Abstract
Modulation of gap junction structures and gap junctional communication is important in maintaining tissue homeostasis and can be controlled via phosphorylation of connexin43 (Cx43) through several different signaling pathways. Transformation of cells by v-src has been shown to down-regulate gap junction communication coincident with an increase in tyrosine phosphorylation on Cx43. Activation of mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) also lead to down-regulation via phosphorylation on specific serine residues. Using phosphospecific anti-Cx43 antibodies generated by the authors' laboratory to specific tyrosines (src substrates) and serine residues (MAPK and PKC substrates) to probe LA-25 cells (which express temperature-sensitive v-src), the authors show that distinct tyrosine and serines residues are phosphorylated in response to v-src activity. They show that tyrosine phosphorylation appears to occur predominantly in gap junction plaques when src is active. In addition, src activation led to increased phosphorylation of apparent MAPK and PKC sites in Cx43. These results indicate all three signaling pathways could contribute to gap junction down-regulation during src transformation in LA-25 cells.
INTRODUCTION
Gap junction-mediated intercellular communication facilitates direct communication among adjacent cells by allowing passage of ions and small metabolites (White and Paul Citation1999; Saez et al. Citation2003; Sohl and Willecke Citation2004). Vertebrate gap junctions, composed of integral membrane proteins from the connexin gene family, are critically important in regulating embryonic development, coordinated contraction of excitable cells, tissue homeostasis, controlled cell growth, and differentiation (Saez et al. Citation2003; Sohl and Willecke Citation2004). Furthermore, connexin mutations have been linked to several diseases (Bergoffen et al. Citation1993; Gong et al. Citation1997; Kelsell et al. Citation1997), including oculodentodigital dysplasia, a disease caused by connexin43 (Cx43) mutations that can lead to atrioseptal defects and arrhythmias (Paznekas et al. Citation2003). Extensive evidence indicates that gap junctions play important roles in the control of cell growth and inhibit tumor formation.
Oncogenes, tumor promoters, and growth factors have been shown to down-regulate gap junctional communication. For example, many reports demonstrate that treatment with the tumor promoter 12-O-tetradeconylphorbol-13-acetate (TPA) leads to decreased gap junctional communication and phosphorylation of Cx43 on serine 368 (S368) (e.g., Lampe and Lau Citation2000; Solan et al. Citation2003; Ek-Vitorin et al. Citation2006) and S262 (Doble et al. Citation2004). Activation of mitogen-activated protein kinase (MAPK) via different growth factors also causes decreased gap junctional communication via phosphorylation at S279/282 (Kanemitsu and Lau Citation1993; Warn-Cramer et al. Citation1996; Cottrell et al. Citation2003). Earlier studies with cells expressing activated src have shown gap junctional communication to be down-regulated (Atkinson and Sheridan Citation1988; Azarnia et al. Citation1988) and Cx43 to be phosphorylated on tyrosine (Swenson et al. Citation1990; Crow et al. Citation1992; Goldberg and Lau Citation1993; Kanemitsu et al. Citation1997; Zhou et al. Citation1999; Lin et al. Citation2001). Cx43 is phosphorylated on Y265 and Y247 by v-src (Lin et al. Citation2001). Src interacts with Cx43 when it is phosphorylated at Y265 and this interaction apparently regulates the interaction between Cx43 and ZO-1 (Giepmans et al. Citation2001; Toyofuku et al. Citation2001). Hypoxia can also lead to increased Cx43-src interaction in a manner dependent on undefined Cx43 dephosphorylation events (Li et al. Citation2005). There is some controversy as to whether src phosphorylation of Cx43 on Y265 and/or Y247 directly inhibits gap junctional communication or whether MAPK phosphorylation of Cx43 plays a more direct role (Zhou et al. Citation1999; Lin et al. Citation2001; Cottrell et al. Citation2003).
