Abstract
In the human heart, ventricular myocytes express connexin43 (Cx43) and traces of Cx45. In congestive heart failure, Cx43 levels decrease, Cx45 levels increase and gap junction size decreases. To determine whether alterations of connexin coexpression ratio influence gap junction size, we engineered a rat liver epithelial cell line that endogenously expresses Cx43 to coexpress inducible levels of Cx45 under stimulation of the insect hormone, ponasterone A. In cells induced to express Cx45, gap junction sizes are significantly reduced (by 15% to 20%; p < 0.001), an effect that occurs despite increased levels of junctional connexons made from both connexins. In contrast, coexpression of Cx40 with Cx43 does not lead to any change in gap junction size. These results are consistent with the idea that increased Cx45 expression in the failing ventricle contributes to decreased gap junction size.
INTRODUCTION
In the heart, gap junctions form sites of electrical coupling between individual myocytes that mediate the orderly spread of electrical excitation throughout the heart (Desplantez et al. Citation2007). Gap junction channels comprise pairs of connexons (hemichannels) made from hexamers of connexin subunits (Goodenough et al. Citation1996). The connexins are a multigene family of conserved proteins; 21 different connexin types are expressed in human and twenty in mouse (Sohl and Willecke Citation2004). Although cells stably transfected with cDNAs encoding different connexins show distinct properties, such as unitary conductance, voltage gating, ionic selectivity, and molecular permeability (Veenstra et al. Citation1995; Harris Citation2001), the precise functional properties of gap junctions in vivo depend on the specific connexins present (Cottrell and Burt Citation2005; Rackauskas et al. Citation2007). Cardiomyocytes express connexins 43, 40, and 45 (Cx43, Cx40 and Cx45) in various combinations, in different specialized regions of the heart (Severs et al. Citation2006; Desplantez et al. Citation2007), which are hypothesized to contribute to the patterns of current flow that govern the normal heart rhythm.
Human working ventricular myocytes predominantly express Cx43, with small quantities of Cx45 (Vozzi et al. Citation1999). In the diseased ventricle, expression of Cx43 is down-regulated, gap junction size is decreased, and Cx45 expression is up-regulated (Kaprielian et al. Citation1998; Dupont et al. Citation2001; Kitamura et al. Citation2002; Yamada et al. Citation2003). Correspondingly, in the heterozygous Cx43 knockout mouse, which expresses half the normal level of Cx43, an increased ratio of Cx43:Cx45 is associated with diminished gap junction size in the neonates (Johnson et al. Citation2002). These results raise the possibility that the coexpression ratio (i.e., Cx43:Cx45) may contribute to regulation of gap junction size. Because this hypothesis cannot easily be investigated in human tissue or in transgenic animals, we have developed two inducible transfected cell models (Halliday et al. Citation2003; Severs et al. Citation2006). In addition to the endogenous Cx43 present in the original rat liver epithelial (RLE) cell line, these models express Cx45 or Cx40 under the control of the ecdysone system (RLE Ind45 and RLE Ind40 respectively). A range of ratios of the inducible transfected connexin to the endogenous Cx43 are thus obtainable using ponasterone A to stimulate the ecdysone promoter.
METHODS
Routine Cell Culture
HeLa cells transfected with a native or V5-tagged version of Cx40, Cx43, Cx45, and RLE cells (McMahon et al. Citation1986) engineered to express mouse Cx40 (RLE Ind40) or Cx45 (RLE Ind45) (Halliday et al. Citation2003; Severs et al. Citation2006), were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. For reference, a generic map of the DNA constructs used and a diagram of the ecdysone system are shown in . Cx40 or Cx45 expression was induced by adding ponasterone A to the medium and maximal induction was obtained at 2 μ M. To maintain homogenous expression, poorly expressing cells were eliminated by seeding in 250 μ g/ml hygromycin B in induction medium. To obtain noninduced cells, RLE Ind40 and RLE Ind45 were maintained for at least 12 hours to remove remnants of hygromycin B, before induction was removed.
