Abstract
The urokinase-type plasminogen activator receptor (uPAR) is a glycosylphosphatidyl inositol–anchored protein that mediates cell adhesion to the extracellular matrix protein vitronectin (VN). We demonstrate here that this cell adhesion process is accompanied by the formation of an adhesion patch characterized by an accumulation of uPAR into areas of direct contact between the cell and the matrix. The adhesion patch requires the glycolipid anchor and develops only on a VN-coated substrate, but not on fibronectin. It consists of detergent-insoluble microdomains that accumulate F-actin and tyrosine-phosphorylated proteins, but not β1 integrins. Lack of inhibition of adhesion in the presence of integrin-blocking reagents and adhesion on a VN fragment without the RGD sequence indicated that the adhesion of uPAR-bearing cells on VN could occur independently of integrins. Hence, uPAR-mediated cell adhesion on VN relies on the formation of a unique cellular structure that we have termed “detergent-insoluble adhesion patch” (DIAP).
INTRODUCTION
Urokinase-type plasminogen activator receptor (uPAR) (CD87) is a multifunctional cell-surface receptor that mediates pericellular proteolysis, signal transduction and regulates cell adhesion (Blasi and Carmeliet Citation2002). uPAR-deficient mice show diminished transmigration in the thioglycolate-induced peritonitis model (May et al. Citation1998) and an interaction between uPAR and β2 integrins was proposed to be the responsible mechanism (Simon et al. Citation1996; Simon et al. Citation2000). In earlier studies, uPAR was shown to directly mediate cell adhesion to VN (Waltz and Chapman Citation1994; Wei et al. Citation1994). Since uPAR is a glycosylphosphatidylinositol (GPI)-anchored protein, it was suggested that the β1, β2, and β3 family of integrins are mediators of the effects of uPAR on cell adhesion (Chaurasia et al. Citation2006; Degryse et al. Citation2005; Tarui et al. Citation2003; Wei et al. Citation2005, Citation1996; Xue et al. Citation1994, Citation1997). Moreover, on intact cells a direct interaction between uPAR and integrins was demonstrated by the high-resolution fluorescence resonance energy transfer technique (Xia et al. Citation2002). The regions in uPAR responsible for the interaction with α5β1 integrin (Chaurasia et al. Citation2006), αVβ3 integrin (Degryse et al. Citation2005) β2 integrins (Simon et al. Citation1996, Citation2000) have been identified.
The evidence that uPAR-mediated cell adhesion on VN is dependent on integrins is that, (a) uPAR or integrin-based peptides inhibit the interactions between uPAR and β1 and β3 integrins and thereby diminish adhesion on VN (Simon et al. Citation2000; Wei et al. Citation1996, Citation2001, Citation2005), (b) over-expression of β 1-integrin dominant negative mutants inhibits uPAR-mediated adhesion on VN (Wei et al. Citation1996) and (c) EDTA and integrin-blocking antibodies inhibit uPAR-dependent adhesion on VN (Simon et al. Citation1996, Citation2000). Complexes between uPAR and adapter proteins are found in microdomain structures such as caveolae and in cholesterol/sphingolipid–rich detergent-insoluble lipid rafts, which have a lower fluidity than the surrounding membrane and seem to be able to sequester glycolipid-anchored proteins (Bohuslav et al. Citation1995; Cunningham et al. Citation2003; Sitrin et al. Citation2003; Wei et al. Citation1999). The emerging paradigm is that integrins, the primary adhesion molecules in cells, transduce uPAR-mediated cell adhesion and that the relevant interactions takes place in lipid rafts.
On the other hand, from a number of previous studies, it appears that uPAR-dependent adhesion on VN must also function independently of integrins, since their inhibition by EDTA did not diminish this adhesion process (Li et al. Citation2003; Montuori et al. Citation2002), and adhesion was observed on a VN fragment without the RGD peptide (Deng et al. Citation2001; Madsen et al. Citation2007). An alanine scan of uPAR showed that only those mutants defective in binding to VN also showed diminished adhesion to VN, whereas putative integrin-binding domain mutants showed unaltered cell adhesion, indicating that this adhesion was independent of integrins (Madsen et al. Citation2007). The mechanism of this type of cell adhesion was not further explored in these publications. We have investigated this phenomenon in more detail and find that this adhesion process is associated with the active clustering of uPAR-rich detergent-insoluble microdomains at the point of contact with the VN substratum. This gives rise to an unique morphological structure that we have termed “detergent-insoluble adhesion patch” (DIAP). The glycolipid anchor is necessary for the formation of DIAP and this cell adhesion is distinct from and independent of integrin-mediated cell adhesion.
MATERIALS AND METHODS
Reagents
The monoclonal antibody (mAb) #3936 against uPAR and mAb #394 to uPA were from American Diagnostica (Pfungstadt, Germany). Anti-mouse uPAR polyclonal antibody was from R&D Systems (Wiesbaden, Germany). VN was purified from human plasma, as has been previously described (Stockmann et al. Citation1993). Anti-uPAR mAb R3 and R4 were given by Dr. Gunilla Hoyer-Hansen (Finsen Laboratory, Copenhagen, Denmark). Rabbit polyclonal antibody against human uPAR was provided by Dr. Douglas Cines (University of Pennsylvania, Philadelphia, PA). Dr. Britta Engelhardt (Max Planck Institute, Bad Nauheim, Germany) provided the anti-VLA4 mAbs (PS/2 and 5/3). Monoclonal antibody Game 46 against mouse β2 integrins, MFR5 against VLA5, M17/4 against CD11a, M1/70 against CD11b, and (2C9.G2) hamster monoclonal anti-mouse β3 integrin was from Pharmingen (Hamburg, Germany). Monoclonal antibody K20 against β1 integrins (CD29) was from Dako (Hamburg, Germany); anti-phosphotyrosine mAb Py20 was from Transduction Laboratories (Heidelberg, Germany); latrunculin A and genistein were from Calbiochem (Heidelberg, Germany). Cholera toxin B subunit, FITC–cholera toxin B subunit and a polyclonal antibody to it were from Sigma (Taufkirchen, Germany). VN 1–44 and VN 1–48 peptides were isolated from human plasma and kindly provided by L. Standker (Standker et al. Citation1996).