Here, we describe the production of three phosphospecific antibodies that react with Cx43 when it is phosphorylated at Y247, Y265, or S279/S282. We demonstrate the specificity of these antibodies and show that both Y247 and 265 are phosphorylated in LA-25 cells (that contain temperature sensitive src) maintained at the permissive temperature but not the nonpermissive temperature. We go on to show that Cx43 in LA-25 cells is also phosphorylated on S279/282 and S368 at the permissive temperature. Thus, active v-src apparently leads to Cx43 phosphorylation via activated MAPK and protein kinase C (PKC) signaling pathways.
METHODS
Antibodies, cDNA Constructs and Other Reagents
All general chemicals, unless otherwise noted, were purchased from Fisher Scientific. Epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and TPA were purchased from Sigma Chemical (St. Louis, MO). Mouse anti-Cx43 antibodies, Cx43CT1 and Cx43IF1, were prepared against amino acids 360-382 of Cx43 and antibody Cx43NT1 against amino acids 1 to 20 (Cx43NT1) of Cx43 (described in Cooper and Lampe 2002; Lampe et al. Citation2006; Sosinsky et al. Citation2007) at the Fred Hutchinson Cancer Research Center Hybridoma Development Facility (Seattle, WA). The pS368 antibody was custom prepared and affinity purified by 21st Century Biochemicals (Marlboro, MA) (Solan et al. Citation2007). A rabbit anti-Cx43 specific for phosphorylation at S262 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). We made rabbit anti-pY247,-pY265, and-pS279/282 Cx43 antibodies by custom commercial preparation (ProSci, Poway, CA; 13-week schedule) against synthetic peptides that were phosphorylated at Y247 (CKSDP(pY)HATT-amide), Y265 (Acetyl-KYA(pY)FNGC-amide), and S279/282 (CAPL(pS)PM(pS)PPGY-amide), which we linked via the N-or C-terminal cysteine to maleimide-activated KLH (Pierce Biotechnology, Rockford, IL) according to manufacturer's instructions. Phosphospecific antibody was affinity purified essentially identically to our previously published method (Lampe et al. Citation2006). Serine to alanine mutations at S279, S282, and the combined mutation at S279/282 were made using the GeneTailor site-directed mutagenesis system (Invitrogen, Carlsbad, CA) on full length Cx43 that had been cloned into the mammalian expression vector pIREShyg (Clontech, Moutain View, CA).
Cell Line Maintenance and Transfection
Normal rat kidney (NRK) epithelial cells (NRK-E51; American Tissue Culture Collection [ATCC], Manassas, VA), 293T cells exogenously expressing Cx43, LA-25 cells (NRK cells containing temperature sensitive v-Src), and Madin-Darby canine kidney (MDCK) cells that do not express Cx43 (Jordan et al. Citation1999) were cultured in Dulbecco's minimal Eagle's medium (Mediatech, Pittsburgh PA) supplemented with 10% fetal calf serum and antibiotics in a humidified 5% CO2 environment. Transient transfections of wild-type and mutant Cx43 into the MDCK cell line were performed by electroporation with a Nucleofector apparatus (Amaxa Biosystems, Gaithersburg, MD), and cells were analyzed 24 h later.
Immunoblotting
Cells were lysed in sample buffer containing 50 mM NaF, 500 μ M Na3VO4, 2 mM phenylmethylsulfonyl fluoride (PMSF), and 1× complete protease inhibitors (Roche Molecular Biochemicals, Alameda, CA) and cellular proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide). After electrophoresis, protein was transferred to nitrocellulose, the membrane was blocked, and antibodies were incubated as previously indicated (Lampe et al. Citation2006). IRDye800 donkey anti-rabbit (Rockland Immunochemicals, Gilbertsville, PA) and AlexaFluor 680 anti-mouse (Molecular Probes, Eugene, OR) secondary antibodies were used to simultaneously visualize the phosphospecific and NT1 antibodies, respectively, and were directly quantified using the Li-Cor Biosciences Odyssey infrared imaging system and associated software (Lincoln, NE).