Figure 1 Diagram of the DNA construct/system use in stable transfection. (A) Constitutive CMV promoter constructs (native and tagged versions, three different antibiotic resistances cloned downstream from an internal ribosome entry site (IRES) are also available) were transfected into HeLa cells. Lysates from stable transfectants expressing Cx40, Cx43, Cx45, and the tagged versions of these connexins were used to calibrate the specific anti-connexin antibodies' reactivity in Western blot experiments and thereby to compare the connexins to each other. The position of the V5 epitope and the six-histidine stretch are indicated. (B) Diagram of the ecdysone system that permits inducible expression of Cx40 and Cx45. RLE cells were first transfected with pVgRXR (Invitrogen) coding for the transcription factors of the ecdysone system (ecdysone receptor, VgEcR; retinoid X receptor [RXR]) and can be selected using bleomycin. One stable clone expressing the transcription factors was retransfected with DNA constructs coding for both a connexin (CxX; Cx40 or Cx45) and the hygromycin resistant gene (Hygro) downstream from the IRES on the bicistronic construct. Upon association with ponasterone A, the VgEcR and RXR proteins form a heterodimer that activates the IND promoter and induces the transcription of an mRNA coding for Cx40 or Cx45 and hygromycin resistance. Selection of inducible RLE clones was therefore done in induction medium since the uninduced cells are not resistant to the antibiotic.
![Figure 1 Diagram of the DNA construct/system use in stable transfection. (A) Constitutive CMV promoter constructs (native and tagged versions, three different antibiotic resistances cloned downstream from an internal ribosome entry site (IRES) are also available) were transfected into HeLa cells. Lysates from stable transfectants expressing Cx40, Cx43, Cx45, and the tagged versions of these connexins were used to calibrate the specific anti-connexin antibodies' reactivity in Western blot experiments and thereby to compare the connexins to each other. The position of the V5 epitope and the six-histidine stretch are indicated. (B) Diagram of the ecdysone system that permits inducible expression of Cx40 and Cx45. RLE cells were first transfected with pVgRXR (Invitrogen) coding for the transcription factors of the ecdysone system (ecdysone receptor, VgEcR; retinoid X receptor [RXR]) and can be selected using bleomycin. One stable clone expressing the transcription factors was retransfected with DNA constructs coding for both a connexin (CxX; Cx40 or Cx45) and the hygromycin resistant gene (Hygro) downstream from the IRES on the bicistronic construct. Upon association with ponasterone A, the VgEcR and RXR proteins form a heterodimer that activates the IND promoter and induces the transcription of an mRNA coding for Cx40 or Cx45 and hygromycin resistance. Selection of inducible RLE clones was therefore done in induction medium since the uninduced cells are not resistant to the antibiotic.](/cms/asset/9254dd97-5c42-4883-ac46-da59b2531cfb/icac_a_301560_uf0001_b.gif)
Antibodies
For the detection of Cx43, either a monoclonal antibody against residues 252 to 270 of rat Cx43 (Chemicon, MAB3067) or a rabbit polyclonal antibody raised against the C-terminus of Cx43 (Sigma, C6219) were used. For Cx45, we used an “in house” monoclonal antibody, (Q14E(mab19-11-5)) (Coppen et al. Citation2003), and for Cx40, a goat polyclonal antibody raised against the C-terminus of Cx40 (Santa Cruz, SC20466) or an “in house” rabbit polyclonal antibody (S15C(R84)) (Severs et al. Citation2001). For immunoconfocal microscopy, appropriate fluorophore-conjugated (FITC or Cy3) secondary antibodies were purchased from Jackson. For immunoblots, appropriate alkaline phosphatase–conjugated antibodies were supplied by Pierce.
Immunoconfocal Analysis
Cells grown on glass coverslips were fixed using either ice-cold methanol or 2% formaldehyde (freshly prepared from paraformaldehyde, in phosphate-buffered saline; PBS) at 4°C. After blocking with 1% bovine serum albumin in PBS, cells were incubated with anti-connexin antibody, followed by the secondary antibody, mounted, and examined using a Leica TCS SP confocal microscope.
