Abstract
Neonatal rat cardiomyocytes mainly coexpress the connexins Cx40, Cx43, and to a small amount Cx45, leading to potential formation of mixed (heteromeric/heterotypic) gap junction channels. Using the dual-voltage clamp technique with switching clamp circuits, the authors investigated voltage sensitivity of gap junction channels between cell pairs of Cx40, Cx43, and Cx45 stably transfected HeLa cells and compared those data to data obtained from cell pairs of cultured neonatal rat cardiomyocytes. In accordance to previously published data, the relationship between normalized conductance and transjunctional voltage (g/Vj) was quasisymmetrical for the transfected HeLa cells, indicating homotypic gap junction channels. Boltzmann curves fitted to data obtained from neonatal rat cardiomyocyte pairs expressing both Cx40 and Cx43 showed an asymmetrical inactivation pattern, which cannot be explained by the presence of pure populations of homotypic gap junction channels of either isoform. In conclusion the authors assume the additional presence of heterotypic and possibly even heteromeric gap junction channels in neonatal rat cardiomyocytes.
INTRODUCTION
Cardiac gap junction channels are the basis of intercellular communication in cardiac tissue. Besides cellular metabolic coupling they allow, being a low-resistance pathway, rapid current flow from cell to cell and thus the spontaneous activity of cardiac pacemaker cells, e.g., in the sinoatrial node of the heart, results in a coordinated action of the whole organ, ensuring a synchronous heartbeat as an important condition for life. A change in function, and especially distribution of cardiac gap junction channels, can be found in chronic heart disease and is believed to be involved in the generation and maintenance of arrhythmia.
Gap junction channels are composed of two hexameric connexons. Each cell provides one hemichannel—the so called connexons—that are paired to form a functional gap junction channel. The elemental parts of those connexons are the connexins—proteins with four transmembranic domains, two extracellular loops, one intracellular loop, and intracellular amino and carboxyl termini.
All of the at least 20 known mammalian connexins share this topology. They differ mainly in the length of the carboxyl terminus and are named by their molecular weight in kDa.
In the heart, at least the connexins Cx30.2, Cx37, Cx40, Cx43, and Cx45 are expressed, each with a different contribution pattern and abundance in the different cells of the heart (Severs et al. Citation2001; Duffy et al. Citation2006). Thus Cx43 and Cx40 are the most abundant cardiac connexins. Cx45 is ubiquitously expressed in early development stages, decreases during development and is then specialized to the conduction system, whereas Cx37 as an endothelial connexin is mainly found in vasculature. Cx30.2 has been reported in intercalated disks and vascular smooth muscle cells (Nielsen et al. Citation2003).
An interesting physiological function of gap junction channels is the phenomenon of voltage-dependent inactivation. If gap junction channels are faced with elevated transjunctional voltage differences, the current through the gap junction channels decreases and the initial instantaneous gap junction conductance drops to a steady-state conductance (del Corsso et al. 2006).
The common way to describe this voltage sensitivity is to plot the transjunctional voltage (Vj) against the normalized gap junction conductance and fit the resulting data to a two-state Boltzman curve, providing parameters for half-maximal inactivation and minimal normalized conductance. Those parameters differ among gap junction channels composed of different connexins (Beblo et al. Citation1995; Bruzzone et al. Citation1993; Moreno et al.Citation1995; Ebihara Citation1995; Valiunas et al. Citation2000; Desplantez et al. Citation2007).
At least in transfected cells, such as connexin-transfected HeLa cells, the occurrence of functional heterotypic gap junction channels has been shown. Heterotypic gap junction channels are composed of two different connexons made of different connexins (e.g., connexon 1: Cx40, connexon 2: Cx43), whereas heteromeric gap junction channels are composed of two identical connexons but with more than one contributing connexin (e.g., connexon 1: Cx40 + Cx43, connexon 2: Cx40 + Cx43). The occurrence of heterotypic and heteromeric gap junction channels is not surprising due to the sequence homology of the different connexins. Because cardiomyocytes can coexpress more than one connexin, the formation of heterotypic and perhaps even heteromeric gap junction channels in vivo is imaginable and strongly assumable from the results of our and other studies examining mammalian cells from primary cell culture (Chen and DeHaan Citation1996; Elenes et al. Citation1999; Polontchouk et al. Citation2002).