Immunofluorescence
Cells were washed twice in phosphate-buffered saline (PBS), and fixed in cold methanol/acetone (50:50) for 1 min, followed by a 1-h block in 1% bovine serum albumin in PBS. Cells were incubated with a rabbit anti-phosphoCx43 antibody (pY247 or pY265) and a total Cx43 anti-mouse antibody (Cx43IF1) in blocking solution for 1 h. Following several PBS washes, the cultures were incubated with Alexa-594-conjugated goat anti-rabbit antibody and/or Alexa 488-conjugated goat anti-mouse antibody for 30 to 60 min and counterstained with DAPI (Molecular Probes), followed by several washes in PBS. The coverslips were mounted onto slides with DABCO antifade medium (25 mg/ml of 1,4-diazobicyclo-(2,2,2)octane [Sigma] diluted in Spectroglycerol [Kodak] and 10% PBS, pH 8.6) and viewed with a Nikon Diaphot TE300 fluorescence microscope.
RESULTS
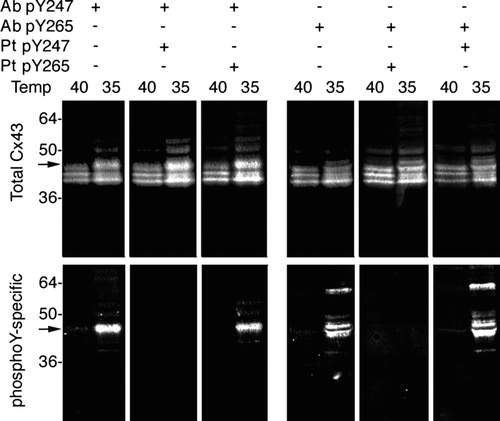
Expression of v-src has been shown to result in phosphorylation at Y247 and Y265 (Swenson et al. Citation1990; Crow et al. Citation1992; Lin et al. Citation2001). We produced two antibodies specific for Cx43 when it is phosphorylated at either Y247 or Y265 in order to better understand their relative contribution to reduced communication and Cx43 localization. We have validated these antibodies in a series of Western blots () with LA-25 cells grown at 40°C (nonpermissive) and 35°C (permissive) that were incubated in the presence (+) or absence (−) of the immunizing peptides (Pt) for each of the antibodies. Blots were simultaneously probed with NT1, an antibody to the N-terminus of Cx43, which recognizes Cx43 independent of phosphorylation status and either the pY247 or pY265 antibodies. For both the pY247 and the pY265 antibodies, there is much more reactivity at 35°C than at 40°C and incubation of the immunizing peptide with each antibody completely blocked the reactivity with the lysate when they were matched (e.g., Ab pY247 and Pt pY247), whereas incubation with the immunizing peptide for the other antibody (e.g., pY247 and Pt pY265) had no effect on the signal. Cx43 from cultured cells commonly demonstrates multiple electrophoretic isoforms when analyzed by SDS-PAGE: a faster migrating form (sometimes referred to as P0 or NP) that includes the nonphosphorylated isoform, and multiple slower migrating forms (sometimes termed P1 and P2; Musil and Goodenough Citation1991). The specificity of the pY247 and pY265 antibodies for Cx43 (i.e., alignment with the P2 isoform(s) of Cx43 indicated by arrow in ) is demonstrated by the fact that reactivity only is present when src is active, and the specificity of each antibody for its immunizing peptide indicates that both antibodies are indeed specific for Cx43 phosphorylated on each of the tyrosines in western immunoblot applications.
Figure 1 Phosphotyrosine antibodies are specific for Cx43 phosphorylated on Y247 or Y265. Immunoblot analysis of LA-25 cell lysates grown at the nonpermissive (40) or permissive (35) temperature. Replicate immunoblots were probed simultaneously with a monoclonal antibody (NT1) to total Cx43 (upper panel) and antibody against Cx43 phosphorylated on Y247 (Ab pY247) or Y265 (Ab pY265) (lower panel) as indicated. Antibodies were coincubated with immunizing peptides (Pt Y247 or Pt Y265) as indicated to demonstrate the specificity of each phosphospecific antibody. Note that antibody binding in the western blots was detected via fluorescence with the Odyssey imaging system allowing simultaneous detection of the phosphospecific and total Cx43 antibodies resulting in a white on black image for each channel.