Quantification of Gap Junction Size
Ten images per slide were taken using the × 63 objective with a detector pinhole of 50 μ m, giving an optical section of 0.5 μ m. A zoom factor of 4 with a 512 × 512-pixel image was used. Images were acquired with high brightness and contrast settings, which were maintained throughout the experiment. Quantification of gap junction size was performed by applying a threshold on a 255-point grey scale to each image to reduce any cell background. The gap junction plaques in the membrane were manually marked and their length measured using PC Image software (Foster Findlay Ass.).
Western Blotting
Western blotting and quantification were carried out as previously described (Dupont et al. Citation2001) using anti-Cx43 (Sigma, C6219), anti-Cx40 (Santa Cruz, SC20466), or anti-Cx45 (Q14E(mab19-11-5)) antibodies, followed by incubation with the appropriate alkaline-phosphatase-conjugated secondary antibody. In order to quantify and compare the amounts of Cx40, Cx43 and Cx45, we used a V5-tagged version of the three connexins transfected into HeLa cells. The anti-V5 antibody (Invitrogen) immunoreactivity was used to calibrate the specific anti-connexin antibodies (Severs et al. Citation2006).
Extraction of Nonjunctional and Junctional Proteins
In order to quantify separately the cytoplasmic from junctional connexins, we used the Triton X-100 differential solubilization method (Musil and Goodenough Citation1991). Cells were scraped in phosphate-buffered saline (PBS) and pelleted (1000 rpm, 5 min). Nonjunctional connexins were extracted by resuspension of the pellet (10 μ l per cm2 of freshly confluent cells) in solubilization buffer (1% [v/v] Triton X-100, 40 mM Tris pH 8, 150 mM sodium chloride, 0.04% [w/v] sodium azide, 1:250 [v/v] mammalian protease inhibitor cocktail [Sigma, P8340]). Samples were homogenized by vortexing and sonication and incubated on ice for 30 min, with vortexing every 5 min, then centrifuged (15000 rpm, 30 min, 4°C). Nonjunctional proteins in the supernatant were collected. Junctional proteins in the pellet were resuspended in an equal volume of SB20 (20% SDS and 0.15 M Tris, pH 6.8) (Coppen et al. Citation1998). Samples were sonicated then incubated at room temperature for 30 min before processing by western blotting as described above.
Statistical Analysis
All analysis was done using GraphPad Prism 4 (GraphPad Software). Data are expressed as mean ± SEM. Statistical significance was evaluated with the unpaired Student t test for comparisons between two means or one-way analysis of variance (ANOVA) (followed by Bonferroni's correction). Statistical differences were judged significant at p ≤ 0.05.
RESULTS
shows western blot analysis of inducible Cx45 and Cx40 and endogenous Cx43 in the RLE Ind45 and RLE Ind40 cell lines. For both cell lines, with no induction, expression of the transfected connexin is undetectable, indicating no leakage of the uninduced ecdysone promoter. Expression of endogenous Cx43 appeared to be slightly suppressed at the highest levels of induction in both clones. The coexpressed Cx43 and Cx45 (, , ) or Cx43 and Cx40 (, , ) are extensively colocalized, indicating that in both RLE Ind40 and RLE Ind45 most if not all gap junctions are made of both connexins.
Figure 2 Dose-dependent expression of transfected connexins upon induction with ponasterone A. The upper immunoblots show the induction of Cx45 or Cx40 in the RLE Ind45 and RLE Ind40 cell lines, respectively. The black gradient slopes indicate increasing ponasterone A concentrations (0, 0.1, 0.25, 0.5, 1, and 2 μ M). Expression of both Cx45 and Cx40 is tightly regulated by the inducer. Endogenous Cx43 expression (detected with the anti-Cx43 Sigma antibody) is shown in the middle panel and equal protein loading is demonstrated by Coomassie blue staining (lower panel).