Cell culture
Mouse pre-B lymphocytes (BAF3), suspension cells, were from American Type Culture Collection (ATCC) (Rockville, MD) and were cultured in RPMI-1640 containing 10% (vol/vol) fetal calf serum (FCS), 100-U/mL penicillin, 100-μ g/mL streptomycin and 2-ng/mL mouse interleukin-3 (Stratham Biotech, Hannover, Germany). Human uPAR cDNA was provided by Dr. Niels Behrendt (Finsen Laboratory, Denmark). The cDNA for the fusion protein between human uPAR (1–281) and the transmembrane as well as the cytoplasmic domain of interleukin-2 receptor α (IL-2Rα) was provided by Dr. Harold Chapman (University of California, San Francisco) and is identical to the construct used in earlier studies (Li et al. Citation1994). uPAR-transfected BAF3 were prepared as has been described before (Kanse et al. Citation2004b). All culture media were from GIBCO (Eggenstein, Germany), and the cell culture plastic was from Nunc (Roskilde, Denmark). Analysis of uPAR and integrin expression on BAF3 cells by flow cytometry was performed as described before (Kanse et al. Citation2004b).
Cell Adhesion-Related Procedures
Tissue culture plates were coated with 2 μ g/ml VN or 10 μ g/ml FN and blocked with 3% (wt/vol) bovine serum albumin (BSA). BSA coated wells were used to measure non-specific adhesion which was subtracted from the adhesion on other substrates. Cells were washed in serum-free medium and plated onto coated wells for 60 min at 37°C in the absence or presence of test substances in serum-free medium containing 0.3% (wt/vol) BSA. In some experiments, HEPES-buffered saline (25 mM HEPES, pH 7.4, and 137 mM NaCl, 10 mM D-glucose and 4 mM KCl) was used as the adhesion buffer. For adhesion assays with BAF3 cells experiments were performed in the presence of 2 ng/ml of IL-3 unless otherwise indicated. Extensive preliminary experiments showed that the adhesion of BAF3 cells under various conditions and on various adhesion substrates was identical in the presence or absence of IL-3 which is in contrast to an earlier publication (Shibayama et al. Citation1998).
Preparation of Detergent Insoluble Fraction for Analysis by Confocal Laser Scanning Microscopy and Western Blotting
Permanox slides (Nunc) were coated with VN (2 μ g/ml) or FN (10 μ g/ml) in PBS overnight at 4°C and blocked with 3% (wt/vol) BSA in PBS. BAF3 cells bearing different forms of uPAR were washed extensively in serum-free medium and were allowed to adhere to the VN- or FN-coated slides for 1 h at 37°C. Thereafter, the slides were washed twice with PBS and the cells were solubilized with PBS containing Triton X-100 (1%, wt/vol), MgCl2 (2mM) and Na3VO4 (1 mM) at 4°C with vigorous mixing, washed again with PBS and then fixed with ice-cold methanol-acetone (1:1, vol/vol) or with paraformaldehyde (3.5%, wt/vol). In some experiments 0.2 or 0.5 % (wt/vol) Triton X-100 or Na2CO3 (0.1 M, pH 11.5) or N-octylglucoside (1%, wt/vol) was used for solubilizing the cells (Nebl et al., Citation2002). In parallel, slides were fixed directly after cell adhesion without any lysis of the cells. After blocking with 3% (wt/vol) BSA the slides were incubated with the primary antibody. After washing, secondary rhodamine or FITC coupled antibodies (Dianova, Hamburg, Germany) were added (1:100 to 1:200). After extensive washing, the cells were counterstained with 4′,6-diamidino-2-phenlyindole dihydrate (DAPI) (Sigma) or rhodamine-labelled phalloidin (Molecular Probes, Leiden, The Netherlands) as indicated and mounted in Vectashield (Vector Laboratories, Burlingame, CA).
Fluorescence microscopy was performed using a Leica DMR microscope (Leica Microsystems, Heidelberg, Germany). Confocal microscopy was performed using a spectral laser-scanning microscope (Leica Microsystems) using plan-Apo 63X objectives (NA 1.4 oil). After defining the top and the bottom plane of the cells, images of red and green fluorescence in approximately 50 sections each of 0.2 μ m thickness were captured using software supplied by the manufacturer. Images from double staining experiments were merged to analyse the extent of colocalization of the different antigens.
Western blotting was performed using a similar set up as above except that 10 cm dishes were used. After lysis of the adherent cells with 1% (wt/vol) Triton X-100 at 4°C with vigorous mixing the lysate was centrifuged (13,000 × g, 4°C, 15 min) to separate the detergent soluble (= supernatant) and insoluble material (= pellet). The supernatants, pellets and the detergent insoluble material bound tightly to the plate (= detergent insoluble adhesion patch, DIAP) were each solubilized with 4% (wt/vol) sodium dodecyl sulphate sulfate (SDS) and boiled. In parallel dishes the number of adherent cells was determined by cell counting. The volume of the different fractions was adjusted to represent identical cell numbers and subjected to Western blotting analysis. After separation on 10% (wt/vol) polyacrylamide gels, proteins were blotted to PVDF membranes, blocked with 5% (wt/vol) skimmed milk powder, 0.1% (wt/vol) Tween-20 in PBS, and incubated with the primary antibody followed by peroxidase-linked secondary antibody and ECL detection (Amersham-Pharmacia, Freiburg, Germany).
Sucrose Density Gradient Centrifugation
This was performed as has been described previously (Cunningham et al. Citation2003) with minor modifications. Briefly, cells were washed with MBS buffer (25-mM MES, 100-mM NaCl, pH 6.5) and solubilized in Triton X-100 (1% vol/vol). Tightly adherent material was scraped together with a rubber policeman to remove any material bound to the dish. Thereafter the lysate was centrifuged gently at 1000 × g for 1 min to remove any insoluble material, e.g., nuclei that would otherwise interfere with the density gradient centrifugation. In preliminary experiments we showed that the detergent-insoluble microdomains were retained in the supernatant for further analysis. The lysates were then loaded on a sucrose gradient and centrifuged as described before (Cunningham et al. Citation2003). The contents of the tubes were harvested as 1-mL fractions and analyzed by Western blotting. The lipid raft fraction was identified by performing dot blots using cholera toxin B subunit–based detection system for GM-1 glycolipid (Thomas et al. Citation2003).
Interference Reflection Microscopy
The contact area of non-transfected (BAF3-WT) and u-PAR transfected BAF3 (BAF3-uPAR) with either vitronectin or fibronectin was obtained by interference reflection microscopy (IRM). Adherent cells were imaged with an Antiflex Plan-Neofluor Ph3, 63x NA 1,25 oil immersion objective, equipped with a λ /4 plate and custom-made polarization and analysis filter, mounted on an Axiovert 100M (both from Carl Zeiss Microimaging, Inc). Images were captured with a 12-bit digital charge-coupled device camera (model ORCA 4742-95; Hamamatsu Photonics) controlled by Openlab software (Improvision). Image analysis was performed using the MetaMorph software package.