METHODS
Cell Line and Culture
Connexin-Transfected HeLa Cells
The three different connexin-transfected HeLa cell lines we have used in our study have been kindly provided by Klaus Willecke (Institute for Genetics, Bonn, Germany). Each cell line was stably transfected with only one of the three connexin isoforms Cx40, Cx43, and Cx45. Connexin fragments for mouse Cx40, mouse Cx45, and rat Cx43 were transfected after ligation into a pBEHpac18 vector. Plasmids contained a puromycin resistance (Cx40, Cx43) or zeocin resistance (Cx45) for growth selection (for reference see Traub et al. Citation1994; Elfgang et al. Citation1995).
The cells were cultured in 60-mm Petri dishes by using M199 medium (Sigma, Deisenhofen, Germany), supplemented with 10% fetal calf serum, 200 U/ml penicillin, 200 μ g/ml streptomycin, and 25 mM Hepes (pH adjusted to 7.4 with NaOH). Cells were passaged weekly, diluted 1:6 and kept at 37°C in an incubator (5% CO2/95% ambient air). Twenty-four hours before patch clamp studies were performed, the cells were trypsinized and diluted to reach a sufficient amount of cell pairs. For easy delivery to the patch clamp recording chamber, those cells were seeded on gelatin-coated glass cover slips.
Neonatal Rat Cardiomyocytes
Neonatal rat cardiomyocytes were isolated from ventricles of maximum 24-hours-old Sprague-Dawley rats. Hearts were chopped into small pieces and then digested with collagenase II solution. Cardiomyocytes were centrifuged, preplated in a cell culture growth bottle to reduce noncardiac cells, and then resuspended in M199 medium (M199 with Earle's Salt, GlutaMAX; Gibco) containing 5% fetal calf serum, 10% horse serum, 200 U/ml penicillin, 200 μ g/ml streptomycin, and 25 mM Hepes (pH 7.4 with NaOH). The cells were seeded on glass cover slips in 60-mm Petri dishes coated with 1% gelatine. Medium was changed three times a week and fetal calf serum concentration was reduced to 1% 24 h after isolation. Growth conditions were 5% CO2, 95% ambient air, 37°C. Patch clamp experiments were carried out on days 1 to 3 after isolation.
Immunocytochemistry
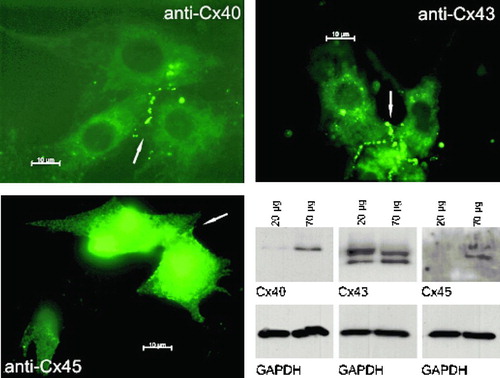
Cells were seeded on cover slips coated with 1% gelatine (50000 cell/cm2), grown for 5 days and then fixed with 4% formalin/phosphate-buffered saline (PBS). They were immunostained using the following primary antibodies: rabbit-anti-Cx40 (1:200; AB1726, Chemicon), rabbit-anti-Cx43 (1:2000; C6219, Sigma-Aldrich, Munich, Germany), rabbit-anti-Cx45 (1:100; AB 1745, Chemicon) and swine-anti-rabbit flurescein isothiocyanate (FITC)-conjugated secondary antibody (1:32; F0205, DAKO, Hamburg, Germany). Cells were viewed with a microscope with fluorescence device and photographed with magnification of 1000× using AxioCam and AxioVision software (both Carl Zeiss MicroImaging, Jena, Germany).
Western Blot Analysis
For Western Blot analysis, 50000 cells/cm2 were seeded on a 6-well-plate and grown for 5 days. Protein extracts were obtained using a lysis puffer containing phosphatase inhibitors (150 mM NaCl, 20 mM Na3PO4, 2 mM MgCl2, 10 mM sodium pyrophosphate, 0.1 mM sodium orthovanadate, 10% TritonX-100, 10% glycerol, 0.1 Nonidet P-40, 10 μ g/ml aprotinin, 10 μ g/ml leupeptin, 10 μ g/ml pepstatin, 10 nM okada acid, 100 μ M phenylarsinoxide, 100 μ M cantharidine). Protein concentration was determined using BCA Protein Assay Kit (Pierce, Rockford, USA). Samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, applying an amount of 20 or 70 μ g protein per slot and transferred by tank-blot using Towbin buffer to polyvinylidene fluoride (PVDF) membrane (Roth, Karlsruhe, Germany). We used the same primary connexin antibodies as for immunocytochemistry, but in higher dilutions (anti-Cx40 1:500; anti-Cx43 1:5000; anti-Cx45 1:1000), mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:10000; 5G4 MAb 6C5, HyTest, Turku, Finland) and peroxidase-conjugated secondary antibodies (goat anti-rabbit, 1:5000, A 0545, Sigma, rabbit anti-mouse, 1:2000, A9044, Sigma) which were detected by Uptilight HRP Blot chemiluminescent substrate (UP99619; Uptima by Interchim, Montluçon cedex, France).