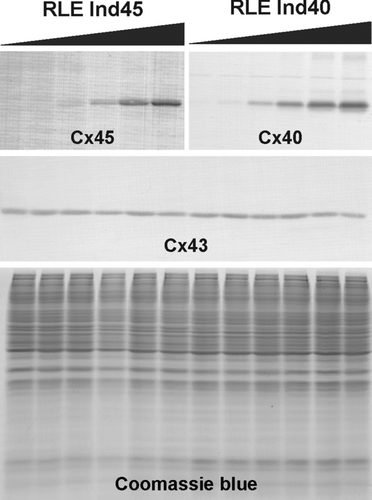
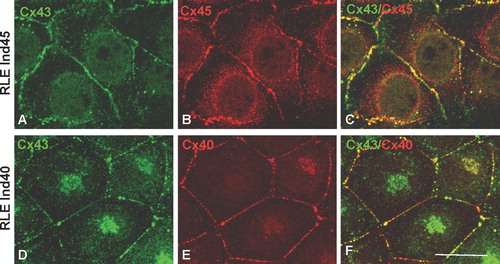
Figure 3 Double immunolabeling of Cx43 and Cx45 in RLE Ind45 cells (A, B, C) and Cx43 and Cx40 in RLE Ind40 cells (D, E, F) at maximal induction. Cx43 (detected using the Chemicon antibody) is colocalized with Cx45 or Cx40 in the same junctions. Scale bar: 25 μ m.
In order to quantify the ratio of expression, we have used our V5-tagged clones to calibrate the specific antibody (anti-Cx43,-Cx40, and-Cx45) reaction, thereby allowing comparative quantification of the three connexins to each other (Severs et al. Citation2006). By using the differential Triton X-100 solubilization method (Musil and Goodenough Citation1991), we have also quantified the proportion of each connexin present in the junctional and nonjunctional fractions in both cell lines and at different levels of induction (, ). Without induction (i.e., Cx43 only), ∼ 55 to 60 arbitrary units (AU) of the total protein is junctional in both clones (100 AU is defined as 100% of endogenous Cx43 expressed in both clones). As induction increases, the total complement of connexins (i.e., Cx43 + Cx40 or Cx43 + Cx45) increases (, dashed line) to ∼ 240 AU in the RLE Ind45 cells and ∼ 180 AU in the RLE Ind40 cells. The amount of connexins used to make gap junctions (, continuous line) remains very similar in the RLE Ind40 clone (from ∼ 58 AU of Cx43 to ∼ 68 AU, with a ratio Cx40/Cx43 of ∼ 1, indicating a loss of ∼ 25 AU of junctional Cx43), whereas in the RLE Ind45 clone this value almost doubles (from ∼ 55 AU of Cx43 to ∼ 107 AU made up of 46 AU of Cx45 and 61 AU of Cx43; see ). Interestingly, lower induction levels produce a more marked influence on Cx43 expression than does maximal induction. At 0.5 μ M ponasterone A, junctional Cx43 + Cx45 is 92 AU, of which Cx43 represents 78 AU and Cx45 only 13 AU. Large amounts of connexins in these two cell lines therefore remain cytoplasmic (, dotted lines; ), though this affects mostly the transfected, inducible connexin. The amounts of nonjunctional Cx43 (∼ 45 AU) remain constant at all levels of induction in both cell lines (; data for RLE Ind40 not shown).
Figure 4 Quantitative analysis of immunoblots to determine total, nonjunctional and junctional connexins in transfected RLE cell lines. (A) In RLE Ind45 cells, total connexin levels are increased by a factor of 2.4 at maximum induction. However, junctional Cx43/Cx45 is increased only by a factor of 2. (B) In RLE Ind40 cells, total connexin levels are increased by a factor of 1.7 at maximum induction, but junctional Cx40/Cx43 remains constant. 100 AU is defined as the total amount of endogenous Cx43 in both cell lines in the noninduced state.