RESULTS
uPAR-Mediated Adhesion on VN Leads to the Formation of Adhesion Patches
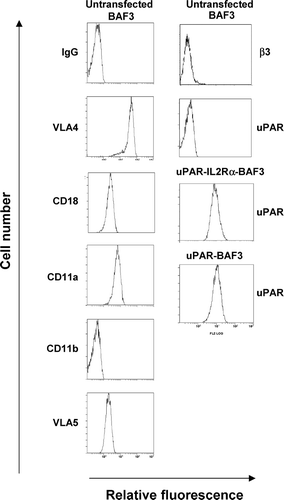
We have previously observed the formation of a distinct uPAR-rich cellular structure in patches during uPAR-dependent adhesion of U937 cells on VN but not on FN (Kanse et al. Citation2004a). In order to investigate this mechanism in more detail we have used a model system based on exogenous human uPAR expression in mouse BAF3 cells that do not express endogenous uPAR (Kanse et al. Citation2004b). Upon transfection with the uPAR cDNA as well as the uPAR-IL-2Rα construct there was a robust and equivalent expression of the GPI-anchored form as well as the transmembrane form of the receptor (). Examination of the integrin expression profile indicated that BAF3 cells express CD18 (β2), LFA-1 (CD18/CD11a), VLA4 (α4β1) and VLA5 (α 5β1), and β1, but not MAC-1 (CD18/CD11b) or the β3 subunit ().
Figure 1 Quantification of the expression of uPAR and integrins. uPAR and uPAR-IL-2Rα expressing BAF3 cells were analyzed by fluorescence-associated cell sorting using a polyclonal anti-uPAR IgG. Integrin expression on BAF3 cells was determined using the following mAbs; PS/2 against VLA4, Game 46 against CD18, M1/74 against CD11a, M1/70 against CD11b, MFR5 against VLA5, and 2C9.G2 against β3-integrin.
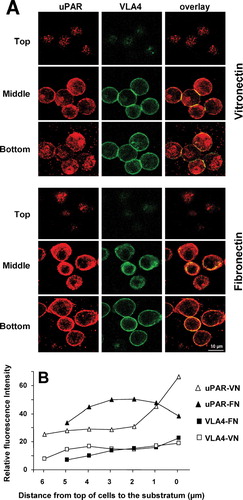
uPAR-BAF3 cells adhere strongly on a VN substrate in accordance with previous reports (Waltz and Chapman Citation1994). Analysis by confocal microscopy showed that after adhesion of uPAR-BAF3 cells on a VN substrate, but not on a FN substrate, there was a rapid and extensive concentration of uPAR at the points of contact between the cells and the matrix (). We have termed this novel structure an “adhesion patch.” Time-course experiments showed that these structures start forming within 5 min after addition of the cells to the slides. These structures were present in > 95% of the cells on a slide and were present in different clones of varying uPAR expression. BAF3 cells express neither the classical VN receptor αVβ3,nor the β2integrin that interacts with uPAR, MAC-1 (CD18/CD11b). Since the β1 integrin very late antigen 4 (VLA4) was the most highly expressed integrin on these cells, we examined the distribution of the VLA4, in the same cells, since there is ample evidence that β1 integrins are physically and functionally linked to uPAR (Chaurasia et al. Citation2006; Degryse et al. Citation2005; Simon et al. Citation2000; Tarui et al. Citation2006; Wei et al. Citation1996, Citation2005; Xue et al. Citation1997). VLA4 was expressed uniformly over the entire cell surface regardless of the adhesion substrate and was not concentrated within the adhesion patches (). Some colocalization between uPAR and VLA4 was observed, particularly on FN and in cell–cell contacts. Non-specific control IgG showed no staining of uPAR-BAF3 cells (data not shown). This substrate-dependent distribution pattern of uPAR, in comparison to VLA4 on VN versus FN substrate, was quantified in the vertical plane () to highlight these differences. These differences were also apparent in cross sections through the vertical plane (data not shown). We observed that, for unknown reasons, the cells were consistently smaller in the vertical axis on FN compared to VN. A similar distribution of uPAR and β 1 integrins was observed in U937 cells on a VN and FN substratum (data not shown). In human peripheral blood monocytes and neutrophils we did not observe such accumulation of uPAR and these cells predominantly use β2 integrins for adhesion on VN as we have shown before (Kanse et al. Citation2004c).
Figure 2 Localization of uPAR and VLA4 to sites of cell adhesion in uPAR-transfected BAF3 cells. (a) uPAR-BAF3 cells were allowed to adhere to VN- or FN-coated slides and adherent cells were fixed and immunostained with anti-uPAR (rabbit polyclonal) IgG and mAb to VLA4 (PS/2) followed by FITC-coupled anti-mouse IgG or rhodamine-coupled anti-rabbit IgG. Confocal microscopy was used to define the localization of the antigens in 0.2-μ m horizontal serial cross sections of the cells. The top, middle, and bottom sections from adherent cells on VN and FN are shown. Panels in the left column show the localization of uPAR, the middle column VLA4 integrin and the right column show the overlay between both. (b) The fluorescence intensity of uPAR and VLA4 staining on VN- or FN-coated slides was quantified in 0.2-μ m serial sections. Relative fluorescence with anti-uPAR IgG on a VN (ρ) or FN substrate (▴)and anti-VLA4 IgG on a VN (□) or FN substrate (▪ is shown. Bar = 10 μ m.
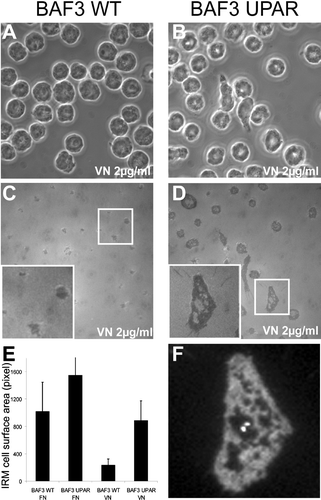
In order to further characterize the uPAR-dependent adhesion on VN we analyzed cell-to-substrate contacts by IRM. BAF3 and uPAR-transfected BAF3 cells were plated onto VN (2 μ g/mL) and FN (10 μ g/mL) substratum and the cells were fixed and the contact surfaces between the cell and the substrate visualized by IRM ((c and d)). Dark areas in the IRM image directly reflect the contact of the cell to the substrate. The dark surface area (including the holes) was quantified, demonstrating an uPAR-dependent increase in cell-to-substrate contact on VN but not on FN ( (e)). The analysis of the uPAR distribution revealed a complete overlap with the IRM-dark regions, suggesting a direct involvement of uPAR in the formation of these irregularly shaped cell substrate adhesion ().