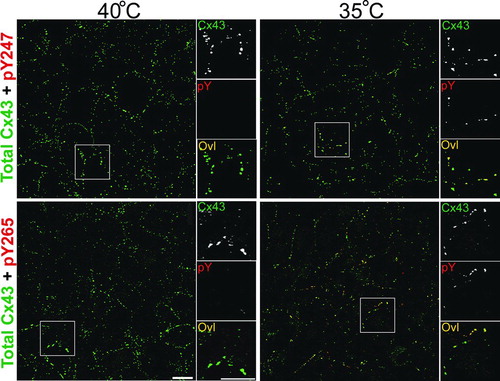
We then wanted to examine whether the antibodies were also useful for immunofluorescence applications. We examined LA-25 cells at permissive (35°C) and non-permissive (40°C) temperatures and found that both antibodies labeled primarily a subset of junctional structures (). All of the pY247 and pY265 antibody (red) labeling overlayed exactly with total Cx43 (green), indicating the specificity of the antibodies for Cx43. Note that pY265 appears to label a larger population of plaques than the pY247 antibody (). In both cases labeling was dramatically diminished at the nonpermissive temperature. These results indicate that the antibodies are specific for Cx43 phosphorylated on tyrosine in immunofluorescence applications.
Figure 2 pY247 and pY265 antibodies recognize Cx43 in a subset of gap junction plaques. Immunofluorescence was performed on LA-25 cells at the permissive (35°C) or nonpermissive temperature (40°C). Large panels show a field of cells labeled with antibody to total Cx43 in green and antibodies to phosphotyrosine 247 (pY247) or 265 (pY265) in red, overlay (Ovl) is seen as yellow. Small panels to the right show a magnified view of boxed areas with individual channels separated to highlight the colocalization of the Cx43 in plaques (magnification bar = 25 μ m).
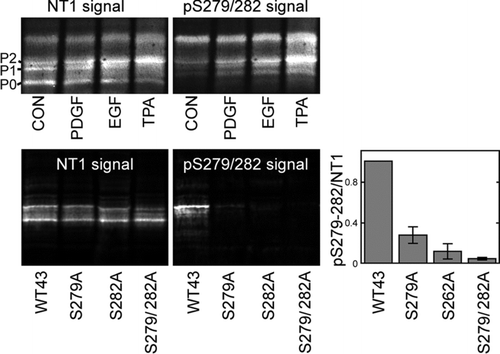
Because there has been some question as to whether src activation might also stimulate other pathways to affect channel closure, particularly in LA-25 cells via the MAPK pathway, we prepared an antibody specific for Cx43 when it is phosphorylated at S279/282. Characterization of this antibody is shown in . In the upper panels, we examined 293T cells transfected with wild-type Cx43 and found that 10-min treatments with PDGF, EGF, or TPA all increased the amount of pS279/282 antibody binding to bands that overlay exactly with bands seen with the NT1 antibody to total Cx43. In particular, pS279/282 showed strong reactivity with the P2 and slower migrating Cx43 isoforms. At this point, we have not identified the modification leading to nor the biological relevance of these higher apparent molecular weight isoforms. Notably, TPA treatment had the most dramatic effect on S279/282 phosphorylation, indicating that MAPK was activated under these conditions. To determine the specificity of the antibody, we transfected MDCK cells with wild-type and S279/282A, S282A, and S279A mutant Cx43 constructs and examined their reactivity with our antibody. We found that none of these mutants reacted near as well with the pS279/282 antibody as the wild type, confirming the specificity of this antibody and indicating that both of these sites are likely involved in antibody binding.
