Abstract
COPD is an inflammatory lung disease that limits airflow and remodels the pulmonary vascular system. This study delves into the therapeutic potential and mechanistic underpinnings of Panax notoginseng Saponins (PNS) in alleviating inflammation and pulmonary vascular remodeling in a COPD rat model. Symmap and ETCM databases provided Panax notoginseng-related target genes, and the CTD and DisGeNET databases provided COPD-related genes. Intersection genes were subjected to protein-protein interaction analysis and pathway enrichment to identify downstream pathways. A COPD rat model was established, with groups receiving varying doses of PNS and a Roxithromycin control. The pathological changes in lung tissue and vasculature were examined using histological staining, while molecular alterations were explored through ELISA, RT-PCR, and Western blot. Network pharmacology research suggested PNS may affect the TLR4/NF-κB pathway linked to COPD development. The study revealed that, in contrast to the control group, the COPD model exhibited a significant increase in inflammatory markers and pathway components such as TLR4, NF-κB, HIF-1α, VEGF, ICAM-1, SELE mRNA, and serum TNF-α, IL-8, and IL-1β. Treatment with PNS notably decreased these markers and mitigated inflammation around the bronchi and vessels. Taken together, the study underscores the potential of PNS in reducing lung inflammation and vascular remodeling in COPD rats, primarily via modulation of the TLR4/NF-κB/HIF-1α/VEGF pathway. This research offers valuable insights for developing new therapeutic strategies for managing and preventing COPD.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a prevalent, preventable, and treatable respiratory condition characterized by persistent signs and airflow limitation. It typically results from prolonged exposure to harmful particles or gases, influenced by various host factors [Citation1]. COPD poses a global health burden, with escalating prevalence and mortality rates. By the end of 2018, over 100 million individuals in China were affected by COPD, severely impacting their quality of life and imposing significant economic burdens on families and society [Citation2].
Long-term exposure to cigarette smoke can activate reactive oxygen species (ROS), induce oxidative stress and inflammation, and lead to cell apoptosis, resulting in the development of enlarged alveolar spaces and COPD - emphysema. Cigarette smoke exposure is a major risk factor for COPD [Citation3]. This process involves both lung parenchyma and interstitium, where lung parenchymal changes include emphysema and airway remodeling, while interstitial changes involve pulmonary vascular remodeling and fibrosis. The pathophysiological mechanisms of COPD involve chronic inflammation, remodeling of airway and alveolar structures, as well as changes in pulmonary vasculature. Inflammatory and hypoxic stimuli affect pulmonary vasculature, leading to the release of inflammatory cells and chemotactic factors that cause endothelial cell, smooth muscle cell, and collagen protein degeneration and abnormal proliferation, ultimately resulting in pulmonary vascular remodeling [Citation4, Citation5]. Although the exact mechanisms by which nonsmokers develop chronic obstructive pulmonary disease (COPD) remains unclear, nonsmokers with COPD exhibit similar morphological characteristics to smokers [Citation3]. This implies that cigarette smoke not only poses significant health risks to smokers but also has a serious impact on the health of nonsmokers through discarded cigarette butts (CBs), secondhand smoke (SHS), and thirdhand smoke (THS) exposure [Citation6]. Gold’s recommended stable-phase COPD drug treatment includes expectorants, bronchodilators, mucolytic/antioxidants, and anti-infectives [Citation1]. While these interventions alleviate signs, they do not impede disease progression or the continuous decline in lung function, and their effects on pulmonary vasculature are limited.
The mechanism of pulmonary vascular remodeling in COPD remains elusive. Recent research suggests the significance of the HIF-VEGF signaling pathway in COPD pulmonary vascular remodeling [Citation7, Citation8]. Hypoxia and inflammation elevate HIF expression, activating downstream target genes such as VEGF [Citation9–11]. NF-κB, regulated by TLR, directly modulates HIF expression during inflammation and hypoxia [Citation12]. Investigating the TLR4/NF-κB/HIF-1α/VEGF pathway’s role in COPD pulmonary vascular remodeling is crucial for understanding this process comprehensively.
Panax notoginseng saponins (PNS), derived from the traditional Chinese herb Panax notoginseng, possess vasodilatory, anti-inflammatory, antioxidative, and blood-activating properties [Citation13–15]. Previous studies demonstrated PNS and curcumin’s potential in slowing pulmonary fibrosis through autophagy activation, reduced collagen gene expression, and decreased inflammatory factors [Citation16–18]. Although PNS exhibit anti-inflammatory and pulmonary vascular remodeling inhibitory effects, detailed research on their impact on the entire pathway in COPD remains limited.
This study aims to explore PNS’s effects on pulmonary lesions in a rat model of COPD, specifically investigating its potential in disease alleviation by modulating the TLR4/NF-κB/HIF-1α/VEGF signaling pathway. The research deepens our understanding of COPD pulmonary vascular remodeling and offers a new direction and experimental basis for developing more effective COPD treatment strategies.
Methods and materials
Network pharmacology and bioinformatics analysis for PNS target genes
Target genes of the traditional Chinese medicine Panax notoginseng were retrieved from the Symmap database (http://www.symmap.org/) [Citation19] and ETCM (http://www.tcmip.cn/ETCM/) [Citation20]. COPD-related genes were obtained from the CTD (https://ctdbase.org/) and DisGeNET (https://www.disgenet.org/home/) databases, filtered based on Inference Score ≥ 20 or Score_gda ≥ 0.1. After obtaining the target genes of Panax notoginseng and COPD-related genes, intersections were drawn using the R software package “venn” (version 1.11) on the Hiplot Pro platform (https://hiplot.com.cn/). The interaction network of proteins encoded by candidate target genes was obtained from the STRING database (https://string-db.org), setting the species condition to “Rattus norvegicus”. The core degree (degree) of the candidate target genes was calculated. The importance of candidate target genes in COPD was analyzed using the Phenolyzer database (https://phenolyzer.wglab.org/).
GO/KEGG enrichment analysis
For the gene intersection, the “clusterProfiler” package (version 4.5.0) in R software (version v.4.2.2) was used to perform GO (Gene Ontology) enrichment analysis, covering biological processes (BP), cellular components (CC), and molecular functions (MF), as well as KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis [Citation21].
Experimental animals
A total of 48 SPF-grade male Wistar rats, weighing 180-200 g and aged 8 weeks, were provided by Liaoning Changsheng Biotechnology Co., Ltd., with license number SCXK (Liaoning) 2015-0001. Rats were acclimated to standard laboratory conditions at 24 ± 2 °C, 55-60% humidity, and a 12-h light/dark cycle. All animal experiments strictly followed the NIH “Guide for the Care and Use of Laboratory Animals” and were approved by the Ethics Committee of Yunnan University of Traditional Chinese Medicine.