Figure 3 Determination of cell-substrate contact area by interference reflection microscopy (IRM). Control (a and c) and uPAR transfected (b, d, and f) BAF3 cells were plated onto 2-μ g/mL VN or 10-μ g/mL FN for one hour. After fixation, cells were imaged by phase contrast (a and b) and IRM (c and d). The inserts in (c) and (d) show a magnified view of the boxed area in (c) and (d), respectively. (e) Quantification of cell/surface contact area from IRM images (n > 100 cells). The error bars represent the standard deviation of the measured surface areas. (f) Anti-uPAR immunohistochemistry of the cell from insert in (d). The width of the fields in (a)–(d) corresponds to 85 μ m.
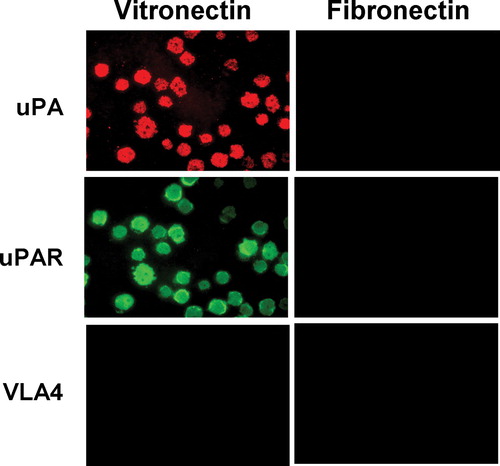
uPAR is known to associate with integrins and colocalize to detergent-insoluble microdomains (Bohuslav et al. Citation1995; Cunningham et al. Citation2003; Wei et al. Citation1996). To further define the nature of the uPAR-rich adhesion patches observed by confocal microscopy, we tested if these structures were resistant to solubilization with detergents. Cells adherent on VN or FN were solubilized extensively with 1% (wt/vol) Triton X-100 and the detergent-resistant fraction left on the adherent substrate was analyzed by immunofluorescence microscopy. After adhesion of uPAR-BAF3 cells on VN, but not on FN, the detergent-insoluble material remaining on the plate was stained strongly for uPAR, but not for VLA4 (). Thus, the adhesion patch seen in intact cells is likely to be composed of detergent-insoluble material; we have termed this the “detergent-insoluble adhesion patch” (DIAP). uPA is known to increase uPAR-dependent cell adhesion on VN by forming a tri-molecular complex with VN and uPAR (Kanse et al. Citation1996; Waltz and Chapman, Citation1994). If uPAR-BAF3 cells were pre-incubated with uPA prior to their adherence to VN or FN followed by solubilization with Triton X-100, uPA was found in DIAP only on a VN but not a FN substrate (). If uPA was omitted from the preincubation medium, no staining with anti-uPA IgG was observed. This indicates that soluble or secretory proteins, upon association with uPAR, can become part of DIAP. Hence, uPAR-dependent adhesion on VN is associated with the formation of DIAP, which contains high levels of uPAR but not β1 integrins.
Figure 4 Localization of uPA, uPAR, and β1 integrin to sites of cell adhesion in detergent-insoluble adhesion patches (DIAP) of uPAR-transfected cells. uPAR-BAF3 cells were allowed to adhere to VN- or FN-coated slides. The cells were preincubated with uPA (50 nM), at 37°C for 30 min and washed extensively before addition to the slides. Adherent cells were lysed with 1% Triton X-100 (wt/vol) at 4°C for 60 min, and the material remaining on the plate (DIAP) was fixed with methanol/acetone and immunostained with anti-uPA (rabbit polyclonal), anti-uPAR (rabbit polyclonal) IgG or anti β1 integrin (mAb PS/2) followed by FITC-coupled anti-mouse IgG or rhodamine-coupled anti-rabbit IgG. Bar = 10 μ m.
Formation and Composition of DIAP
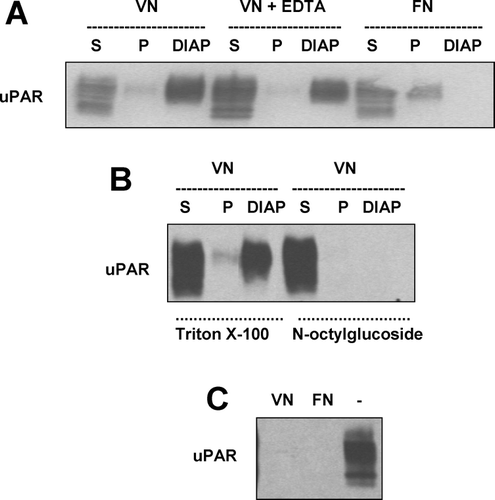
Following cell adhesion on an appropriate substrate, cell lysates were separated into fractions consisting of detergent-soluble lysates (supernatants), detergent-insoluble fraction (pellet), as well as the detergent-insoluble fraction bound tightly to the adhesion substrate (DIAP). After adhesion of uPAR-BAF3 cells to VN, some uPAR was found in the supernatant and very little in the pellet, whereas the majority of uPAR was associated with DIAP ().This distribution did not change significantly when cell adhesion was carried out in the presence of EDTA ((a)), which does not influence uPAR-dependent adhesion but inactivates integrins (see below). In contrast, on FN the majority of uPAR was found in the supernatant, some in the pellet, and no uPAR was associated with the substratum ((a)). If the lysates were prepared with n-octyl glucoside, which solubilizes lipid microdomains, no uPAR remained on the substratum, indicating that this was indeed a Triton X-100–insoluble fraction ((b)). If Triton X-100 lysates were prepared from cells in suspension and then added to VN-coated dishes, no uPAR was found associated with the plate ((c)). Hence, the formation of DIAP is not a passive process occurring in the presence of VN and uPAR, but is engineered in an active manner by the cell during cell adhesion.