Figure 3 pS279/282 specifically recognizes Cx43 phosphorylated on S279/282 in response to PDGF, EGF, or TPA stimulation. (Upper panel) Immunoblot analysis of 293T cell lysates after 10 min treatment with PDGF, EGF or TPA (all 10 ng/ml). Blots were probed with antibody to total Cx43 (NT1 signal) and pS279/282 (pS279/282 signal). (Lower panel) MDCK cells were transfected with wild-type Cx43 (WT43) or Cx43 containing serine to alanine mutations at S279, S282, or both (S279A, S282A, and S279/282A, respectively). Blots were probed with antibody to total Cx43 (NT1 signal) or pS279/282 (pS279/282 signal). Signals were quantified and ratios of pS279/282 to total Cx43 were calculated (graph, lower panel). Error bars depict standard error and differences between WT43 and all 3 mutants were statistically significant (Student's t test p value < 0.0001, n = 3).
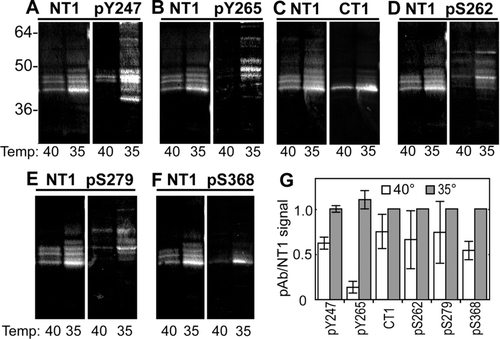
We then examined LA25 cells at permissive and nonpermissive temperature with a panel of phosphospecific antibodies to get an overview of what pathways are activated via v-src. We utilized the pY247, pY265, and pS279/282 antibodies, described here, as well as pS262, and pS368 and CT1 (which recognizes Cx43 specifically not phosphorylated at S364/S365; Sosinsky et al. Citation2007) antibodies all in combination with our antibody to total Cx43 (NT1). We found that all of these antibodies showed increased labeling at 35°C (). We also saw the appearance of very slow migrating isoforms of Cx43, which were particularly well-recognized by pY265. It is interesting to note that unlike pY265 levels, the pY247 signal was not completely abrogated at the nonpermissive temperature (, , and ). It has been previously shown that MAPK can phosphorylate Cx43 on S279/282 (Warn-Cramer et al. Citation1996) and that TPA treatment leads to phosphorylation on S368 (Lampe et al. Citation2000; Solan et al. Citation2003) and S262 (Doble et al. Citation2004), indicating that these pathways are activated in response to active v-src (, , and ). Our results also indicate that at least some of the reduction in communication may be due directly to phosphorylation at S279/282 in LA-25 cells. Furthermore, some could be due to PKC phosphorylation at S368 and src phosphorylation at Y247 and Y265, consistent with the observation that some functional blockage of channel activity still occurs in src expressing cells in the presence of MAPK inhibitors (Zhou et al. Citation1999). We have previously shown that CT1 labels predominantly intracellular Cx43 (Sosinsky et al. Citation2007). The CT1 and pS368 signal are predominately at the P0 position, as previously reported (Sosinsky et al. Citation2007 and Solan et al. 2003, respectively). The pS262 and pS279/282 antibodies are strongly reactive with the P2 form of Cx43 as well as labeling slower migrating forms. The pY antibodies also label slower migrating bands. Note that although several of the antibodies recognize slow migrating forms of Cx43, the pattern of isoforms recognized appear distinct for each phospho-antibody reaffirming their specificity.
Figure 4 Active v-src leads to increased phosphorylation on specific tyrosine and serine residues in Cx43. Immunoblot analysis of LA-25 cells grown at the permissive (35) and nonpermissive (40) temperature. Replicate blots were probed with antibodies to total Cx43 (NT1) and a series of phosphospecific antibodies. (Upper panels) NT1 and phosphotyrosine antibodies pY247 (A) and pY265 (B), CT1, recognizing unmodified S364/365 (C) and phosphorylated S262 (pS262) (D). (Lower panels) NT1 and antibodies recognizing phosphorylated S279/282 (pS279) (E) and S368 (pS368) (F). Signals were quantified and the ratio of phosphospecific antibodies to total Cx43 were calculated (G). Differences between permissive and non-permissive temperature was significant for both the pY247 and pY265 antibodies (Student's t test p value < 0.001, n = 3).