Construction of COPD rat model
Rats were randomly divided into six groups, each with eight rats: control group, model group, Roxithromycin group (positive control), and three PNS groups with low (2.36 mg/ml), medium (4.73 mg/ml), and high (9.45 mg/ml) doses. Using Hongta brand cigarettes (Hongta Tobacco Co., Ltd.), the COPD rat model was replicated using the smoke exposure combined with airway instillation of lipopolysaccharide (LPS) method [Citation22]. On the 1st and 14th days of the experiment, each group of animals was anesthetized with 3% isoflurane (792632, Sigma Aldrich, USA, https://www.sigmaaldrich.cn/CN/zh) and instilled with 200 μL of LPS into the trachea. From the 2nd to 13th and 15th to 56th days, rats were placed in a custom-made organic glass smoke box (patent applied; ZL201921358122.2) for smoke exposure once a day, 10 cigarettes each time, for 45 min each, continuously for 8 weeks.
Intervention protocols
From the ninth week of the experiment, following the successful replication of the model, a four-week drug intervention commenced. In the blank and model groups, rats were administered 2 ml/day of physiological saline via gavage. The Roxithromycin capsule dosage required for the rats was determined based on the adult dose, adjusted for the rat’s body surface area (calculated as 6.17 times the adult dose, with the rat unit weight dose conversion factor being 0.018, assuming an average rat weight of 200 g). This calculation resulted in a dose of 4.73 mg/ml per rat per day. Prior to administration, a suspension was prepared using physiological saline, and PNS was administered through gavage. The dosage ratios for the low, medium, and high dose groups were set at 0.5:1:2, respectively. The medium dose corresponded to the clinical equivalent dose (as per KPC Pharmaceuticals, Inc GMP Z20040016 Xuesaitong soft capsule, with clinical use of 100 mg, three times a day, totaling 300 mg). The daily required dose for rats in the medium dose group was calculated to be 9.45 mg, derived using the formula 300 mg × 0.018 × 5 × 0.35. Upon the initiation of drug treatment in the ninth week, the average weight of the rats was 350 g. For each administration, the drug was dissolved in 2 ml of physiological saline to achieve a concentration of 4.73 mg/ml. The daily required dose for the low-dose group was half that of the medium dose, equating to 2.36 mg/ml, while for the high-dose group, it was twice the medium dose, totaling 9.45 mg/ml.
Lung function assessment
The lung function was evaluated using the ratio of the forced expiratory volume in 0.2 s (FEV0.2) to the forced vital capacity (FVC), and the peak expiratory flow (PEF). Briefly, each rat was injected with 1% sodium pentobarbital (40 mg/kg, intraperitoneally) to induce anesthesia, followed by a period of maintenance to ensure adequate sedation. Afterward, a tracheotomy was performed on each rat between the second and third tracheal cartilage rings using an inverted T-shaped incision. The rats were then intubated and connected to a ventilator, with a set breathing rate of 75 breaths per min and a tidal volume of 5 ml/kg. Under these conditions, the values for FEV0.2/FVC and PEF were measured using the AniRes2005 Pulmonary Mechanics Analyzer (Beijing Bestlab High-Tech). The operation of this device was carried out strictly according to the manufacturer’s instructions [Citation23].
Specimen collection
After 4 weeks of drug intervention, rat specimens were collected. After the last gavage, water and food were withheld for 24 h, and after weighing, rats were anesthetized with 3% isoflurane. The rats’ limbs and heads were fixed, the skin was prepared, and routine disinfection was performed. A longitudinal incision was made along the midline to expose the neck white line, and after blunt dissection of the muscles, the abdominal cavity was exposed under direct vision. The large and small intestines were moved aside to expose the abdominal aorta, from which blood was drawn. Afterward, the supernatant was centrifuged at 4 °C, 3000 r/min, for 10 min, and the supernatant was aliquoted into 1.5EP tubes and stored at −80 °C for ELISA detection. The thoracic cavity was opened, and the right upper lung was taken and soaked in 4% polyformaldehyde for pathological examination. The remaining lungs were washed with 0.9% Sodium Chloride Injection, aliquoted into 1.5 ml EP tubes, rapidly frozen in liquid nitrogen, and stored at −80 °C for RT-qPCR and Western blot detection.
Hematoxylin and eosin (H&E) Staining
Lung tissue sections were dehydrated in a gradient of alcohol, cleared, and routinely embedded in paraffin. Continuous sections with a thickness of 4 µm were cut on a paraffin slicer, and the sections were baked at 60 °C for 1 h and then dewaxed in xylene. After hydration, routine H&E staining was performed. The tissue was stained with hematoxylin solution for 2 min, rinsed with tap water for 10 s, and differentiated with 1% hydrochloric acid ethanol for 10 s. After washing with distilled water for 1 min, the tissue was stained with eosin solution for 1 min, briefly rinsed with distilled water for 10 s, dehydrated in a gradient of alcohol, cleared in xylene, and sealed with neutral gum. After sealing, histomorphological changes were observed under an optical microscope (XP-330, Shanghai Bingyu Optical Instrument Co., Ltd., Shanghai, China) [Citation24, Citation25].
Victoria Blue + Van Gieson Method for Collagen Fiber Staining to observe vascular lesions in Rat lung tissue
Victoria Blue Elastic Fiber Staining: First, the sections were oxidized with Tanake oxidant (A1:A2 = 1:1, freshly prepared, Sigma-Aldrich) for 5 min. This oxidant should be stored in the dark at 4 °C. Then, the sections were bleached with Tanake bleach (Sigma-Aldrich) for several min until the color of the oxidant was completely removed. The sections were immersed in Victoria Blue staining solution (VB001, Thermo Fisher Scientific) for 24 h. Then, the cell nuclei were counterstained with Nuclear Fast Red staining solution (Sigma-Aldrich) for 5-10 min. Finally, routine dehydration, clearing, and sealing procedures were performed.
Van Gieson Method for Collagen Fiber Staining: The sections stained with Victoria Blue were stained with modified Harris hematoxylin solution (Thermo Fisher Scientific) for 3 min. Differentiation was performed with 0.5% hydrochloric acid ethanol (Sigma-Aldrich) for a few s. The sections were stained with Fast Green solution (Thermo Fisher Scientific) for 4 min. The sections were washed with 1% acetic acid (Sigma-Aldrich) for 2 min. Finally, routine dehydration, clearing in xylene, and sealing procedures were performed.
Immunohistochemistry (IHC)
Lung tissues were embedded in OCT (Sunny Biotech, 4583) and frozen at −80 °C for storage. Frozen sections with a thickness of approximately 8 mm were prepared using the Leica Cryostat (Leica Biosystems, CM1950). To block endogenous peroxidase activity, lung tissue sections were incubated with 3% H2O2 (McLean, 7722-84-1) for 15 min, followed by three washes with 1X PBS (Biowork, B548117-0500) for 5 min each. Subsequently, sections were blocked with 5% goat serum (ThermoFisher, 16210064) at room temperature. For overnight incubation at 4 °C, sections were probed with primary antibodies: Mouse anti-TLR4 (1:200, 66350-1-Ig, Proteintech, Wuhan, China), Rabbit anti-NF-κBp65 (1:400, 8242, CST, UK), Rabbit anti-HIF-1α (1:100, ab51608, Abcam, UK), or Rabbit anti-VEGF (1:200, 19003-1-AP, Proteintech, Wuhan, China). After three washes with 1X PBS for 5 min each, corresponding biotinylated secondary antibodies, anti-Mouse (1:100, A0288, Biyun Tian, China) or anti-Rabbit (1:100, A0279, Biyun Tian, China), were applied and incubated for 1 h at room temperature. Following three washes with 1X PBS for 5 min each, sections were incubated with biotinylated horseradish peroxidase (HRP) (1:200, A0308, Biyun Tian, China) for 30 min, followed by three washes with 1X PBS for 5 min each. Finally, sections were subjected to DAB chromogenic solution (A:B = 1:1, P0201, Biyun Tian, China) and stained for 15 min. The sections were observed using an Olympus light microscope (Olympus Corporation, BX63) [Citation26].