Double-Cell Voltage Clamp
The method for the determination of gap junction conductance via double-cell voltage clamp has been described previously (Metzger and Weingart Citation1985; Muller et al. Citation1999; Weng et al. Citation2002). Thus we only give a brief description of the method here and focus on the assessment of voltage-dependent inactivation of gap junction channels.
For our measurements, we used two synchronized switch clamp amplifiers (SEC05, switching frequency 30 kHz; NPI electronic, Tamm, Germany), which have been shown to overcome series resistance problems due to their discontinuous operation principle and thereby allow precise determination of gap junction currents independent from series resistance with an error < 5% under usual experimental conditions (series/access resistance < 50 MΩ; Muller et al. Citation1999). Data were low-pass filtered at 1 kHz and sampled at 10 kHz by using CellWorks (NPI electronic).
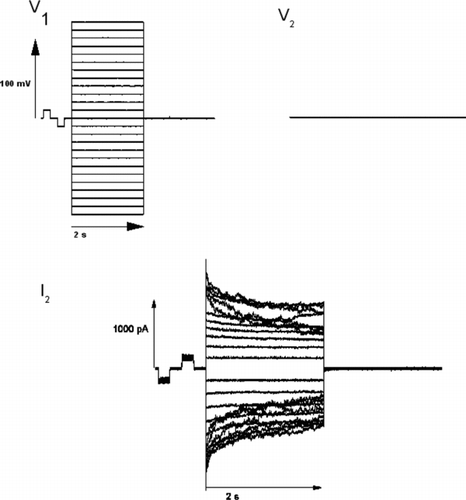
Cover slips with adherent cells were transferred to a 1-ml perfusion chamber mounted on the stage of an inverted microscope (Axiovert; Zeiss). Cells were superfused with Tyrode's solution supplemented with 1 mM BaCl2 to block K+ conductance. On each cell of a pair, whole-cell configuration was established via glass suction pipettes (resistance 3 to 5 MΩ, filled with “intracellular” solution containing amphotericin B [perforated patch method Rae et al. Citation1991], seal resistance > 1 GΩ) after careful pipette capacity compensation. So every cell was connected to and controlled by a separate headstage and amplifier. Each cell was initially clamped to a holding potential of −40 mV. Then one cell was stepped to different test potentials so that the following transjunctional voltage differences between the cell pair were established: −120 to 120 mV (10-mV steps, pulse duration 2 s). A smaller and shorter prepulse was performed right before each 2-s step for later normalization (± 10 mV tranjunctional, 10 ms). The current recorded in the nonpulsed cell (I2) (see ) was taken as the gap junction current. Junctional conductance (Gj) was calculated from Gj = I2/Vj with the transjunctional voltage difference Vj = V2 − V1 as described (Valiunas et al. Citation1997; del Corrso et al. 2006).
Current and voltage traces of both cells were stored to a computer hard disk drive for later data analysis.
Experiments were carried out in the same way for pairs of neonatal rat cardiomyocytes and transfected Cx40, Cx43, and Cx45 HeLa cells.
Solutions
Experiments were carried out in Tyrode's solution with calcium containing (in mM) NaCl 135, KCl 4, CaCl2 2, MgCl2 1, Na2HPO2 0.33, NaH2PO4 1.47, Hepes 10, Glucose 10 pH 7.4 with NaOH at 37°C.
Pipette solution contained (in mM) CsCl 125, Hepes 10, NaCl 8, CaCl2 1, EGTA 10, MgATP 3, Na2ATP 2, Na2GTP 0.1, pH 7.2 with CsOH at 37°C.