DISCUSSION
The observation that src activity leads to an acute down-regulation of gap junction communication was initially made over 20 years ago (Atkinson et al. Citation1981; Azarnia et al. Citation1988). Since that time several studies have examined the molecular basis for these effects. It has been shown that src can bind to Cx43 and that src kinase activity results in phosphorylation at Y247 and Y265 (Swenson et al. Citation1990; Lin et al. Citation2001). Studies have also shown an increase in serine phosphorylation on Cx43 in response to src activity (Kurata and Lau Citation1994). There are conflicting views regarding the role of these phosphorylation events in gap junction closure. This is likely due to differences in experimental systems and differences in acute versus chronic regulation of Cx43 through src. For example, in experiments utilizing Xenopus oocytes, one group found that coexpression of v-src and Cx43 resulted in dramatic down-regulation of gap junction communication after 18 h (Swenson et al. Citation1990) and that this effect was dependent on Y265. However, more recently, another group performed experiments examining acute regulation by expressing v-src after gap junctions were allowed to form, and in this case, Y265 did not appear to be required for gap junction closure, rather residues involved in SH3 domain binding and MAPK phosphorylation appeared to be required (Zhou et al. Citation1999). In this same study, they also showed that treatment of LA25 cells with the MAPK kinase (MEK) inhibitor PD98059 inhibited most of the apparent v-src effects on Cx43. However, studies utilizing Cx43 KO cells stably transfected with v-src and wild-type Cx43 or site-directed mutants showed no effects of PD98059 or S255/279/282 and a requirement for Y247 and Y265 (Lin et al. Citation2001, Citation2006). These studies all indicate a strong role for src in down-regulating gap junction communication, but indicate that this can occur through different mechanisms.
Thus, there has been extensive analysis of the effects of src using site-directed mutants and deletion constructs to dissect the mechanism of gap junction closure but a consistent picture has not emerged. Whereas one reason for this is likely due to different experimental systems, another reason is likely that although mutants are extremely useful, alteration of primary amino acid sequences can have unforeseen consequences which may affect protein function and protein-protein interactions. This may be especially pertinent in light of the finding that v-src binds to the region of Cx43 (), which is phosphorylated by MAPK (Kanemitsu et al. Citation1997). Phosphospecific antibodies pose an advantage in that one can examine intrinsically expressed Cx43 and directly ascertain the phosphorylation state of specific residues, which may yield some insight as to the structural consequences of these events. This can be extremely useful during acute regulation since one can perform time course experiments to gain an understanding of the kinetics of different phosphorylation events. Phosphospecific antibodies can also be used to look at regulation in vivo via immunohistochemistry and immunoblotting of tissue derived lysates. We have developed many phosphospecific antibodies to both tyrosine and serine residues on Cx43, several of which lie in the domains deemed important in src regulation, as well as an antibody specific for Cx43 not modified on S364/S365. We believe these antibodies will be critical tools in gaining a clear picture of how gap junctions are regulated and how these events are coordinated by different signaling pathways, including MAPK and src.
Figure 5 Model of Cx43 with phosphorylated residues and SH3 binding domains indicated (modified from Lampe and Lau Citation2000). Residues to which phosphospecific antibodies have been made are shown in large bold print. Other serine residues are circled. A SH3 binding domain involved in v-src interaction is noted (Kanemitsu et al. Citation1997).