Detection of serum inflammatory factor concentrations
Rat serum ELISA kits: IL-1β (MB-1588A), IL-8 (MB-1716A), TNF-α (MB-1721A), ICAM-1 (MB-1740A), SELE (MB-1943A), and VEGF (MB-1720A) were purchased from Jiangsu Enzyme Labeling Biotechnology Co., Ltd. (http://mbbiology.com/). First, blank wells, standard wells, and sample wells were set up on the ELISA plate, with no reagents added to the blank wells. Different concentrations of standard substances were sequentially added to the standard wells. 40 μl of sample diluent was added to the sample wells to be tested, and 10 μl of serum to be tested was added to achieve a 5-fold dilution of the serum. Subsequently, 100 μl of enzyme-labeled reagent, purchased from Thermo Fisher Scientific, was added to each well except the blank wells. Incubation was performed at 37 °C for 60 min. After incubation, the wells were washed multiple times with washing solution and blotted dry. The color development process used color-developing solutions A and B from Sigma-Aldrich, adding 50 μl of each and mixing in the dark, then incubating at 37 °C for 10 min. After color development, 50 μl of stop solution provided by Sigma-Aldrich was immediately added to stop the reaction. Finally, the absorbance of each well was measured at a wavelength of 450 nm using a microplate reader from BioTek, and the OD values were recorded. During the data analysis phase, the concentration of inflammatory factors in the samples was calculated using the standard curve and linear regression equation (R-value approaching 1), where the sample concentration needs to be multiplied by the dilution factor of 5 to obtain the actual concentration [Citation27].
RT-qPCR detection of key factor mRNA expression in lung tissue
Total RNA from lung tissue was extracted using Trizol reagent, and the concentration and purity of the extracted total RNA were detected using a Nanodrop 2000 micro UV spectrophotometer. According to the instructions of the PrimeScript RT reagent Kit (RR036A, Takara, Japan, https://www.takarabiomed.com.cn/), RNA was reverse transcribed into cDNA. qRT-PCR detection was performed using the ABI PRISM 7300 RT-PCR system (Applied Biosystems). Three replicates were set for each well. β-actin was used as an internal reference, and the relative expression of genes was analyzed using the 2-ΔΔCt method. The primer sequences are shown in Table S1.
Western blot
Rat lung tissue was lysed on ice for 30 min with RIPA lysis buffer (P0013B, Biyuntian, Shanghai, China) containing 1% PMSF. After centrifugation at 14,000 g, the supernatant was collected. The protein extract was measured for protein concentration using the BCA method (P0012, Biyuntian, Shanghai, China). An appropriate 4 × Loading Buffer (9173, Takara, Japan) was added and boiled at 100 °C for 10 min to denature the protein. SDS-PAGE gel (P0012A, Biyuntian, Shanghai, China) was prepared, with 80 V voltage electrophoresis for 20 min for the stacking gel and 120 V voltage electrophoresis for 40 min for the separating gel. The protein was transferred to a PVDF membrane by wet transfer at a constant current of 260 mA for 2 h. After blocking with 5% skim milk in TBST for 1 h, the membrane was incubated with primary antibodies Rabbit anti-TLR4 (1:1000, ab217274, Abcam, UK), Rabbit anti-NF-κBp65 (1:1000, 3033S, CST, UK), Rabbit anti-HIF-1α (1:1000, ab179483, Abcam, UK), and Rabbit anti-VEGF (1:1000, 19003-1-AP, Proteintech, Wuhan Sanying, China) overnight at 4 °C. The membrane was washed with TBST and incubated with a secondary antibody sheep anti-rabbit (1:2000, ab6721, Abcam, UK) for 1 h. The membrane was washed with TBST, and the ECL luminescent reagent was used for luminescence. The film was exposed, developed, and fixed. Images were captured, and the results were scanned for grayscale values using gel image analysis software as normalized to β-actin [Citation28, Citation29].
Ultra-high-performance liquid chromatography-QTOF-MS analysis
UHPLC-QTOF-MS analysis was performed using the UHPLC system (1290, Agilent Technologies) and UPLC BEH Amide column (1.7 μm 2.1*100 mm, Waters) in conjunction with the TripleTOF 6600 (Q-TOF, AB Sciex). The elution gradient was: 0.5 min, 95% B; 7 min, 65% B; 8 min, 40% B; 9 min, 40% B; 9.1 min, 95% B; 12 min, 95% B, with a flow rate of 0.5 ml/min. The mobile phase was 25 mM NH4Ac + 25 mM NH4OH in water (PH = 9.75) (A) and acetonitrile (B). The TripleTOF mass spectrometer was used to obtain MS/MS spectra, and the acquisition software (Analyst TF 1.7, AB Sciex) was used to continuously evaluate the full scan survey MS data during the collection of MS data spectra and to trigger the collection of MS/MS. In all cycles, 12 precursor ions with an intensity greater than 100 were fragmented at a collision energy of 30 V. Gas 2 was set at 60Psi, curtain gas at 35Psi, the source temperature at 600 °C, and the ion spray voltage at 5000 V or −4000 V in positive or negative mode, respectively.
Statistical analysis
Experimental data were expressed as “mean ± standard deviation” and were statistically analyzed using SPSS 23.0 software. When the data followed a normal distribution and met the chi-square criterion, a one-way analysis of variance was used to compare differences among multiple groups, and the LSD method was used for pairwise comparisons between groups. When the data did not follow a normal distribution or the variance was not uniform, non-parametric tests were used for group comparisons, and the Kruskal-Wallis H test was used for comparisons among multiple groups. p < 0.05 indicated statistically significant differences. Statistical graphs were drawn using GraphPad Prism 8.0.1.
Results
Network pharmacology and bioinformatics reveal PNS can regulate the TLR4/NF-κB pathway
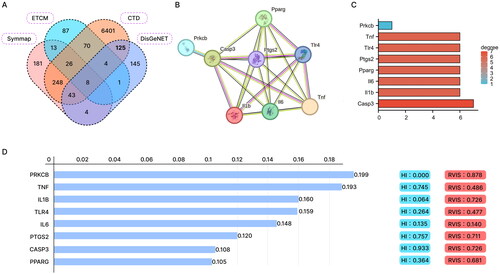
To explore the molecular mechanism by which PNS regulates COPD, we first searched for related targets of the traditional Chinese medicine SAN QI in the syntax and ETCM databases, obtaining 523 and 209 target genes, respectively. We retrieved COPD-related genes from the CTD and DisGeNET disease-related databases, obtaining 9016 and 330 disease-related genes, respectively. The intersection yielded 8 target genes SAN QI might regulate in COPD (). Importing these 8 target genes into the STRING database for protein interaction network analysis, we found that Casp3, Il1b, Il6, Pparg, Ptgs2, Tlr4, and Tnf had high Degree values (). Analysis in the Phenolyzer database revealed that Prkcb, Tnf, Il1b, and Tlr4 were of higher importance in the disease ().