Figure 5 Characterization of the detergent-insoluble adhesion patch (DIAP) of uPAR expressing cells on a VN and FN matrix. (a) uPAR expressing BAF3 were allowed to adhere on a VN- (2 μ g/mL) or an FN- (10 μ g/mL) coated plate in the presence of EDTA (10 mM) where indicated. Thereafter, nonadherent cells were washed away and the adherent cells were lysed with 1% (wt/vol) Triton X-100 at 4°C for 60 min with extensive mixing and this lysate was collected. The lysate was separated into a pellet and supernatant by centrifugation. The plate was extensively washed with fresh solutions of Triton X-100 as above and then the material remaining on the plate (DIAP) extracted with SDS sample buffer. In parallel plates the number of adherent cells was quantified under the different adhesion conditions and this information was used to adjust the volumes of the different extracts. Equivalent amounts of the different fractions were analyzed by Western blotting with antibodies against uPAR. (b) In a similar experiment, as above, n-octyl glucoside (1% wt/vol) was used to lyse the cells adherent on a VN substratum instead of Triton X-100. (c) In a similar experiment to above, instead of adding cells to VN- or FN-coated dishes, pre-prepared Triton X-100 lysates of the same number of growing cells were added to VN- or FN-coated plates. An aliquot of this lysate was also analyzed as a positive control.
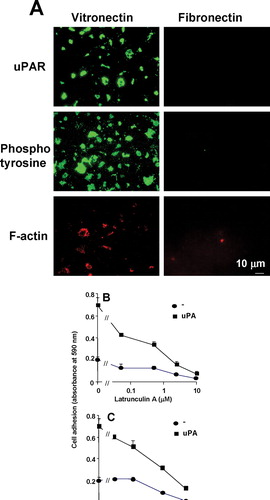
Since cell adhesion is generally associated with the reorganization of the actin cytoskeleton (Zamir et al. Citation2000) and protein tyrosine phosphorylation (Mondal et al. Citation2000), their role in the uPAR-dependent cell-adhesion process was investigated. On a VN but not FN substratum some F-actin staining was detected after Triton X-100 extraction to be associated with the substrate ((a)). Similarly, tyrosine-phosphorylated proteins appeared in DIAP on the VN but not on the FN substrate (). Based on these results, we investigated whether functional inhibition of these components may influence uPAR-dependent cell adhesion. Both, latrunculin A, which disrupts actin polymerization (Zamir et al. Citation2000), and genistein, an inhibitor of protein tyrosine phosphorylation (Mondal et al. Citation2000), decreased uPAR-dependent cell adhesion on VN in a dose-dependent manner ( and c). The glycolipid GM1 is a marker for some types of detergent-insoluble microdomains; we examined the staining of FITC-cholera toxin-B subunit that binds to GM1, in DIAP (Thomas et al. Citation2003). In uPAR-rich DIAP on a VN substrate no GM1 could be detected (data not shown), indicating that DIAP is distinct from GM1-containing lipid rafts.
Figure 6 Localization of uPAR, F-actin and tyrosine phosphorylated proteins to sites of cell adhesion in uPAR-BAF3 cells. (a) uPAR-BAF3 cells were allowed to adhere to VN- or FN-coated slides. DIAP associated with the substratum were fixed and immunostained with anti-uPAR (rabbit polyclonal) IgG, Phalloidin-conjugated rhodamine and anti-phosphotyrosine IgG followed by FITC-coupled anti-mouse IgG or rhodamine-coupled anti-rabbit IgG. Fluorescence microscopy was used to define the localization of the antigens in DIAP, as indicated in the figure. uPAR-BAF3 cells were preincubated with different concentrations of (b) latrunculin A or (c) genistein at 37 °C for 30 min, as indicated. After extensive washing cell adhesion on VN was determined in the absence (•) or presence of uPA (50nM) (▪) and is expressed as absorbance at 590 nm (mean ± SEM, n = 3).
Manipulation of the GPI Anchor Inhibits DIAP Formation
Since uPAR-dependent cell adhesion leads to an accumulation of uPAR in DIAP, we investigated the effect of disrupting the GPI anchor on this cell adhesion process. In these experiments, cells bearing uPAR with a GPI anchor were compared with uPAR with a transmembrane protein domain from IL-2-R-α. We found that uPAR-IL-2 Rα fusion protein supports adherence
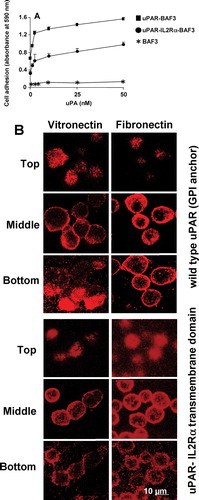
to a VN substrate, but to a lower extent than the GPI-anchored receptor, and this adhesion could be increased in the presence of uPA ((a)). However, confocal microscopy indicated that the uPAR-IL-2 Rα receptor did not form DIAP as compared to the GPI-anchored uPAR ((b)). Similarly, after solubilizing the adherent cells with 1% (wt/vol) Triton X-100, no uPAR-rich DIAP-like structures were observed (data not shown). Hence, the GPI anchor is necessary for strong adhesion and DIAP formation.
Figure 7 Localization of uPAR to the sites of cell adhesion in uPAR-transfected BAF3 cells with or without the GPI anchor. (a) Non-transfected BAF3 cells (*), cells expressing GPI-anchored uPAR (▪) or cells expressing uPAR as a fusion protein with the transmembrane domain of the IL-2 Rα receptor (•) were allowed to adhere to VN-coated wells in the presence of the indicated concentrations of uPA and the cell adhesion was quantified. (b) Similarly, the cells were allowed to adhere on VN- or FN-coated slide and immunostained with anti-uPAR (rabbit polyclonal) IgG followed by FITC-coupled anti-mouse IgG. Confocal microscopy was performed as described above. The top, middle, and bottom sections from adherent cells on VN and FN are shown in the figure. Bar = 10 μ m.
Partitioning of uPAR-rich DIAP in Distinct Membrane Domains
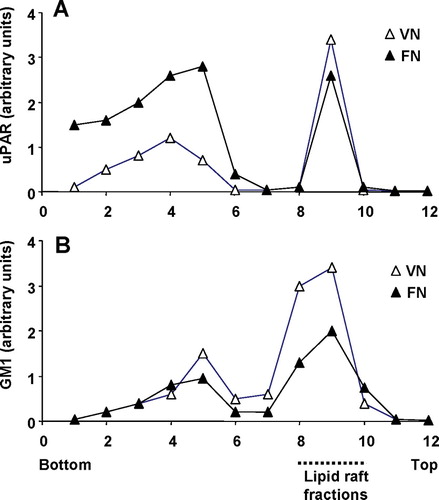
Since uPAR has been shown to partition into distinct microdomains on sucrose density gradients (Cunningham et al. Citation2003), we tested if DIAP-like structures are analogous to these microdomains. GM1 was used as a marker for raft fractions. We could confirm the previously reported observation that wild-type GPI-anchored uPAR partitioned into lipid rafts whereas the uPAR with a transmembrane protein did not (Cunningham et al. Citation2003). In accordance with their results we also found uPA in the lipid raft fractions (data not shown). On a VN matrix uPAR-BAF3 cells had more uPAR in the raft fraction (50% of total uPAR) versus non-raft fraction compared to cells on a FN substrate (20% of total uPAR in the raft fraction) (). On a VN matrix 70% of total GM1 was in the raft fraction on a VN matrix compared with 62% on a FN matrix (). Hence, on a VN substrate, uPAR partitions more into lipid rafts than on a FN substrate.