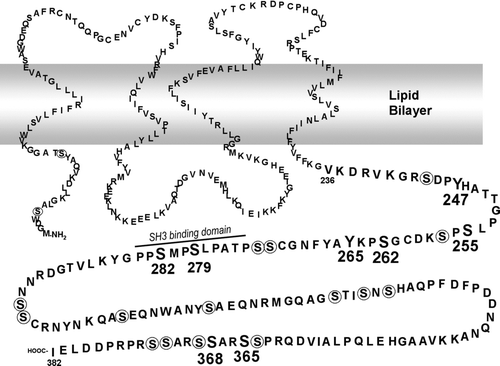
Clearly Cx43 regulation can be complex. In we show that phosphorylation on S279/282 is increased in response to MAPK activation via PDGF or EGF, as expected, but we see the most dramatic increase in response to TPA, a PKC activator. This is noteworthy as there has been widespread interest in the ability of TPA to down-regulate gap junctional communication that has focused primarily on PKC-mediated effects, especially its clear ability to phosphorylate Cx43 on S368 (Lampe et al. Citation2000; Solan et al. 2003). This illustrates the importance of understanding how multiple signaling pathways can affect Cx43 and gap junctional communication. In , we show that v-src activity leads to phosphorylation on Y247 (), Y265 (), S262 (), S279/282 (), and S368 () and leads to a decrease in phosphorylation at S364/365 (as shown by an increase in CT1 signal; ). We show that these phosphotyrosine antibodies work in immunofluorescence and that phosphorylation is occurring on Cx43 in the gap junction plaque (). Because some plaques are entirely green, indicating no phosphotyrosine labeling, whereas others are yellow (due to approximately equal overlay of the total Cx43 antibody [green] and phosphotyrosine antibodies [red] signals), phosphotyrosine is only abundant in a subset of gap junction plaques. If phosphorylation on tyrosines is solely responsible for gap junction closure and abundant phosphorylation is required, these data indicate that some plaques could still be communication competent. There also appears to be differential regulation between pY247 and pY265 as pY265 labels a large proportion of plaques, whereas pY247 labels fewer plaques. This result is consistent with the idea that phosphorylation on Y265 may precede that on Y247 (Lin et al. Citation2001). We also found that pY247 signal, unlike pY265, was not completely inhibited at the nonpermissive temperature (, , and ). One interesting possibility is that phosphorylation on Y247 requires src binding, but not kinase activity, a situation that could occur at the nonpermissive temperature. LA-25 cells were derived from NRK cells several decades ago (Kurth Citation1975). Immunoblots performed with NRK cells show extensive Cx43 expression but no labeling with the pY247 antibody (data not shown), so Y247 phosphorylation does require activated src in some form.
and exemplify the need to analyze the effects of kinase activation and inhibition experiments on Cx43 properties with a panel of phosphospecific antibodies to determine what could be considered a phosphorylation profile prior to making conclusions about the kinase systems and residues involved. Here, we show examples of phosphorylation sites associated with decreased gap junctional communication. We have previously shown that phosphorylation on S325/328/330 (Lampe et al. Citation2006) and S365 (Solan et al. Citation2007) are positively associated with gap junctional communication both in cell culture and in tissue. In this paper, we show that activation of v-src leads to large increases in phosphorylation of several serines and tyrosines known to regulate gap junctional communication. These results help unravel the seemingly disparate results from different studies and lead to a more comprehensive picture of Cx43 regulation.
These studies were supported by grants GM55632 and AR047963 from the National Institutes of Health.
REFERENCES
- Atkinson M M, Menko A S, Johnson R G, Sheppard J R, Sheridan J D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol 1981; 91: 573–578
- Atkinson M M, Sheridan J D. Altered junctional permeability between cells transformed by v-ras, v-mos or v-src. Am J Physiol 1988; 255: C674–C683
- Azarnia R, Reddy S, Kmiecik T E, Shalloway D, Loewenstein W R. The cellular src gene regulates junctional cell-to-cell communication. Science 1988; 239: 398–400
- Bergoffen J, Scherer S S, Wang S, Oronzi Scott M, Bone L J, Paul D L, Chen K, Lensch M W, Chance P F, Fishbeck K H. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 1993; 262: 2039–2042
- Cooper C D, Lampe P D. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem 2002; 277: 44962–44968
- Cottrell G T, Lin R, Warn-Cramer B J, Lau A F, Burt J M. Mechanism of v-Src-and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol 2003; 284: C511–C520