Figure 1. Network pharmacology and bioinformatics analysis to screen Panax notoginseng-related target genes for COPD regulation.
Note: (A) Venn diagram showing the intersection of Panax notoginseng’s related targets and COPD disease-related genes. (B) Interaction network diagram of proteins encoded by the 8 candidate target genes. (C) Bar chart showing the degree ranking of the 8 candidate target genes. (D) Importance ranking of the 8 candidate target genes in COPD as shown by the Phenolyzer database.
Further, GO and KEGG enrichment analyses of the 8 candidate target genes showed that in BP, they were mainly enriched in response to mechanical stimulus, hypoxia, response to decreased oxygen levels, and positive regulation of nitric oxide biosynthetic process. In CC, they were primarily enriched in the membrane raft, membrane microdomain, receptor complex, and external side of the plasma membrane. In MF, they were mainly enriched in cytokine receptor binding, cytokine activity, tumor necrosis factor receptor superfamily binding, growth factor receptor binding, and lipopolysaccharide immune receptor activity (). These genes primarily participate in responses to hypoxia, cytokines, tumor necrosis factors, growth factors, and LPS immune receptor activities.
Figure 2. GO and KEGG enrichment analysis of candidate target genes.
Note: (A-C) GO function analysis shows the enrichment of the 8 candidate target genes in BP, MF, and CC. (D) KEGG pathway enrichment analysis of the 8 candidate target genes.
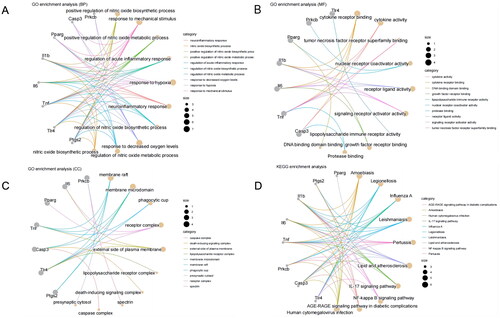
KEGG pathway enrichment analysis showed that the 8 candidate target genes were primarily enriched in inflammation-related pathways such as the IL-17 signaling pathway, NF-kappa B signaling pathway, and AGE-RAGE signaling pathway in diabetic complications (). Previous studies have reported that the main active ingredient of SAN QI, PNS, can mediate Tlr4-regulated inflammatory responses in various diseases [Citation30–32]. KEGG analysis also found Tlr4 to be primarily enriched in the NF-κB signaling pathway. Concurrently, using ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS), we screened the main active components of Panax notoginseng saponins. With 40 distinct peaks, 20 primary components were detected. Ginsenoside R1, Ginsenoside Rb1, Ginsenoside Rb2, Ginsenoside Rf, Ginsenoside Re, Ginsenoside Rg1, Ginsenoside Rg3 (R-FORM), and Ginsenoside Rg5 were identified as potential main active ingredients (Supplementary Figure 1, Table S2). Thus, we hypothesize that PNS might affect COPD by regulating the TLR4/NF-κB pathway.
PNS Alleviates COPD signs and improves COPD-related weight loss
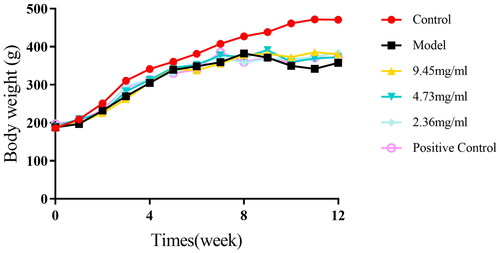
At the beginning of the study, rats in the control group displayed good overall vitality, shiny fur, and normal eating habits. Contrastingly, rats in the model group exhibited several COPD signs: dull fur, significant appetite loss, slow or stagnant weight gain, increased cough frequency, and oral and nasal secretions. Subsequently, we ascertained whether PNS conferred the efficacy to alleviate signs of COPD. Roxithromycin, an antibiotic commonly used to alleviate pulmonary inflammation and decelerate the progression of COPD, was employed as a positive control [Citation33, Citation34]. Our findings revealed that following the PNS intervention, notable improvements were observed in the rats of the treatment group, including enhanced fur quality, a slight increase in body weight, and a significant reduction in the quantity of nasal and oral secretions. At week 0, there was no significant difference in weight among the groups, providing a solid baseline for the experiment. However, by the end of week 8, compared to the control group, the rats in the model and treatment groups displayed a significant weight reduction (p < 0.01), further confirming the successful establishment of the COPD model. Notably, by week 8, there was no significant difference in weight among the treatment groups compared to the model group, suggesting that the therapeutic effects of PNS might take longer to manifest. However, by the end of week 12, the weight of the rats in the treatment groups had increased, albeit still lower than the control group (p < 0.01). This change indicates that PNS might have a reversing effect on COPD-induced weight loss. From week 8 to week 12, the weight of the rats in the model group slightly decreased, while that of the rats in the treatment groups increased (Table S3, ). These results confirm the potential utility of PNS in alleviating COPD signs and improving COPD-related weight loss.
PNS improves pathological lung damage in COPD rats
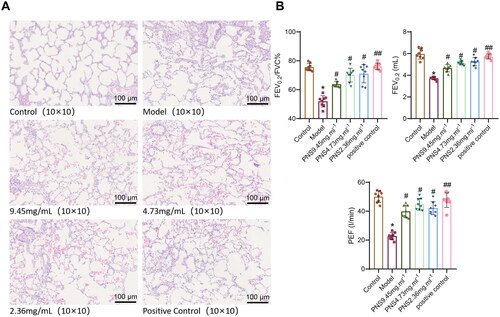
In the control group, lung tissue structures appeared normal, with no evident inflammation around the trachea and blood vessels. In contrast, the model group displayed significant pathological changes in the lung tissue, such as widened alveolar septa, inflammatory cell infiltration around blood vessels, thickened walls of some arteries, inflammatory cell infiltration around bronchi, expanded alveolar cavities, and fused and thinned alveolar walls. Compared to the model group, the drug-treated groups showed reduced epithelial hyperplasia in the bronchioles, narrowed bronchial lumens, thickened walls, and decreased inflammatory cell infiltration (). Compared to the blank group, the model group exhibited a notable reduction in lung function indices (FEV0.2/FVC% and PEF), with a marked decrease in FEV0.2 values. In contrast, all drug-treated groups, including the positive control group, showed a significant increase in these lung function indices (FEV0.2/FVC%, FEV0.2, and PEF) (). These results suggest that PNS may have therapeutic potential in ameliorating the pathological changes in lung tissue and improving lung function associated with COPD. It was also observed that despite treatment with PNS or the positive control drug, the PEF values could not be restored to the levels seen in the blank control group. This suggests that lung function in rats remained somewhat impaired, and that PNS treatment could only provide relief and improvement. The reason for the higher FEV0.2/FVC ratio in all treatment groups compared to the control group could be attributed to this ratio being a composite of two parameters. An increase in FEV0.2 or a decrease in FVC can both contribute to an increase in this value, leading to variability within a certain range.