Figure 8 uPAR partitioning into lipid raft and non-raft domains in uPAR-BAF3 cells adherent on a VN or FN substrate. Cells adherent on a VN substrate (▵) or FN substrate (▴) were lysed and fractionated by sucrose density gradient centrifugation. Equal volumes were probed for (a) uPAR and (b) GM1 glycolipid by Western blotting and dot blotting respectively. The blots were scanned and the relative density for uPAR and GM1 was measured for each fraction. The fractions were numbered from bottom to the top with fractions 1–6 representing non-rafts and fractions 8–10 representing lipid rafts.
Role of Integrins in uPAR-Mediated Adhesion on VN
We then examined the functional role of integrins in the uPAR-mediated adhesion on VN. The adhesion of uPAR-expressing BAF-3 cells on VN was not influenced by EDTA, the mAb PS/2 against VLA4 or the monoclonal antibody Game 46 against β2integrins (). For comparison, the monoclonal antibody PS/2 inhibited cell adhesion on FN (). uPAR-blocking mAb R3 (D1 specific) and mAb 3936 (D2-D3 specific) reduced cell adhesion on VN, both in the absence or presence of uPA, confirming the specificity of uPAR-dependent cell adhesion (). Higher concentrations of mAb R3 (25 μ g/mL) completely inhibited adhesion indicating that uPAR is the primary, if not the only, adhesion receptor involved (data not shown).
Figure 9 Characteristics of the adhesion of uPAR-BAF3 cells on VN and FN. Cells were allowed to adhere on (a) VN (2μ g/mL) or (b) FN (10 μ g/mL) in the presence of control IgG (20 μ g/mL), the anti-uPAR mAb 3936, R3 or R4 (each at 20 μ g/mL), anti-VLA4/ α4β1 integrin mAb PS/2 at 20 μ g/mL), anti-β2 integrin mAb Game 46 (15 μ g/mL), or EDTA (10 mM). In (a) experiments were performed in the absence (dotted bars) or presence (hatched bars) of 50-nM uPA and in (b) in the absence (dotted bars) or presence (hatched bars) of 50-ng/mL PMA. (c) Adhesion of cells on VN, FN, VN 1–44 (RGD–), VN 1–48 (RGD+) and BSA was performed as described above. Cell adhesion is expressed as absorbance at 590 nm (mean ± SEM, n = 3).
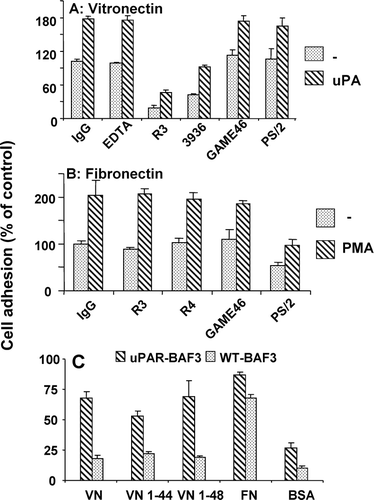
The N-terminal somatomedin B domain of VN contains the RGD sequence and is the primary interaction site for interaction with integrins. The somatomedin B domain of VN is found naturally in human plasma as a peptide with RGD sequence (VN 1–48) or without it (VN 1–44) (Standker et al. Citation1996). The adhesion of BAF3 cells, with and without uPAR, was investigated on VN, FN, BSA, VN 1–44 (RGD−) and VN 1–48 (RGD+). Both the VN peptides, with and without the RGD sequence, could mediate uPAR-dependent cell adhesion (). A cyclic RGD peptide that is a specific inhibitor of αVβ3 integrin did not influence uPAR-BAF3 adhesion on VN but the adhesion of cells bearing αVβ3 integrin was inhibited (data not shown). Hence, uPAR-dependent cell adhesion on VN is a result of a direct interaction between uPAR and VN independently of integrins.
DISCUSSION
Cell-to-matrix adhesion is mediated by point-like focal complexes associated with lamellipodia and streak-like focal adhesions at the periphery of adherent cells. Cell-to-substrate adhesion can also be mediated by podosomes and invadopodia (Linder and Kopp Citation2005). uPAR-mediated adhesion of cells on a VN substrate induces the accumulation of uPAR into irregularly sized patches often connected by undulating projections, directly in contact with the adhesion substrate. The formation of these detergent-insoluble adhesion patches is caused by a direct interaction of uPAR with VN and leads to strong cell adhesion. The shape and appearance of DIAPs is not consistent with focal adhesions, podosomes or invadopodia. However, similar irregular adhesion patches have been observed in β 3-GFP integrin and talin-FERM domain–expressing cells
(Cluzel et al. Citation2005). In our system, the formation of DIAP is mediated by a lipid raft-associated cell-surface receptor, whose immobilization on a substratum seems to recruit more lipid rafts. During this adhesion process the distribution of β1integrins was not different on FN or VN substratum. More colocalization between uPAR and β1 integrins was observed on a FN substrate compared to VN substrate and this colocalization was prominent in cell-to-cell contacts and generally on the whole cell surface. Co-immunoprecipitation between uPAR and β1and β2integrins was also observed (data not shown). The morphological distribution of integrins and their functional analysis indicates that the formation of DIAP is a novel cell-adhesion structure/mechanism which functions through lipid raft–associated receptors, providing a new paradigm for cell-to-substrate adhesion.
Detergent insolubility of the uPAR-rich clusters on VN was established by density gradient centrifugation and selective solubilization with 1% Triton X-100, hence the name DIAP. uPAR is often expressed on the leading edge of migrating cells (Kjoller and Hall Citation2001). This edge is characterized by the presence of the GM1 ganglioside (Gomez-Mouton et al. Citation2001). No GM1 staining was observed, suggesting that DIAPs were not GM1-lipid rafts. This is also in agreement with an earlier report that uPAR is not found in GM1-lipid rafts in migrating lymphocytes (Gomez-Mouton et al. Citation2001). However, we did find uPAR in the same fraction as GM1-lipid rafts upon sucrose density gradient centrifugation, hence DIAP is likely to be a subset of lipid rafts. The presence of increased levels of uPAR together with GM1 in the lipid raft fraction on a VN rather than a FN substrate indicates that the VN-uPAR interaction may influence the formation of lipid rafts.
