10.1016/j.rmed.2016.06.016.
- Rinaldo RF, Mondoni M, Comandini S, et al. The role of phenotype on ventilation and exercise capacity in patients affected by COPD: a retrospective study. Multidiscip Respir Med. 2020;15(1):476. doi:10.4081/mrm.2020.476.
- Tanabe N, Rhee CK, Sato S, et al. Disproportionally impaired diffusion capacity relative to airflow limitation in COPD. COPD. 2020;17(6):627–634. doi:10.1080/15412555.2020.1845639.
- Kirby M, Owrangi A, Svenningsen S, et al. On the role of abnormal DL(CO) in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax. 2013;68(8):752–759. doi:10.1136/thoraxjnl-2012-203108.
- Xie C, Rong Z, Li Z, et al. Measurements of membrane diffusing capacity and pulmonary capillary blood volume in normal subjects and patients with mild emphysema. Chin Med J (Engl). 1996;109:840–847.
- Barbera JA, Ramirez J, Roca J, et al. Lung structure and gas exchange in mild chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(4 Pt 1):895–901. doi:10.1164/ajrccm/141.4_Pt_1.895.
- Hirai DM, Jones JH, Zelt JT, et al. Oral N-acetylcysteine and exercise tolerance in mild chronic obstructive pulmonary disease. J Appl Physiol (1985). 2017;122(5):1351–1361. doi:10.1152/japplphysiol.00990.2016.
- Kirby M, Pike D, Sin DD, et al. COPD: do imaging measurements of emphysema and airway disease explain symptoms and exercise capacity? Radiology. 2015;277(3):872–880. doi:10.1148/radiol.2015150037.
- Ostridge K, Gove K, Paas KHW, et al. Using novel computed tomography analysis to describe the contribution and distribution of emphysema and small airways disease in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(8):990–997. doi:10.1513/AnnalsATS.201810-669OC.
- Barberà JA, Riverola A, Roca J, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149(2 Pt 1):423–429. doi:10.1164/ajrccm.149.2.8306040.
- Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277–1281. doi:10.1056/NEJM197806082982303.
- Santos S, Peinado VI, Ramirez J, et al. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(9):1250–1256. doi:10.1164/rccm.200210-1233OC.
- Borek I, Birnhuber A, Voelkel NF, et al. The vascular perspective on acute and chronic lung disease. J Clin Invest. 2023;133(16):e170502. doi:10.1172/JCI170502.
- Aaron CP, Hoffman EA, Lima JAC, et al. Pulmonary vascular volume, impaired left ventricular filling and dyspnea: the MESA lung study. PLoS ONE. 2017;12(4):e0176180. doi:10.1371/journal.pone.0176180.
- Hueper K, Vogel-Claussen J, Parikh MA, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med. 2015;192(5):570–580. doi:10.1164/rccm.201411-2120OC.
- Ross BA, Brotto AR, Fuhr DP, et al. The supine position improves but does not normalize the blunted pulmonary capillary blood volume response to exercise in mild COPD. J Appl Physiol (1985). 2020;128(4):925–933. doi:10.1152/japplphysiol.00890.2019.
- Phillips DB, Brotto AR, Ross BA, et al. Inhaled nitric oxide improves ventilatory efficiency and exercise capacity in patients with mild COPD: a randomized-control cross-over trial. J Physiol. 2021;599(5):1665–1683. doi:10.1113/JP280913.
- Tedjasaputra V, van Diepen S, Phillips DB, et al. Pulmonary capillary blood volume response to exercise is diminished in mild chronic obstructive pulmonary disease. Respir Med. 2018;145:57–65. doi:10.1016/j.rmed.2018.10.015.
- Díaz AA, Pinto-Plata V, Hernández C, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med. 2015;109(7):882–889. doi:10.1016/j.rmed.2015.04.009.
- Hughes JMB, Bates DV. Historical review: the carbon monoxide diffusing capacity (DLCO) and its membrane (DM) and red cell (Theta.Vc) components. Respir Physiol Neurobiol. 2003;138(2–3):115–142. doi:10.1016/j.resp.2003.08.004.
- Elbehairy AF, Vincent SG, Phillips DB, et al. Pulmonary vascular volume by quantitative CT in dyspneic smokers with minor emphysema. COPD. 2023;20(1):135–143. doi:10.1080/15412555.2023.2169121.