Figure 4. HE staining and lung function measurements were obtained for the lung tissue of each group of rats.
Note: A: HE staining results (10 × 10) of the lung tissue in each group of rats; B: Lung function (FEV0.2/FVC%, PEF) measurements in each group of rats; compared to the Control group, *P < 0.01, P < 0.05; compared to the Model group, ##p < 0.01, #p < 0.05.
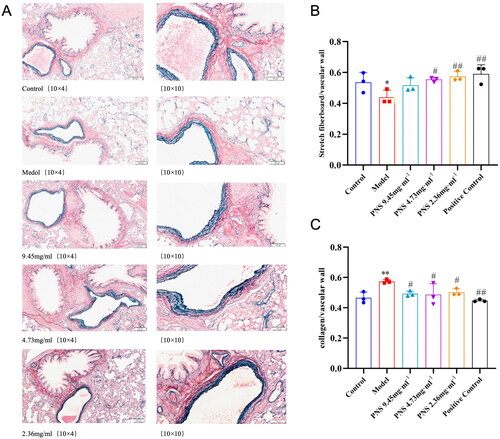
PNS alleviates pulmonary vascular remodeling induced by COPD in rats
In the normal group, the morphological structure of the lung tissue remained intact, including the integrity of the bronchial epithelium and elastic fibers. In contrast, the model group rats displayed evident abnormalities in the lung tissue, such as epithelial shedding in the trachea, fusion of large alveoli, narrowing of the bronchioles, reduced elastic fibers, and inflammation around the vessels. Compared to the model group, the PNS-treated group showed improvements in bronchiole and vessel periphery inflammation, the number of elastic fibers, and the fusion of large alveoli (). It further supports the potential utility of PNS in alleviating lung tissue remodeling and inflammation induced by COPD. The model group rats displayed a significant difference in the proportion of elastic fibers compared to the control group, specifically a notable reduction (p < 0.05). However, with PNS intervention, the proportion of elastic fibers in the treated groups significantly increased (p < 0.05, p < 0.01), though there was no significant difference among the treatment groups. Additionally, the proportion of collagen in the model group rats significantly increased, but with PNS intervention, the proportion of collagen decreased (p < 0.05, p < 0.01) (). It suggests that PNS might counteract the structural changes in the pulmonary vessels induced by COPD.
Figure 5. PNS’s effect on pulmonary vascular remodeling induced by COPD in rats.
Note: (A) Victoria + VG staining results of lung tissues from rats in each group (10 × 10). (B) Proportion of elastic fibers in lung tissues from rats in each group. (C) Proportion of collagen fibers in lung tissues from rats in each group. Compared to Control, **p <0.01, *p <0.05; compared to Model, ##p <0.01, #p <0.05.
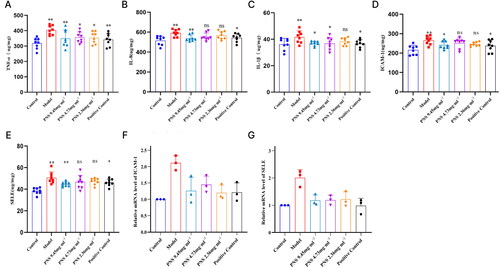
PNS inhibits the expression of inflammatory factors and adhesion molecules in COPD rats
Compared to the control group, the serum concentrations of IL-1β, IL-8, TNF-α, ICAM-1, SELE, IL-6, IL-17A, IFNγ, and TGFβ in the model group rats were significantly increased (p < 0.01). In comparison to the model group, the serum concentrations of IL-1β, IL-8, TNF-α, ICAM-1, SELE, IL-6, IL-17A, IFNγ, and TGFβ were decreased in the 9.45 mg/ml dosage group (p < 0.05, p < 0.01). Similarly, the concentrations of TNF-α, IL-1β, IL-6, IL-17A, IFNγ, and TGFβ were reduced in the 4.73 mg/ml dosage group (p < 0.05), while IL-8, ICAM-1, and SELE showed decreased expression trends. In the 2.36 mg/ml dosage group, the serum expression of TNF-α, IL-6, IL-17A, IFNγ, and TGFβ was decreased (p < 0.05), along with a decreasing trend in the expression of ICAM-1, SELE, IL-8, and IL-1β. In the positive control group, the expression of TNF-α, IL-8, IL-1β, ICAM-1, SELE, IL-6, IL-17A, IFNγ, and TGFβ was reduced (p < 0.05, p < 0.01) (, Supplementary Figure 2A). Compared to the control group, the relative expression levels of ICAM-1, SELE, IL-6, IL-17A, IFNγ, and TGFβ mRNA in the lung tissue of the model group rats were increased. However, compared to the model group, the administration group rats showed a decrease in the relative expression levels of ICAM-1, SELE, IL-6, IL-17A, IFNγ, and TGFβ mRNA in the lung tissue (, Supplementary Figure 2B). These results indicate that PNS exhibits significant anti-inflammatory and anti-cellular adhesion effects.
Figure 6. PNS affects the expression of related inflammatory factors and adhesion molecules in COPD rats.
Note: (A–C) ELISA detection of TNF-α, IL-8, and IL-1β levels in the serum of rats from each group. (D–E) ELISA detection of ICAM-1 and SELE levels in the serum of rats from each group. (F–G) RT-qPCR detection of ICAM-1 and SELE mRNA expression levels in lung tissues from rats in each group. Compared to Control, **p <0.01, *p <0.05; compared to Model, ##p <0.01, #p <0.05.
PNS inhibits the expression of TLR4/NF-κB/HIF-1α/VEGF pathway factors in COPD rats
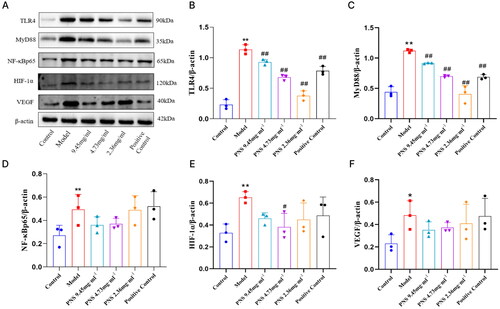
Compared to the control group, the concentration of VEGF in the serum of the model group rats significantly increased (p < 0.01). However, compared to the model group, the concentration of VEGF in the serum of the treated group rats decreased (p < 0.01, p < 0.05) (). Compared to the control group, the relative expression of TLR4, NF-κB, HIF-1α, and VEGF mRNA in the lung tissue of the model group rats increased (p < 0.05). In contrast, compared to the model group, the relative expression of TLR4, NF-κB, HIF-1α, and VEGF mRNA in the lung tissue of the treated group rats decreased (p < 0.05) (). Compared to the control group, the relative expression of TLR4/MyD88/NF-κBp65/HIF-1α/VEGF protein in the lung tissue of the model group rats significantly increased (p < 0.01, p < 0.05). However, compared to the model group, the relative expression of TLR4/MyD88/NF-κBp65/HIF-1α/VEGF protein in the lung tissue of the treated group rats decreased (p < 0.01, p < 0.05) (, Supplementary Figure 3).