Data Analysis
The initial instantaneous gap junction conductance (GJinst, from prepulse) and the steady-state conductance at the end of each voltage step (GJss) were calculated from the recorded current and voltage traces. The normalized conductance (gss/ginst) of each voltage step then was averaged for all examined cell pairs and plotted against the transjunctional voltage difference. Finally the data were fitted to the two-state Boltzmann equation (van Veen et al. Citation2006).where
gmax = maximal normalized conductance (set to one)
gmin = minimal normalized conductance
A = constant = zq/kT (z = number of equivalent electron charges; q = voltage sensor; k = Boltzmann constant; T = temperature)
Vj = transjunctional voltage
V0 = voltage with half maximal inactivation
Data analysis was performed via CellWorks (NPI electronic), MS Excel (Microsoft), and GraphPad Prism (GraphPad Software).
RESULTS
Immunocytochemistry and Western Blot Analysis
Western Blot analysis clearly shows expression of Cx43 in neonatal rat cardiomyocytes () as well as expression of Cx40 and Cx45. The latter ones were only clearly detectable when 70 μ g of total protein amount was used, but there is also a small amount of Cx40 detectable when 20 μ g of total protein amount was applied. Immunocytochemical staining shows integration of connexins Cx40, Cx43, and Cx45 into cell membrane, especially on contact zones of two or more cells (). Both experiments show expression of connexins Cx40, Cx43, and Cx45 and their incorporation into cell membrane.
Voltage-Dependent Inactivation of Gap Junction Channels in Cx40-, Cx43-, and Cx45-Transfected HeLa cells
During the 2-s duration voltage steps, the gap junction channels in connexin-transfected HeLa cell pairs showed the following behavior. For lower transjunctional voltage differences (−20 to 20 mV), the recorded gap junction current remained stable, the gap junction conductance decreased only slightly, and the normalized conductance was close to 1.0. Reaching higher voltage differences, the current started to decrease during the voltage step, from an instantaneous level to a steady-state level, reflecting the voltage-dependent inactivation of the gap junction channels. The instantaneous gap junction conductance dropped to a steady-state conductance that, however, never reached zero. The normalized conductance consequently became lower than 1.0 ().
Figure 2 Original superimposed traces for voltage of the pulsed cell V1, voltage of the nonpulsed cell V2 = −40 mV and current recorded in the non-pulsed cell I2. Junctional conductance (Gj) is calculated as follows: Gj = I2/Vjwith the transjunctional voltage difference Vj = V2 − V1.
As a result of this behavior, the plotted graph of the averaged normalized gap junction conductance of all examined HeLa cell pairs in each group (Cx40, Cx43, and Cx45) against the respective transjunctional voltage difference showed a bell-shaped appearance (, , ). The data could easily be fitted to the above mentioned Boltzmann equation (see Equation 1). Comparing the results of the three different HeLa cell lines (Cx40, Cx43, and Cx45), the resulting inactivation curve for Cx45 was the narrowest, followed by Cx40 and then Cx43. Looking at the data gained from the curve fitting, V0 values as the parameter for half-maximal inactivation were consequently closest to zero for Cx45, followed again by Cx40, and then Cx43 (). Values for gmin reflecting the amount of inactivation were lowest for Cx45 and slightly higher for Cx40, compared to Cx43 in our study ().
Figure 3 (a–c) Summarized data gained from Cx40-, Cx43-, and Cx45-transfected HeLa cells showing dependence of gap junction conductance on junctional potential Vj with a quasisymmetrical relationship for gj (normalized) = f (Vj). Average instantaneous gap junction conductance of each group was (mean ± SEM): HeLa Cx40: Gj = 5.18 ± 1.89 nS, n = 8; HeLa Cx43: Gj = 12.24 ± 2.92 nS, n = 9; HeLa Cx45: Gj = 2.84 ± 1.24, n = 10.
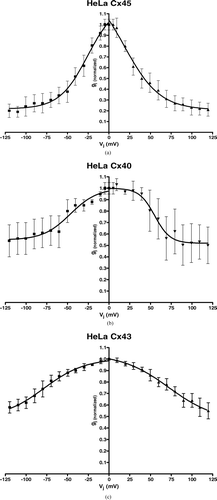
TABLE 1 Inactivation parameters
The shape of each inactivation curve gained from the three different HeLa cell lines transfected with Cx40, Cx43, or Cx45 was quasisymmetrical and gmin values were only slightly different for positive and negative area of the curves.