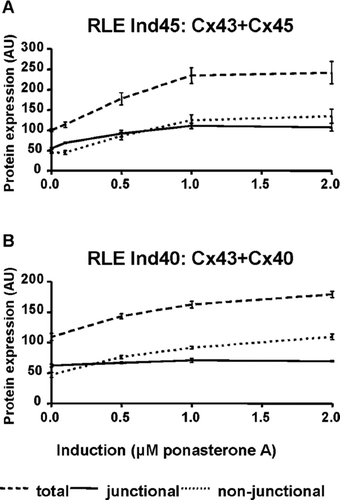
TABLE 1 Proportion of nonjunctional and junctional Cx43 (endogenous) and Cx45 with increasing levels of induction
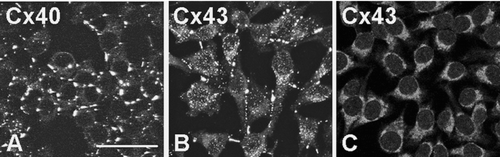
The size of the Cx43/Cx45 gap junctions at different levels of induction was measured following immunolabeling with anti-Cx43 (Chemicon or Sigma) or anti-Cx45 antibodies (). Induction of Cx45 significantly reduced the size of gap junctions by 15% to 20% (p < 0.001; ), and maximal effect was observed at low induction (0.5 μ M ponasterone A).
Figure 5 Representative images used to measure gap junction size. In this example, RLE Ind45 cells at maximal induction were labeled for either Cx45 (A) or Cx43 (B; Chemicon). Images were thresholded before measuring gap junction size with PC Image software (C). Scale bar: 5 μ m.
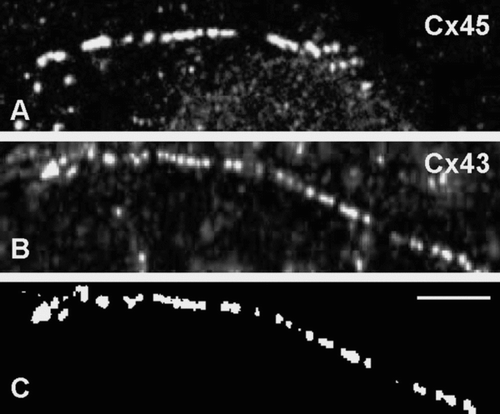
Figure 6 Gap junction sizes measured in RLE Ind45 cells at varying levels of induction. In order to compare gap junction size labeled with the anti-Cx43 and anti-Cx45 antibodies, data were normalized to the average size at maximum induction (100 arbitrary units [AU]). At both medium and high levels of induction Cx43-labeled gap junction size (measured with both the Chemicon and Sigma antibodies) was significantly reduced compared to noninduced cells. The size of Cx45-labeled gap junctions in induced cells was comparable to those measured for Cx43. The concentrations (0, 0.5, 2 μ M) of ponasterone A used are indicated. * p < 0.001.
![Figure 6 Gap junction sizes measured in RLE Ind45 cells at varying levels of induction. In order to compare gap junction size labeled with the anti-Cx43 and anti-Cx45 antibodies, data were normalized to the average size at maximum induction (100 arbitrary units [AU]). At both medium and high levels of induction Cx43-labeled gap junction size (measured with both the Chemicon and Sigma antibodies) was significantly reduced compared to noninduced cells. The size of Cx45-labeled gap junctions in induced cells was comparable to those measured for Cx43. The concentrations (0, 0.5, 2 μ M) of ponasterone A used are indicated. * p < 0.001.](/cms/asset/791a4344-583d-4b06-8af3-473930834c1a/icac_a_301560_uf0006_b.gif)
At high levels of induction in the RLE Ind40 cell line (i.e., more Cx40 in the junctions), the average size of gap junctions detected with the Chemicon anti-Cx43 antibody was significantly smaller, but when detected using the Santa Cruz anti-Cx40 antibody, gap junctions were larger (data not shown). These observations suggest that in this case, there is artefactual steric hindrance of antibody binding. Because all the gap junctions contain both Cx43 and Cx40 (), their size should not vary in opposite directions when different antibodies are used for detection. Therefore, we used a third antibody that detects Cx43 and cross-reacts with Cx40 (anti-Cx43, Sigma; ). This antibody is raised against the C-terminus of Cx43, which is similar to that of Cx40 (Cx40-SKASSKARSDDLSV and Cx43-SRASSRPRPDDLEI; i.e., 11 out of 14 amino acid residues are either identical or similar). Using this antibody, gap junction sizes in the RLE Ind40 cell line were identical regardless of the respective amounts of Cx40 and Cx43 expressed. This demonstrates that the differences seen by using the other, more specific, primary antibodies were artifactual.