Figure 7. PNS’s regulation of TLR4/NF-κB/HIF-1α/VEGF pathway factor expression in COPD rats.
Note: (A) ELISA detection of VEGF concentration in the serum of rats from each group.
(B-D) RT-qPCR detection of TLR4, NF-κB, HIF-1α, and VEGF mRNA expression levels in lung tissues from rats in each group. Compared to Control, **p <0.01, *p <0.05; compared to Model, ##p <0.01, #p <0.05.
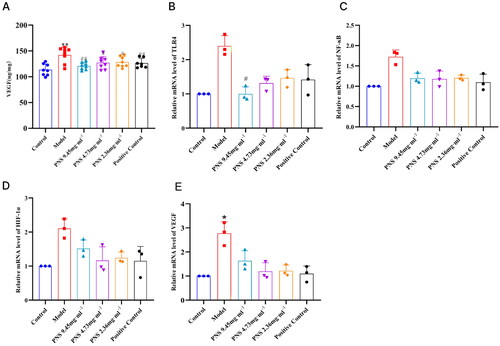
Figure 8. Changes in TLR4/NF-κB/HIF-1α/VEGF protein expression in lung tissues from rats in each group.
Note: (A) Western blot detection of TLR4, MyD88, NF-κB, HIF-1α, and VEGF protein expression in lung tissues from rats in each group. (B-F) Protein expression charts of TLR4, MyD88, NF-κB, HIF-1α, and VEGF in lung tissues from rats in each group. Compared to Control, **p <0.01, *p <0.05; compared to Model, ##p <0.01, #p <0.05.
Discussion
Chronic inflammation in COPD is a critical factor leading to the aggregation of inflammatory cells in the lungs and increased secretion of inflammatory factors. These changes exacerbate lung tissue damage and contribute to both airway and pulmonary vascular remodeling, as evidenced in studies [Citation35, Citation36]. The dynamic process of inflammation in COPD not only damages the bronchiolar epithelium but also leads to structural changes in the pulmonary vasculature, which are key aspects in the progression of the disease. Therefore, our study aimed to elucidate the mechanism of action of PNS in treating COPD-related vascular remodeling lesions.
In this study, we investigated the effects of PNS, an active ingredient of Panax notoginseng [Citation37] and a primary component of the clinically used medication Xuesaitong Soft Capsules, on vascular remodeling lesions in COPD rats. PNS has been demonstrated to impart PNS) an anti-inflammatory effect and can suppress Th17 cell differentiation [Citation38]. Following the administration of PNS in this study, there was a marked improvement in the general health of the rats, as indicated by increased food and water intake and weight gain, suggesting an overall positive impact of PNS on health status. More critically, the COPD rats treated with PNS showed substantial improvements in lung pathology. There was a notable reduction in the proliferation of the bronchiolar epithelium, a decrease in the narrowing of airway lumens, a reduction in the thickening of the airway walls, and a lesser infiltration of inflammatory cells into the lung tissue. These findings are particularly relevant as they indicate that PNS treatment not only alleviates the symptomatic aspects of COPD but also addresses underlying structural changes.
The alleviatory effects of PNS have been documented in prior research [Citation16], but the mechanistic underpinnings remain largely unclear. During the inflammatory process characteristic of COPD, the integrity of vascular endothelial cells is significantly compromised, leading to heightened capillary permeability. This disruption is primarily mediated by cytokines like IL-1 and TNF-α, along with adhesion molecules such as ICAM-1, chemokines, and E-selectin [Citation39]. This sequence of events underscores the pivotal role of endothelial cell function in the pathogenesis of COPD, and the subsequent increase in vascular permeability signifies a major shift in pulmonary pathology. Notably, the observed decrease in levels of inflammatory and adhesion factors of the present study reinforces the potential of PNS as a therapeutic agent, effectively mitigating pulmonary inflammatory lesions in COPD. This novel insight into the efficacy of PNS in treating vascular remodeling lesions in COPD adds a valuable dimension to the understanding and management of this complex disease.
Pulmonary vascular remodeling, an integral part of COPD pathology, begins in the early stages of lung inflammation and continues to progress throughout the disease. This remodeling is not merely a consequence of ongoing inflammation but also contributes to a feedback loop that further drives the inflammatory process [Citation40]. In the early stages of COPD, key pathological changes such as endothelial cell dysfunction, elastosis, and abnormal collagen deposition are already evident [Citation41, Citation42]. Our study brings to light the significant impact of PNS treatment in this context. We observed that PNS effectively reduces elastosis in the bronchi and pulmonary vessels of COPD rats, indicating an improvement in the elastic properties of these structures. Furthermore, PNS treatment led to a decrease in collagen deposition, a factor often associated with the stiffening of lung tissues in COPD. These findings collectively illustrate the potential of PNS in improving the extent of pulmonary vascular remodeling.
The ability of PNS to mitigate these key pathological changes holds substantial promise as a therapeutic strategy for COPD, particularly in addressing the vascular remodeling aspects of the disease. These results open new avenues for further research and clinical exploration, potentially leading to improved treatments for the pulmonary complications associated with COPD. The mechanistic pathways through which PNS exerts these effects seem to involve the TLR4/NF-κB/HIF-1α/VEGF pathway. Interestingly, the HIF-1α and NF-κB have been recognized as central players in the orchestration of cellular responses to hypoxia and inflammation. HIF-1α, a critical transcription factor in regulating oxygen balance, plays a substantial role in various processes, including vascular remodeling, cell proliferation, and migration [Citation43, Citation44]. This is particularly relevant in the pathophysiology of COPD, where oxygen imbalance and vascular changes are prominent features. NF-κB, another key transcription factor, is induced by various inflammation-related cytokines and is central in driving inflammatory responses. Studies have highlighted the interaction between NF-κB and HIF-1α, where NF-κB modulates HIF-1α expression during inflammation and hypoxia, with TNF-α inducing HIF-1 even under normoxic conditions [Citation12, Citation45]. TLR4, integral to innate immunity, acts as an upstream regulator of NF-κB. Activation of TLR4 by its ligands initiates a cascade leading to the activation and expression of NF-κB and related nuclear factors [Citation46–48]. This activation results in the transcription of various target genes, including inflammatory cytokines such as TNF-α, IL-1, IL-6, and adhesion molecules like ICAM-1 and E-selectin [Citation49]. In the development of COPD, both inflammation and hypoxia enhance NF-κB expression, thereby elevating HIF-1α levels [Citation50]. Upon reaching a critical threshold, HIF-1α translocates to the cell nucleus, activating downstream target genes, including VEGF, which is crucial for vascular remodeling and inflammation [Citation51].