The average instantaneous gap junction conductance of each group was (mean ± SEM) HeLa Cx40: Gj = 5.18 ± 1.89 nS, n = 8; HeLa Cx43: Gj = 12.24 ± 2.92 nS, n = 9; HeLa Cx45: Gj = 2.84 ± 1.24, n = 10.
Voltage-Dependent Inactivation of Gap Junction Channels in Neonatal Rat Cardiomyocytes
The gap junction channels in neonatal rat cardiomyocytes showed in principle the same behavior as seen in connexin-transfected HeLa cell pairs in our study.
In the neonatal rat cardiomyocytes, the gap junction currents also decreased during the 2-s voltage steps, with elevated voltage gradients across the gap and the instantaneous gap junction conductance decreased to a steady-state conductance.
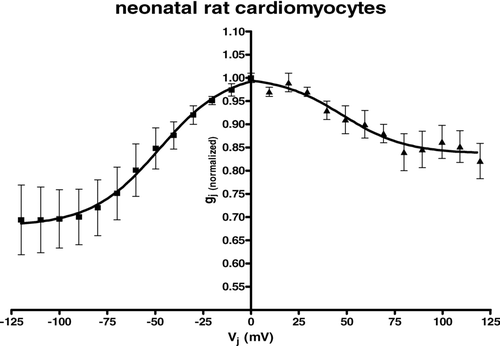
Absolute values for V0 obtained from curve fitting of the averaged data from all examined neonatal rat cardiomyocyte pairs were almost identical for positive and negative Vj. gmin values were higher than those obtained from the different HeLa cell groups. Interestingly enough, the gmin value for the positive area of the curve was > 0.15 higher than for the part of the curve reflecting negative transjunctional voltages (). The resulting curve of the averaged data of all carried out experiments was asymmetrical ().
Figure 4 Summarized data gained from neonatal rat cardiomyocytes showing dependence of gap junction conductance on junctional potential Vj with an asymmetrical relationship for gj(normalized) = f(Vj). Average instantaneous gap junction conductance was (mean ± SEM): Gj = 14.01 ± 2.41 nS, n = 16.
Looking at separate inactivation curves of separate cell pairs, we found not only gap junctions with quasisymmetrical inactivation behavior (), but also cell pairs that showed extreme asymmetry even with rising gap junction conductances at the end of the 2-s steps, with rising transjunctional voltage gradients instead of an inactivation ().
Figure 5 Examples taken from separate recordings of neonatal rat cardiomyocyte cell pairs with (a) quasisymmetrical gating behavior and (b) asymmetrical gating behavior.
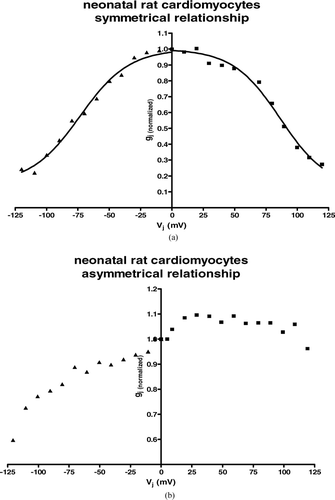
Most cell pairs showed at least a small asymmetry, with an elevated gmin value at the part of the inactivating curve belonging to positive transjunctional voltage gradients.
The average instantaneous gap junction conductance was (mean ± SEM): Gj = 14.01 ± 2.41 nS, n = 16.
DISCUSSION
Cx40-, Cx43-, and Cx45-Transfected Hela Cells Showed Typical Behavior Regarding Voltage-Dependent Inactivation of Gap Junction Channels
Cells expressing solely one connexin isoform, such as the connexin-transfected HeLa cells examined in our study, form homomeric-homotypic gap junction channels. Each hemichannel (connexon) is composed of only one isoform (homomeric) and the gap junction channel is built up out of two identical hemichannel (homotypic). These channels have been studied by many authors (e.g., Bukauskas et al. Citation1995; Moreno et al. Citation1995; Valiunas et al. Citation1997; Desplantez et al. Citation2004). Most of these studies were carried out on connexin-transfected cells.