It has been reported that exogenously added uPA was found to partition into lipid raft fractions together with uPAR upon density gradient centrifugation (Cunningham et al. Citation2003). Similarly, we observed that exogenously added uPA accumulates in DIAP on a VN but not a FN substrate. This is in accordance with the function of uPA as a stimulator of direct binding between uPAR and VN and a promoter of this cell adhesion process.
uPAR-IL-2 Rα transfectants have been shown to exhibit unaltered uPA-binding capability (Li et al. Citation1994), but no cell adhesion on VN (Wei et al. Citation1996). However, we found weak cell adhesion of cells expressing this form of uPAR. These cells did not form DIAP-like structures, indicating that the formation of DIAP is dependent on the GPI anchor. Cholesterol-modifying agents, cyclodextrin and cholesterol oxidase, inhibited adhesion of GPI-anchored uPAR cells much more efficiently than the transmembrane-anchored uPAR cells (unpublished observations) further supporting the concept of uPAR clustering in DIAPs. Our results are supported by previous observations that the transmembrane form of uPAR does not accumulate in lipid rafts (Cunningham et al. Citation2003; Wei et al. Citation1996). Presumably the GPI anchor allows higher lateral mobility and a greater level of clustering of uPAR in DIAP compared with the transmembrane anchor.
Evidence for a macroscopic linkage between uPAR and the cytoskeleton has been provided by magnetic twisting cytometry experiments (Wang et al. Citation1995). In line with this observation we found that F-actin was localized in detergent-resistant structures on a VN but not an FN substrate, and cell adhesion was inhibited by latrunculin A, an actin-depolymerizing agent. Similarly, tyrosine-phosphorylated proteins were also found in detergent-resistant structures on a VN but not an FN substratum, and cell adhesion was diminished by the tyrosine kinase inhibitor, genistein. These results suggest that actin polymerization and tyrosine phosphorylation is involved in uPAR-dependent cell adhesion on VN.
Previous reports in the literature have provided evidence that uPAR-mediated adhesion on VN can occur upon inhibition of integrins with EDTA (Li et al. Citation2003; Montuori et al. Citation2002) as well as on a VN peptide without the RGD sequence (Deng et al. Citation2001; Madsen et al. Citation2007). In another study uPAR was present in the raft fractions and β1integrins were exclusively located in non-raft fractions (Cunningham et al. Citation2003). Our own evidence that this cell adhesion is independent of integrins is that (a) the uPAR-VN-dependent adhesion remained completely intact in the absence of divalent cations, which are essential for maintaining the functional structure of integrins; (b) specific integrin-blocking reagents did not influence the uPAR-mediated adhesion; (c) major integrins on the investigated cells are not found in DIAP; (d) uPAR-dependent adhesion was observed on a VN peptide from the somatomedin B domain that does not contain the RGD sequence. During the revision of this manuscript Madsen et al. (Citation2007) have published data that supports our hypothesis. They performed an alanine scan of the entire uPAR gene and found defective adhesion of those mutants that did not bind VN. However, all mutants with an alteration in the putative integrin binding region showed normal cell adhesion on VN.
In conclusion, we have presented evidence that uPAR-dependent cell adhesion is driven by the formation of a “detergent-insoluble adhesion patch” (DIAP) where GPI-anchored uPAR is clustered and forms contacts to the VN in the substratum. This type of uPAR-mediated adhesion can occur independently of integrins, a finding that contradicts the prevailing notion that integrins play an obligatory role in mediating uPAR-dependent adhesion. DIAP formation and cell adhesion was observed when human uPAR was expressed in mouse (BAF3, FDCP-1, and 32D) or human cells (HEK 293 and Raji) and when mouse uPAR was expressed in BAF3 cells indicating that there is no species specificity. In uPAR-transfected HEK-293 cells uPAR-rich DIAP were observed on VN, but integrins contributed largely to cell adhesion. However, in the presence of RGD peptides or EDTA to inhibit integrins, uPAR-mediated adhesion was predominant (data not shown). Therefore our conclusion, that is similar to the one from Madsen et al (Madsen et al. Citation2007), is that uPAR-mediated DIAP formation supports adhesion via integrins that are the workhorse of adhesion and spreading processes. Similar observations were made in U937 cells which naturally express uPAR and β2 (MAC-1) integrins (Kanse et al. Citation2004a); however, the adhesion of human monocytes and neutrophils to VN was mediated mainly by β2 integrins, and no uPAR-rich DIAP formation was observed, presumably due to low levels of uPAR expression on these cells (Kanse et al. Citation2004c). It is likely that a critical expression level of uPAR is required for DIAP formation and cell adhesion. Hence, cell adhesion to VN can be mediated by uPAR, β 2 integrins as well as αvβ3 integrins. Tumor cells (Yang et al. Citation2006) and stem cells (Basire et al. Citation2006) express very high levels of uPAR, and the formation of uPAR-rich DIAP and the associated cell adhesion in these cells is likely.
The skilful technical assistance of Susanne Tannert-Otto and Thomas Schmidt-Wöll is greatly appreciated. This work was supported by grants, Ka 1468/2-2, from the Deutsche Forschungsgemeinschaft (Bonn, Germany) to S.M.K., and from the Swiss National Science Foundation (3100A0-103805) to B.W.H.