Figure 7 Anti-Cx43 antibody (Sigma) cross-reacts with Cx40. Screening of Cx40 transfected HeLa cells using (A) anti-Cx40 (S15C(R84)), (B) anti-Cx43 (Sigma), and (C) anti-Cx43 (Chemicon). Cx40 is detected at cell interfaces (A). Labeling is also seen at cell interfaces when using the anti-Cx43 antibody (Sigma) (B). However, labeling with anti-Cx43 antibody (Chemicon) indicates that Cx43 is not expressed and the apparent anti-Cx43 Sigma antibody labeling is due to cross-reactivity with Cx40 (C). Scale bar: 100 μ m.
In the RLE Ind45 cell line, all three antibodies (anti-Cx45 and anti-Cx43, Chemicon and Sigma) gave almost identical results (), confirming a true decrease in gap junction size upon Cx45 induction. Without induction of the second connexin, Cx43 gap junction sizes were virtually identical in both RLE Ind45 and RLE Ind40 cell lines (data not shown).
DISCUSSION
The normal spread of the action potential between myocytes, including the specialized nodal and conduction cells, depends in part on the size, distribution, and connexin composition of gap junctions. Gap junctions in slow conducting tissues, e.g., the sinoatrial and atrioventricular nodes, are small and mostly made of Cx45, whereas large gap junctions made of Cx43 and/or Cx40 are expressed in faster conducting tissues (i.e., conduction system, atria, ventricle (Severs et al. Citation2006; Desplantez et al. Citation2007)). Our cell lines allow a graded, homogenous expression of Cx40 or Cx45 on a background of endogenous Cx43 expression. Using these cell lines, we have demonstrated that in the setting of increased Cx45 expression, gap junction size is significantly decreased. We also show that measuring stoichiometry in the total or nonjunctional Triton X-100 soluble fractions does not accurately represent the stoichiometry of the gap junction ().
Two key properties of our cell model permitted these findings. First, the original RLE cell line is a nontransformed cell line that communicates extensively, suggesting tight regulation of intercellular communication and/or connexin amounts in these cells. Secondly, our plasmid systems are designed specifically to obtain a homogenous level of the transfected connexin so that the stoichiometries of connexins we observe correspond to cells expressing similar ratios and not to averaged stoichiometries of cells that express vastly different amounts of the transfected connexin. The level of junctional Cx43 was altered in both cell lines, a feature that would probably have been overlooked with cells expressing heterogeneous levels of the transfected, inducible connexin.
Cx43 is reported to form heteromers with Cx40 (He et al. Citation1999; Valiunas et al. Citation2001) and with Cx45 (Martinez et al. Citation2002). Our immunoconfocal data show that Cx40 + Cx43 or Cx45 + Cx43 are extensively colocalized in the same gap junction plaques, suggesting, yet not proving, heteromerization in this particular cell line. That Cx40 and Cx43 coexpression leads to steric hindrance of antibody binding implies that the two connexin epitopes are very close to each other and is consistent with the presence of heteromers. Furthermore, homomeric, heterotypic Cx43/Cx40 channels are undetectable by immunofluorescence in other transfection systems (Elfgang et al. Citation1995; Rackauskas et al. Citation2007) and so are unlikely to contribute significantly to the co-localization seen in our model. For Cx45 and Cx43 coexpression, the increased amounts of both connexins in the pool of junctional connexons would fit the notion that less permeable heteromers would be needed in greater number to maintain junctional coupling. However, because Cx45 and Cx43 homomers are fully compatible, and if homomers were the predominant configuration, then the gap junctions would be made mostly of Cx43 or Cx45 homomers with the formation of both heterotypic and homotypic channels.