Thus equilibrium parameters for voltage-dependent inactivation of different homomeric-homotypic channels containing Cx40, Cx43, and Cx45 gained from curve fitting, as has been done in our study, have been published. They differ among laboratories, which could reflect species orthologs, differences in expression systems, experimental technique, as well as the conductance state of the examined cell pairs. It is known from the results of different studies that the phenomenon of voltage-dependent inactivation of gap junction channels best can be observed in low coupled cell pairs. Consequently, some authors work with induced or de novo formed cell pairs (Bukauskas et al. Citation1995) to reach even the single-channel level of gap junctions. Others use uncoupling agents to reduce intercellular conductance to better quantify voltage sensitivity, such as n-heptanol or palmitoleic acid.
All authors come to the same conclusion that voltage sensitivity differs among the various homomeric-homotypic channels and is Cx specific, and all show the same rank order of voltage sensitivity, i.e., Cx45 > Cx40 > Cx43, in accordance with our data (see , , , ). The strongest inactivation is reported for Cx45-Cx45 channels, followed by Cx40, and then Cx43 (Desplantez et al. Citation2007). Stronger inactivation in this context means that the voltage sensitivity appears at lower absolute Vj values, resulting in smaller absolute V0 values. The bell-shaped inactivation curve for Cx45 HeLa cells is narrower than the one for the Cx40-transfected cells and the curve for Cx43 HeLa cell pairs is the widest. If we compare our values for V0 and gmin to the findings of other authors, especially the values for Cx40 and Cx43, the values in our study are slightly higher, but show the same rank order as reported by Moreno et al. (Citation1995), Beblo et al. (Citation1995), and Ebihara (Citation1995).
This might be explained by the higher conductance level of the cell pairs examined in our study. Moreno and coworkers (1994) reported in their study that for pairs of Cx43-transfected SKHep1 cells with gap junctions cord conductance (GJ) ranging from > 2 to < 90 nS, the ratio gmin/gmax was found to be relatively constant, about 0.4 to 0.5, which exactly fits with our data gained from Cx40-and Cx43-transfected HeLa cells.
Because we did not focus on reducing gap junction conductance via uncoupling to the extent needed to investigate single-channel kinetics, we do not give any statement regarding single-channel conductance states in our study.
What we want to stress at this point is one result that all authors have in common looking at inactivation kinetics of homomeric-homotypic gap junction channels: the relationship of the respective inactivation curves was always quasisymmetrical. This is the main result we share with all other authors when looking at homomeric-homotypic gap junction channels, which is the only channel type present in the three different HeLa cell lines investigated in our study.
Voltage Sensitivity of Gap Junctions in Neonatal Rat Cardiomyocytes
Neonatal rat cardiomyocytes coexpress more than one connexin isoform, as we have shown in our Western Blot and immunocytochemistry results (see also Dhein Citation1998, Citation2004, and Salameh et al. Citation2004 for review). Thus they are theoretically able to form homomeric-heterotypic, potentially heteromeric-homotypic, and heteromeric-heterotypic gap junction channels apart from homomeric-homotypic channels.
Heterotypic gap junction channels have been studied by many authors (Valiunas et al. Citation2000; Cottrell, Burt Citation2001; Cottrell et al. Citation2002; Elenes et al. Citation2001; Desplantez et al. Citation2004). All authors describe a nonsymmetrical gating behavior of the different investigated heterotypic channels (Cx40-Cx43, Cx40-Cx45, and Cx43-Cx45). Valiunas et al. (Citation2000), for example, used a coculture of Cx40-HeLa and Cx43-HeLa or Cx43-RIN cells to study the formation of heterotypic gap junction channels between Cx40 and Cx43 via dual-voltage clamp method. They analyzed single-channel currents as well as multichannel currents. Focusing on their multichannel results, they observed a strong asymmetry in the inactivation kinetics of Cx40-Cx43 gap junction channels. The inactivation was more prominent for negative Vj as for positive Vj, resulting in an inactivation curve that has almost exactly the same shape as the one summarizing our findings for neonatal rat cardiomyocytes. Cottrell and Burt (Citation2001) found the same strong asymmetry for Cx40-Cx43 homomeric-heterotypic gap junction channels in coculture of Cx40-and Cx43-transfected RIN cells. The gmin values published by these authors again are smaller than those we found in our experiments. But again this might be reflected by a much higher conductance level between the cell pairs physiologically expressing these connexins (neonatal rat cardiomyocytes) examined in our study. Although it has been discussed that the measurement of voltage sensitivity can be contaminated by series resistance and cannot be detected in cells well coupled by a large number of channels (Jongsma Citation1993), in our measurement system the series resistance is overcome by the electrical circuit provided by the switch clamp system (Muller et al. Citation1999).