- Hueper K, Parikh MA, Prince MR, et al. Quantitative and semiquantitative measures of regional pulmonary microvascular perfusion by magnetic resonance imaging and their relationships to global lung perfusion and lung diffusing capacity: the multiethnic study of atherosclerosis chronic obstructive pulmonary disease study. Invest Radiol. 2013;48(4):223–230. doi:10.1097/RLI.0b013e318281057d.
- Pompe E, Strand M, van Rikxoort EM, et al. Five-year progression of emphysema and air trapping at CT in smokers with and those without chronic obstructive pulmonary disease: results from the COPDGene study. Radiology. 2020;295(1):218–226. doi:10.1148/radiol.2020191429.
- Biederer J. MR imaging of the airways. Br J Radiol. 2023;96(1146):20220630. doi:10.1259/bjr.20220630.
- Elbehairy AF, Webb KA, Laveneziana P, et al. Acute bronchodilator therapy does not reduce wasted ventilation during exercise in COPD. Respir Physiol Neurobiol. 2018;252-253:64–71. doi:10.1016/j.resp.2018.03.012.
- Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80(1, Part 2):67–93. doi:10.1164/arrd.1959.80.1P2.67.
- Ohno Y, Seo JB, Parraga G, et al. Pulmonary functional imaging: part 1-State-of-the-Art technical and physiologic underpinnings. Radiology. 2021;299(3):508–523. doi:10.1148/radiol.2021203711.
- Gefter WB, Lee KS, Schiebler ML, et al. Pulmonary functional imaging: part 2—State-of-the-Art clinical applications and opportunities for improved patient care. Radiology. 2021;299(3):524–538. doi:10.1148/radiol.2021204033.
- Kirby M, Yin Y, Tschirren J, et al. A novel method of estimating small airway disease using inspiratory-to-expiratory computed tomography. Respiration. 2017;94(4):336–345. doi:10.1159/000478865.
- Guo F, Capaldi D, Kirby M, et al. Development of a pulmonary imaging biomarker pipeline for phenotyping of chronic lung disease. J Med Imaging (Bellingham). 2018;5(2):026002. doi:10.1117/1.JMI.5.2.026002.
- MacNeil JL, Capaldi DPI, Westcott AR, et al. Pulmonary imaging phenotypes of chronic obstructive pulmonary disease using multiparametric response maps. Radiology. 2020;295(1):227–236. doi:10.1148/radiol.2020191735.
- Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79(4):2306–2314. doi:10.1002/mrm.26893.
- Kaireit TF, Voskrebenzev A, Gutberlet M, et al. Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients. J Magn Reson Imaging. 2019;49(4):1122–1132. doi:10.1002/jmri.26342.
- Klimeš F, Voskrebenzev A, Gutberlet M, et al. Repeatability of dynamic 3D phase-resolved functional lung (PREFUL) ventilation MR imaging in patients with chronic obstructive pulmonary disease and healthy volunteers. J Magn Reson Imaging. 2021;54(2):618–629. doi:10.1002/jmri.27543.
- Pöhler GH, Löffler F, Klimeš F, et al. Validation of phase-resolved functional lung (PREFUL) magnetic resonance imaging pulse wave transit time compared to echocardiography in chronic obstructive pulmonary disease. J Magn Reson Imaging. 2021;56(2):605–615. doi:10.1002/jmri.28016.
- Pöhler GH, Klimeš F, Behrendt L, et al. Repeatability of phase-resolved functional lung (PREFUL)-MRI ventilation and perfusion parameters in healthy subjects and COPD patients. J Magn Reson Imaging. 2021;53(3):915–927. doi:10.1002/jmri.27385.
- Neder JA, Berton DC, Muller PT, et al. Incorporating lung diffusing capacity for carbon monoxide in clinical decision making in chest medicine. Clin Chest Med. 2019;40(2):285–305. doi:10.1016/j.ccm.2019.02.005.
- O’Donnell DE. Assessment of bronchodilator efficacy in symptomatic COPD: is spirometry useful? Chest. 2000;117:42S–47S.
- Elbehairy AF, Webb KA, Neder JA, et al. Should mild COPD be treated? Evidence for early pharmacological intervention. Drugs. 2013;73(18):1991–2001. doi:10.1007/s40265-013-0145-9.