Our observation that gap junction size diminishes when Cx45 but not Cx40 is coexpressed with Cx43 is consistent with a tight regulation of connexon aggregation in these cell lines. One protein that has received particular attention as a possible regulator of Cx43 gap junction size is zonula occludens-1 (ZO-1), which binds to the C-terminus of Cx43 through its second PDZ domain (Toyofuku et al. Citation1998; Giepmans and Moolenaar Citation1998). Blocking the interaction between ZO-1 and Cx43 results in the formation of exceptionally large Cx43 gap junction plaques (Hunter et al. Citation2005); hence, transgenic mice engineered to express a truncated Cx43 that does not contain the ZO-1 binding site display very large gap junctions at the periphery of intercalated disks (Maass et al. Citation2007). Cx45 also binds to ZO-1, but it is thought that the interaction is mediated by the first, not the second PDZ domain of ZO-1 (Laing et al. Citation2001). Binding of ZO-1 to connexins through different PDZ domains (or possibly to two PDZ domains in coexpressing cells) may conceivably change its signalling ability and therefore its capacity to regulate gap junction size. In the failing left ventricle, the decreased Cx43/Cx45 ratio and gap junction size (Dupont et al. Citation2001; Yamada et al. Citation2003) correlates with an increased association of Cx43 with ZO-1 (Bruce et al. 2007). Similar mechanisms are likely to operate in our cell system to regulate gap junction size. Because Cx45 can be induced at varied levels, the model is particularly suitable to investigate further (1) if Cx43 and Cx45 form significant numbers of heteromers; (2) if the association between ZO-1 and Cx43 or Cx45 or both is altered; and (3) if alteration of ZO-1 or Cx43 expression leads to altered gap junction size. Conversely, the PDZ binding domain of Cx43 is well conserved in Cx40 (hence the cross-reactivity of the Sigma anti-Cx43 antibody). Cx40 probably interacts with ZO-1 through the same PDZ domain as does Cx43. The size of gap junctions made from Cx40 or Cx40/Cx43 may be regulated in a similar way. This would explain our observation that Cx40 and Cx43 coexpressing cells do not modify either the amounts of junctional connexons or the size of the gap junctions.
We also observed that junctional Cx43 is increased in the presence of low levels of Cx45. This may be caused by the longer half life of Cx45 compared to Cx43 (Berthoud et al. Citation2004) and raises the possibility that the half life of connexins aggregated in gap junction channels may be different to the half life of nonjunctional connexins.
In the failing human left ventricle, Cx43 is expressed at an average of half the level of control ventricles (Dupont et al. Citation2001). Transgenic mice expressing half the normal level of Cx43 (Cx43+/−) similarly display decreased ventricular gap junction size as measured after labeling for Cx43 or Cx45 (Johnson et al. Citation2002). In the RLE-inducible cell lines, the Cx43 levels remain constant or are slightly increased. The reduction of gap junction size may well be larger in the presence of decreased Cx43 levels. Interestingly, relatively low levels of Cx45 expression exhibited the maximal effect on gap junction size and on Cx43 expression. At this level, Cx45 expression is not high enough for every connexon to contain Cx45. The presence of low levels of Cx45 in the presence of diminished Cx43 expression, as seen in a range of settings of ventricular disease, may therefore have a disproportionately large effect on gap junction size and distribution.
Miss K. Grikscheit and Dr. N. Thomas contributed equally, as first authors, to this study.
This study was supported by the British Heart Foundation (project grants PG/05/003 and PG/05/111). We thank Dr. J. Trosko (Michigan State University) for kindly providing the RLE cell line.