REFERENCES
- Basire A, Sabatier F, Ravet S, Lamy E, Mialhe A, Zabouo G, Paul P, Gurewich V, Sampol J, Dignat-George F. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb Haemost 2006; 95: 678–688
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3: 932–943
- Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle U, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med 1995; 181: 1381–1390
- Chaurasia P, Aguirre-Ghiso J A, Liang O D, Gardsvoll H, Ploug M, Ossowski L. A region in urokinase plasminogen receptor domain III controlling a functional association with alpha5beta1 integrin and tumor growth. J Biol Chem 2006; 281: 14852–14863
- Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof B A, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol 2005; 171: 383–392
- Cunningham O, Andolfo A, Santovito M L, Iuzzolino L, Blasi F, Sidenius N. Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. Embo J 2003; 22: 5994–6003
- Degryse B, Resnati M, Czekay R P, Loskutoff D J, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem 2005; 280: 24792–24803
- Deng G, Curriden S A, Hu G, Czekay R P, Loskutoff D J. Plasminogen activator inhibitor-1 regulates cell adhesion by binding to the somatomedin B domain of vitronectin. J Cell Physiol 2001; 189: 23–33
- Gomez-Mouton C, Abad J L, Mira E, Lacalle R A, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez A C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci U S A 2001; 98: 9642–9647
- Kanse S M, Chavakis T, Al-Fakhri N, Hersemeyer K, Monard D, Preissner K T. Reciprocal regulation of urokinase receptor (CD87)-mediated cell adhesion by plasminogen activator inhibitor-1 and protease nexin-1. J Cell Sci 2004a; 117: 477–485
- Kanse S M, Chavakis T, Kuo A, Bdeir K, Cines D B, Preissner K T. Variability in the expression of urokinase receptor (CD87) mutants on cells: relevance to cell adhesion. Cell Biochem Funct 2004b; 22: 257–264
- Kanse S M, Kost C, Wilhelm O G, Andreasen P A, Preissner K T. The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp Cell Res 1996; 224: 344–353
- Kanse S M, Matz R L, Preissner K T, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the beta2 integrin Mac-1 (alphaMbeta2, CD11b/CD18). Arterioscler Thromb Vasc Biol 2004c; 24: 2251–2256
- Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol 2001; 152: 1145–1157
- Li H, Kuo A, Kochan J, Strickland D, Kariko K, Barnathan E S, Cines D B. Endocytosis of urokinase-plasminogen activator inhibitor type 1 complexes bound to a chimeric transmembrane urokinase receptor. J Biol Chem 1994; 269: 8153–8158
- Li Y, Lawrence D A, Zhang L. Sequences within domain II of the urokinase receptor critical for differential ligand recognition. J Biol Chem 2003; 278: 29925–29932
- Linder S, Kopp P. Podosomes at a glance. J Cell Sci 2005; 118: 2079–2082
- Madsen C D, Ferraris G M, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol 2007; 177: 927–939
- May A E, Kanse S M, Lund L R, Gisler R H, Imhof B A, Preissner K T. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J Exp Med 1998; 188: 1029–1037
- Mondal K, Sirenko O I, Lofquist A K, Morris J S, Haskill J S, Watson J M. Differential role of tyrosine phosphorylation in adhesion-induced transcription, mRNA stability, and cytoskeletal organization in human monocytes. J Leukoc Biol 2000; 67: 216–225
- Montuori N, Carriero M V, Salzano S, Rossi G, Ragno P. The cleavage of the urokinase receptor regulates its multiple functions. J Biol Chem 2002; 277: 46932–46939
- Nebl T, Pestonjamasp K N, Leszyk J D, Crowley J L, Oh S W, Luna E J. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem 2002; 277: 43399–43409
- Shibayama H, Anzai N, Ritchie A, Zhang S, Mantel C, Broxmeyer H E. Interleukin-3 and Flt3-ligand induce adhesion of Baf3/Flt3 precursor B- lymphoid cells to fibronectin via activation of VLA-4 and VLA-5. Cell Immunol 1998; 187: 27–33
- Simon D I, Rao N K, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L, Chapman H A. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood 1996; 88: 3185–3194
- Simon D I, Wei Y, Zhang L, Rao N K, Xu H, Chen Z, Liu Q, Rosenberg S, Chapman H A. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. J Biol Chem 2000; 275: 10228–10234
- Sitrin R G, Johnson D R, Pan P M, Harsh D M, Huang J, Petty H R, Blackwood R A. Lipid raft compartmentalization of urokinase receptor signaling in human neutrophils. Am J Respir Cell Mol Biol 2003; 30: 233–241
- Standker L, Enger A, Schulz-Knappe P, Wohn K D, Germer M, Raida M, Forssmann W G, Preissner K T. Structural and functional characterization of vitronectin-derived RGD-containing peptides from human hemofiltrate. Eur J Biochem 1996; 241: 557–563
- Stockmann A, Hess S, Declerck P, Timpl R, Preissner K T. Multimeric vitronectin. Identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem 1993; 268: 22874–22882
- Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar A P, Bdeir K, Kuo A, Yarovoi S V, Cines D B, Takada Y. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost 2006; 95: 524–534
- Tarui T, Andronicos N, Czerkay R P, Mazar A P, Bdeir K, Parry G C, Kuo A, Loskutoff D J, Cines D B, Takada Y. Critical role of integrin alpha 5beta 1 in urokinase (uPA) /urokinase receptor (uPAR, CD87) signaling. J Biol Chem 2003; 278: 29863–29872
- Thomas S, Kumar R S, Casares S, Brumeanu T D. Sensitive detection of GM1 lipid rafts and TCR partitioning in the T cell membrane. J Immunol Methods 2003; 275: 161–168
- Waltz D A, Chapman H A. Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J Biol Chem 1994; 269: 14746–14750
- Wang N, Planus E, Pouchelet M, Fredberg J J, Barlovatz-Meimon G. Urokinase receptor mediates mechanical force transfer across the cell surface. Am J Physiol 1995; 268: C1062–C1066
- Wei Y, Czekay R P, Robillard L, Kugler M C, Zhang F, Kim K K, Xiong J P, Humphries M J, Chapman H A. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol 2005; 168: 501–511
- Wei Y, Eble J A, Wang Z, Kreidberg J A, Chapman H A. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell 2001; 12: 2975–2986
- Wei Y, Lukashev M, Simon D I, Bodary S C, Rosenberg S, Doyle M V, Chapman H A. Regulation of integrin function by the urokinase receptor. Science 1996; 273: 1551–1555
- Wei Y, Waltz D A, Rao N, Drummond R J, Rosenberg S, Chapman H A. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem 1994; 269: 32380–32388
- Wei Y, Yang X, Liu Q, Wilkins J A, Chapman H A. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol 1999; 144: 1285–1294
- Xia Y, Borland G, Huang J, Mizukami I F, Petty H R, Todd R F, 3rd, Ross G D. Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol 2002; 169: 6417–6426
- Xue W, Kindzelskii A L, Todd R F, 3rd, Petty H R. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol 1994; 152: 4630–4640
- Xue W, Mizukami I, Todd R F, 3rd, Petty H R. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res 1997; 57: 1682–1689
- Yang L, Avila H, Wang H, Trevino J, Gallick G E, Kitadai Y, Sasaki T, Boyd D D. Plasticity in urokinase-type plasminogen activator receptor (uPAR) display in colon cancer yields metastable subpopulations oscillating in cell surface uPAR density–implications in tumor progression. Cancer Res 2006; 66: 7957–7967
- Zamir E, Katz M, Posen Y, Erez N, Yamada K M, Katz B Z, Lin S, Lin D C, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol 2000; 2: 191–196