Our findings from the study on PNS treatment in COPD rats are remarkable in this molecular context. We observed that PNS downregulates the expression of molecules within the TLR4/NF-κB/HIF-1α/VEGF pathway, leading to a reduction in pulmonary inflammation and vascular remodeling in COPD rats (). These results suggest that the molecular mechanism by which PNS ameliorates COPD signs and alleviates pulmonary inflammation in rats is likely linked to the reduced activation of the TLR4/NF-κB/HIF-1α/VEGF pathway. This insight provides a deeper understanding of PNS’s potential therapeutic role in COPD treatment, particularly in addressing the inflammatory and hypoxic aspects of the disease.
Figure 9. Schematic diagram of PNS’s molecular mechanism in alleviating COPD by regulating the TLR4/NF-κB/HIF-1α/VEGF pathway.
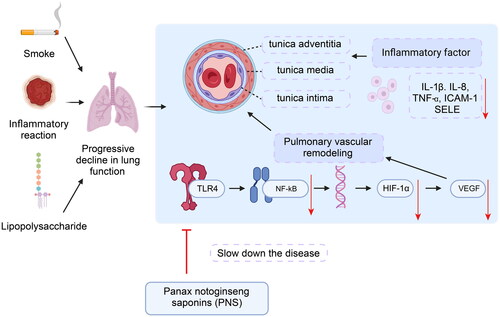
It should be noted that the limited sample size and the specificities of the rat model used in our study might not include all the variations seen in human COPD. Future research should aim to broaden these findings, exploring the effects of PNS in more diverse COPD models and potentially in clinical trials. Given the significant roles of hypoxia and oxidative stress in the pathogenesis of COPD, future studies should delve deeper into these aspects, which may reveal new avenues for therapeutic intervention. Our study also observed that in some instances, such as the results depicted in , PNS did not demonstrate a clear dose-response relationship in alleviating COPD. This finding could be attributed to the narrow range of doses used in our experiments (between 2.36 mg/ml and 9.45 mg/ml), where both high and low doses were within the same order of magnitude. Moving forward, and ensuring the safety of PNS use, we plan to explore a broader spectrum of doses. Investigating both higher and lower doses of PNS might provide a more accurate reflection of the dose-response relationship and enhance our understanding of its therapeutic potential.
Conclusions
In conclusion, our study sheds new light on the potential mechanisms by which PNS could be utilized in the treatment of COPD. We have identified PNS as a promising adjunct therapy, showing potential in reducing pulmonary inflammation and vascular remodeling, key aspects of COPD pathology. However, it’s essential to acknowledge the limitations of our research. While the therapeutic efficacy of PNS is evident in cellular and animal models, it may not fully represent the complexity of human COPD. This gap underscores the need for further investigation into the pharmacokinetics and safety profile of PNS in human subjects. A comprehensive understanding of how PNS is absorbed, distributed, metabolized, and excreted in the human body is critical to fully assess its effectiveness and safety.
Ethical approval
All animal experiments strictly followed the NIH “Guide for the Care and Use of Laboratory Animals” and were approved by the Ethics Committee of Yunnan University of Traditional Chinese Medicine.
Authors’ contributions
YNH, NPH and XMZ designed the study. QYF, BQ, OX and BJL collated the data, carried out data analyses and produced the initial draft of the manuscript. YNH, NPH and XMZ contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Supplemental Material
Download Zip (6 MB)Acknowledgement
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):1–15. doi: 10.1164/rccm.202009-3533SO.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9.
- Vij N, Chandramani-Shivalingappa P, Van Westphal C, et al. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol. 2018;314(1):C73–C87. doi: 10.1152/ajpcell.00110.2016.
- Dransfield M, Rowe S, Vogelmeier CF, et al. Cystic fibrosis transmembrane conductance regulator: roles in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2022;205(6):631–640. doi: 10.1164/rccm.202109-2064TR.
- El Agha E, Thannickal VJ. The lung mesenchyme in development, regeneration, and fibrosis. J Clin Invest. 2023;133(14):e170498. Published 2023 Jul 17. doi: 10.1172/JCI170498.
- Soleimani F, Dobaradaran S, De-la-Torre GE, et al. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: a comprehensive systematic review. Sci Total Environ. 2022;813:152667. doi: 10.1016/j.scitotenv.2021.152667.
- Kang HS, Kwon HY, Kim IK, et al. Intermittent hypoxia exacerbates tumor progression in a mouse model of lung cancer. Sci Rep. 2020;10(1):1854. Published 2020 Feb 5. doi: 10.1038/s41598-020-58906-7.
- Pasupneti S, Tian W, Tu AB, et al. Endothelial HIF-2α as a key endogenous mediator preventing emphysema. Am J Respir Crit Care Med. 2020;202(7):983–995. doi: 10.1164/rccm.202001-0078OC.
- Fu X, Zhang F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp Ther Med. 2018;16(6):4553–4561. doi: 10.3892/etm.2018.6785.
- Yu ZG, Wang BZ, Cheng ZZ. The association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: a case-control study. Kaohsiung J Med Sci. 2017;33(9):433–441. doi: 10.1016/j.kjms.2017.05.014.
- Fricker M, Goggins BJ, Mateer S, et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018;3(3):e94040. Published 2018 Feb 8. doi: 10.1172/jci.insight.94040.
- Korbecki J, Simińska D, Gąssowska-Dobrowolska M, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. 2021;22(19):10701. Published 2021 Oct 2. doi: 10.3390/ijms221910701.
- Feng L, Han F, Zhou L, et al. Efficacy and safety of panax Notoginseng Saponins (Xueshuantong) in patients with acute ischemic stroke (EXPECT) trial: rationale and design. Front Pharmacol. 2021;12:648921. Published 2021 Apr 22. doi: 10.3389/fphar.2021.648921.
- Wang J, Zeng L, Zhang Y, et al. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol. 2022;13:975784. Published 2022 Sep 5. doi: 10.3389/fphar.2022.975784.
- Zhang X, Zhang B, Zhang C, et al. Effect of panax notoginseng saponins and major anti-obesity components on weight loss. Front Pharmacol. 2020;11:601751. Published 2021 Mar 25. doi: 10.3389/fphar.2020.601751.
- Li H, Wang YG, Chen TF, et al. Panax notoginseng saponin alleviates pulmonary fibrosis in rats by modulating the renin-angiotensin system. J Ethnopharmacol. 2024;318(Pt B):116979. doi: 10.1016/j.jep.2023.116979.
- Liu M, Zhang T, Zang C, et al. Preparation, optimization, and in vivo evaluation of an inhaled solution of total saponins of panax notoginseng and its protective effect against idiopathic pulmonary fibrosis. Drug Deliv. 2020;27(1):1718–1728. doi: 10.1080/10717544.2020.1856222.
- Gao J, Yao M, Zhang W, et al. Panax notoginseng saponins alleviates inflammation induced by microglial activation and protects against ischemic brain injury via inhibiting HIF-1α/PKM2/STAT3 signaling. Biomed Pharmacother. 2022;155:113479. doi: 10.1016/j.biopha.2022.113479.