- Crow D S, Kurata W E, Lau A F. Phosphorylation of connexin43 in cells containing mutant src oncogenes. Oncogene 1992; 7: 999–1003
- Doble B W, Dang X, Ping P, Fandrich R R, Nickel B E, Jin Y, Cattini P A, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci 2004; 117: 507–514
- Ek-Vitorin J F, King T J, Heyman N S, Lampe P D, Burt J M. Selectivity of connexin 43 channels is regulated through protein kinase c-dependent phosphorylation. Circ Res 2006; 98: 1498–1505
- Giepmans B N, Hengeveld T, Postma F R, Moolenaar W H. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J Biol Chem 2001; 276: 8544–8549
- Goldberg G S, Lau A F. Dynamics of connexin43 phosphorylation in pp60v-src-transformed cells. Biochem J 1993; 295: 735–742
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar N M, Horwitz J, Gilula N B. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 1997; 91: 833–843
- Jordan K, Solan J L, Dominguez M, Sia M, Hand A, Lampe P D, Laird D W. Trafficking, assembly and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell 1999; 10: 2033–2050
- Kanemitsu M Y, Lau A F. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoyl 13-acetate-sensitive protein kinase C: The possible involvement of mitogen-activated protein kinase. Mol Biol Cell 1993; 4: 837–848
- Kanemitsu M Y, Loo L W, Simon S, Lau A F, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biol Chem 1997; 272: 22824–22831
- Kelsell D P, Dunlop J, Stevens H P, Lench N J, Laing J N, Parry G, Mueller R F, Leigh I M. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997; 387: 80–83
- Kurata W E, Lau A F. p130gag − fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v − src. Oncogene 1994; 9: 329–335
- Kurth R. Differential induction of tumour antigens by transformation-defective virus mutants. J Gen Virol 1975; 28: 167–177
- Lampe P D, Cooper C D, King T J, Burt J M. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci 2006; 119: 3435–42
- Lampe P D, Lau A F. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 2000; 384: 205–215
- Lampe P D, TenBroek E M, Burt J M, Kurata W E, Johnson R G, Lau A F. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 2000; 126: 1503–1512
- Li W, Hertzberg E L, Spray D C. Regulation of connexin43-protein binding in astrocytes in response to chemical ischemia/hypoxia. J Biol Chem 2005; 280: 7941–7948
- Lin R, Martyn K D, Guyette C V, Lau A F, Warn-Cramer B J. v-Src tyrosine phosphorylation of connexin43: Regulation of gap junction communication and effects on cell transformation. Cell Commun Adhes 2006; 13: 199–216
- Lin R, Warn-Cramer B J, Kurata W E, Lau A F. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol 2001; 154: 815–827
- Musil L S, Goodenough D A. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J Cell Biol 1991; 115: 1357–1374
- Paznekas W A, Boyadjiev S A, Shapiro R E, Daniels O, Wollnik B, Keegan C E, Innis J W, Dinulos M B, Christian C, Hannibal M C, Jabs E W. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 2003; 72: 408–418
- Saez J C, Berthoud V M, Branes M C, Martinez A D, Beyer E C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 2003; 83: 1359–1400
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 2004; 62: 228–232
- Solan J L, Fry M D, TenBroek E M, Lampe P D. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci 2003; 116: 2203–2211
- Solan J L, Marquez-Rosado L, Sorgen P L, Thornton P J, Gafken P R, Lampe P D. Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol 2007; 179: 1301–1309
- Sosinsky G E, Giepmans B N, Deerinck T J, Gaietta G M, Ellisman M H. Markers for correlated light and electron microscopy. Methods Cell Biol 2007; 79: 575–591
- Sosinsky G E, Solan J L, Gaietta G M, Ngan L, Lee G J, Mackey M R, Lampe P D. The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem J 2007; 408: 375–385
- Swenson K I, Piwnica-Worms H, McNamee H, Paul D L. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul 1990; 1: 989–1002
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem 2001; 276: 1780–1788
- Warn-Cramer B J, Lampe P D, Kurata W E, Kanemitsu M Y, Loo L WM, Eckhart W, Lau A F. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem 1996; 271: 3779–3786
- White T, Paul D. Genetic diseases and gene knockouts reveal diverse connexin functions. Ann Rev Physiol 1999; 61: 283–310
- Zhou L, Kasperek E M, Nicholson B J. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol 1999; 144: 1033–1045