REFERENCES
- Berthoud V M, Minogue P J, Laing J G, Beyer E C. Pathways for degradation of connexins and gap junctions. Cardiovasc Res 2004; 62: 256–267
- Bruce A F, Rothery S, Dupont E, Severs N J. Gap junction remodeling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res 2008; 77: 757–765
- Coppen S R, Dupont E, Rothery S, Severs N J. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res 1998; 82: 232–243
- Coppen S R, Kaba R A, Halliday D, Dupont E, Skepper J N, Elneil S, Severs N J. Comparison of connexin expression patterns in the developing mouse heart and human foetal heart. Mol Cell Biochem 2003; 242: 121–127
- Cottrell G T, Burt J M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta 2005; 1711: 126–141
- Desplantez T, Dupont E, Severs N J, Weingart R. Gap junction channels and cardiac impulse propagation. J Membr Biol 2007; 218: 13–28
- Dupont E, Matsushita T, Kaba R, Vozzi C, Coppen S R, Khan N, Kaprielian R, Yacoub M H, Severs N J. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol 2001; 33: 359–371
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein R A, Hülser D F, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 1995; 129: 805–817
- Giepmans B N, Moolenaar W H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 1998; 8: 931–934
- Goodenough D A, Goliger J A, Paul D L. Connexins, connexons, intercellular communication. Annu Rev Biochem 1996; 65: 475–502
- Halliday D, Dupont E, Coppen S R, Severs N J. Development of a cell model for functional and structural analysis of connexin co-expression: Achieving homogeneous and inducible expression of multiple connexins in stable transfectants. Cell Commun Adhes 2003; 10: 311–317
- Harris A L. Emerging issues of connexin channels: Biophysics fills the gap. Q Rev Biophys 2001; 34: 325–472
- He D S, Jiang J X, Taffet S M, Burt J M. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci U S A 1999; 96: 6495–6500
- Hunter A W, Barker R J, Zhu C, Gourdie R G. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 2005; 16: 5686–5698
- Johnson C M, Kanter E M, Green K G, Laing J G, Betsuyaku T, Beyer E C, Steinberg T H, Saffitz J E, Yamada K A. Redistribution of connexin45 in gap junctions of connexin43-deficient hearts. Cardiovasc Res 2002; 53: 921–935
- Kaprielian R R, Gunning M, Dupont E, Sheppard M N, Rothery S M, Underwood R, Pennell D J, Fox K, Pepper J, Poole-Wilson P A, Severs N J. Down-regulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation 1998; 97: 651–660
- Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano E J, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol 2002; 13: 865–870
- Laing J G, Manley-Markowski R N, Koval M, Civitelli R, Steinberg T H. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J Biol Chem 2001; 276: 23051–23055
- Maass K, Shibayama J, Chase S E, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res 2007; 101: 1283–1291
- Martinez A D, Hayrapetyan V, Moreno A P, Beyer E C. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res 2002; 90: 1100–1107
- McMahon J B, Richards W L, del Campo A A, Song M K, Thorgeirsson S S. Differential effects of transforming growth factor-beta on proliferation of normal and malignant rat liver epithelial cells in culture. Cancer Res 1986; 46: 4665–4671
- Musil L S, Goodenough D A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 1991; 115: 1357–1374
- Rackauskas M, Kreuzberg M M, Pranevicius M, Willecke K, Verselis V K, Bukauskas F F. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J 2007; 92: 1952–1965
- Severs N J, Dupont E, Thomas N, Kaba R, Rothery S, Jain R, Sharpey K, Fry C H. Alterations in cardiac connexin expression in cardiomyopathies. Adv Cardiol 2006; 42: 228–242
- Severs N J, Rothery S, Dupont E, Coppen S R, Yeh H-I, Ko Y-S, Matsushita T, Kaba R, Halliday D. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech 2001; 52: 301–322
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 2004; 62: 228–232
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 1998; 273: 12725–12731
- Valiunas V, Gemel J, Brink P R, Beyer E C. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol (Heart Circ Physiol) 2001; 281: H1675–H1689
- Veenstra R D, Wang H Z, Beblo D A, Chilton M G, Harris A L, Beyer E C, Brink P R. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res 1995; 77: 1156–1165
- Vozzi C, Dupont E, Coppen S R, Yeh H-I, Severs N J. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol 1999; 31: 991–1003
- Yamada K A, Rogers J G, Sundset R, Steinberg T H, Saffitz J E. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol 2003; 14: 1205–1212