- Wu Y, Zhang F, Yang K, et al. SymMap: an integrative database of traditional chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019;47(D1):D1110–D1117. doi: 10.1093/nar/gky1021.
- Xu HY, Zhang YQ, Liu ZM, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–D982. doi: 10.1093/nar/gky987.
- Wu T, Hu E, Xu S, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2(3):100141. Published 2021 Jul 1. doi: 10.1016/j.xinn.2021.100141.
- Wang J, Ren C, Jin L, et al. Seabuckthorn Wuwei pulvis attenuates chronic obstructive pulmonary disease in rat through gut microbiota-short chain fatty acids axis. J Ethnopharmacol. 2023;314:116591. doi: 10.1016/j.jep.2023.116591.
- Yu N, Sun YT, Su XM, et al. Treatment with eucalyptol mitigates cigarette smoke-induced lung injury through suppressing ICAM-1 gene expression. Biosci Rep. 2018;38(4): BSR20171636. Published 2018 Jul 6. doi: 10.1042/BSR20171636.
- Xu S, Zhu W, Shao M, et al. Ecto-5’-nucleotidase (CD73) attenuates inflammation after spinal cord injury by promoting macrophages/microglia M2 polarization in mice. J Neuroinflammation. 2018;15(1):155. Published 2018 May 22. doi: 10.1186/s12974-018-1183-8.
- Ganbold T, Bao Q, Zandan J, et al. Modulation of microglia polarization through silencing of NF-κB p65 by functionalized curdlan nanoparticle-mediated RNAi. ACS Appl Mater Interfaces. 2020;12(10):11363–11374. doi: 10.1021/acsami.9b23004.
- Guo Z, Liu L, Li S, et al. Effect of BDNF on airway inflammation in a rat model of COPD. Exp Ther Med. 2021;22(4):1116. doi: 10.3892/etm.2021.10550.
- Xu X, Gao W, Cheng S, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14(1):167. Published 2017 Aug 23. doi: 10.1186/s12974-017-0934-2.
- Salem M, Shan Y, Bernaudo S, et al. miR-590-3p targets cyclin G2 and FOXO3 to promote ovarian cancer cell proliferation, invasion, and spheroid formation. Int J Mol Sci. 2019;20(8):1810. Published 2019 Apr 12. doi: 10.3390/ijms20081810.
- Shu M, Zheng X, Wu S, et al. Targeting oncogenic miR-335 inhibits growth and invasion of malignant astrocytoma cells. Mol Cancer. 2011;10(1):59. Published 2011 May 19. doi: 10.1186/1476-4598-10-59.
- Xu Y, Wang N, Tan HY, et al. Gut-liver axis modulation of panax notoginseng saponins in nonalcoholic fatty liver disease. Hepatol Int. 2021;15(2):350–365. doi: 10.1007/s12072-021-10138-1.
- Chen J, Lu P, Liu J, et al. 20(S)- protopanaxadiol saponins isolated from panax notoginseng target the binding of HMGB1 to TLR4 against inflammation in experimental ulcerative colitis. Phytother Res. 2023;37(10):4690–4705. doi: 10.1002/ptr.7938.
- Bai X, Fu R, Duan Z, et al. Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 2021;12(7):2874–2885. doi: 10.1039/d1fo00242b.
- Huckle AW, Fairclough LC, Todd I. Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respir Care. 2018;63(5):609–619. doi: 10.4187/respcare.05943.
- Zhang X, Dong Y, Li WC, et al. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol Sin. 2021;42(12):2058–2068. doi: 10.1038/s41401-021-00618-3.
- Ma H, Zhou Z, Chen L, et al. Anemoside B4 prevents chronic obstructive pulmonary disease through alleviating cigarette smoke-induced inflammatory response and airway epithelial hyperplasia. Phytomedicine. 2022;107:154431. doi: 10.1016/j.phymed.2022.154431.
- Love ME, Proud D. Respiratory viral and bacterial exacerbations of COPD-the role of the airway epithelium. Cells. 2022;11(9):1416. Published 2022 Apr 22. doi: 10.3390/cells11091416.
- Xu Y, Wang N, Tan HY, et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–11323. Published 2020 Sep 14. doi: 10.7150/thno.47746.
- Shen MY, Di YX, Wang X, et al. Panax notoginseng saponins (PNS) attenuate Th17 cell differentiation in CIA mice via inhibition of nuclear PKM2-mediated STAT3 phosphorylation. Pharm Biol. 2023;61(1):459–472. doi: 10.1080/13880209.2023.2173248.
- Oates JC, Russell DL, Van Beusecum JP. Endothelial cells: potential novel regulators of renal inflammation. Am J Physiol Renal Physiol. 2022;322(3):F309–F321. doi: 10.1152/ajprenal.00371.2021.
- Karnati S, Seimetz M, Kleefeldt F, et al. Chronic obstructive pulmonary disease and the cardiovascular system: vascular repair and regeneration as a therapeutic target. Front Cardiovasc Med. 2021;8:649512. Published 2021 Apr 12. doi: 10.3389/fcvm.2021.649512.
- Lange P, Ahmed E, Lahmar ZM, et al. Natural history and mechanisms of COPD. Respirology. 2021;26(4):298–321. doi: 10.1111/resp.14007.
- Jung T, Vij N. Early diagnosis and real-time monitoring of regional lung function changes to prevent chronic obstructive pulmonary disease progression to severe emphysema. J Clin Med. 2021;10(24):5811. Published 2021 Dec 12. doi: 10.3390/jcm10245811.
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021.
- Yang C, Zhong ZF, Wang SP, et al. HIF-1: structure, biology and natural modulators. Chin J Nat Med. 2021;19(7):521–527. doi: 10.1016/S1875-5364(21)60051-1.
- Li ZL, Ji JL, Wen Y, et al. HIF-1α is transcriptionally regulated by NF-κB in acute kidney injury. Am J Physiol Renal Physiol. 2021;321(2):F225–F235. doi: 10.1152/ajprenal.00119.2021.
- Zusso M, Lunardi V, Franceschini D, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16(1):148. Published 2019 Jul 18. doi: 10.1186/s12974-019-1538-9.
- Xu X, Piao HN, Aosai F, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. 2020;177(22):5224–5245. doi: 10.1111/bph.15261.
- Jiang B, Wang D, Hu Y, et al. Serum amyloid A1 exacerbates hepatic steatosis via TLR4-mediated NF-κB signaling pathway. Mol Metab. 2022;59:101462. doi: 10.1016/j.molmet.2022.101462.
- Vercellotti GM, Dalmasso AP, Schaid TR, Jr, et al. Critical role of C5a in sickle cell disease. Am J Hematol. 2019;94(3):327–337. doi: 10.1002/ajh.25384.
- Jiang H, Zhu Y, Xu H, et al. Activation of hypoxia-inducible factor-1alpha via nuclear factor-kappa B in rats with chronic obstructive pulmonary disease. Acta Biochim Biophys Sin . 2010;42(7):483–488. doi: 10.1093/abbs/gmq041.
- Tirpe AA, Gulei D, Ciortea SM, et al. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci. 2019;20(24):6140. Published 2019 Dec 5. doi: 10.3390/ijms20246140.