Most authors include only cell pairs in their observations with a gap junction cord conductance of < 1 nS, whereas some of our cell pairs reach conductance levels above 14 nS. Thus, most published data regarding the connexin-specific parameters of voltage sensitivity are derived from very low coupled cell pairs recorded with continuous voltage clamp amplifiers, which could be vulnerable for series resistance problems if junctional conductance is too high, requiring extensive offline correction methods, which cannot completely eliminate measurement errors due to series resistance (Wilders and Jongsma Citation1992; Veenstra Citation2001).
This aspect, however, does not interfere with the asymmetrical gating behavior we clearly found in our study examining gap junction channels in neonatal rat cardiomyocytes.
As we demonstrated the expression of Cx45 and its insertion into the membrane, one has also to discuss the occurrence of heterotypic gap junction channels containing Cx45.
Differently from Cx40-Cx43 heterotypic gap junctions, those channels are reported to exhibit also increasing currents with decreasing (Cx40-Cx45; Valiunas et al. Citation2000) or increasing Vj (Cx43-Cx45; Desplantez et al. Citation2004), resulting in a GJss that is higher than the GJinst.
Such voltage sensitivity was not found in our examined cell pairs except from one recording showed in ), which has some similarities with data published by Desplantez et al. (Citation2004), with increasing gj for positive Vjfound in Cx43-Cx45 gap junctions.
In the same study, the authors show some recordings from cell pairs with heteromeric-heterotypic gap junction channels from HeLa cells coexpressing Cx43 and Cx45. They observed quasisymmetrical gating as well as asymmetrical gating.
In any case, one has to keep in mind that Cx45 is first ubiquitously expressed in the heart, decreases throughout development, and then is restricted to the cardiac conduction system (Alcolea et al. Citation1999; Miquerol et al. 2003). In addition, we have demonstrated that the expression level of Cx45 in the neonatal rat cardiomyocytes examined in our study is very low, whereas levels of Cx40 and Cx43 are much higher, confirming data from previous studies of our group (Salameh et al. Citation2006). In conclusion and in comparison to data published by other authors, we postulate that the asymmetry found in the voltage sensitivity of neonatal rat cardiomyocytes examined in our study is due to the presence of heterotypic gap junction channels—homomeric-heterotypic or even heteromeric-heterotypic besides homotypic channels. This statement is supported at least by two studies drawing the same conclusion investigating embryonic chick cardiomyocytes (Chen and DeHaan Citation1996) and ventricular myocytes isolated from adult rat hearts (Polontchouk et al. Citation2002).
Taken together, we showed functional evidence for heterotypic gap junction channels in neonatal rat cardiomyocytes.
REFERENCES
- Alcolea S, Theveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin J P, et al. Downregulation of connexin 45 gene products during mouse heart development. Circ Res 1999; 84: 1365–1379
- Beblo D A, Wang H Z, Beyer E C, Westphale E M, Veenstra R D. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circ Res 1995; 77: 813–822
- Bruzzone R, Haefliger J A, Gimlich R L, Paul D L. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell 1993; 4: 7–20
- Bukauskas F F, Elfgang C, Willecke K, Weingart R. Biophysical properties of gap junction channels formed by mouse connexin40 in induced pairs of transfected human HeLa cells. Biophys J 1995; 68: 2289–2298
- Chen Y H, DeHaan R L. Asymmetric voltage dependence of embryonic cardiac gap junction channels. Am J Physiol 1996; 270: C276–C285, (1 Pt 1)
- Cottrell G T, Burt J M. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol 2001; 281: C1559–C1567
- Cottrell G T, Wu Y, Burt J M. Cx40 and Cx43 expression ratio influences heteromeric/ heterotypic gap junction channel properties. Am J Physiol Cell Physiol 2002; 282: C1469–C1482
- del Corsso C, Srinivas M, Urban-Maldonado M, Moreno A P, Fort A G, Fishman G I, Spray D C. Transfection of mammalian cells with connexins and measurement of voltage sensitivity of their gap junctions. Nat Protoc 2006; 1: 1799–1809
- Desplantez T, Dupont E, Severs N J, Weingart R. Gap junction channels and cardiac impulse propagation. J Membr Biol 2007; 218: 13–28
- Desplantez T, Halliday D, Dupont E, Weingart R. Cardiac connexins Cx43 and Cx45: formation of diverse gap junction channels with diverse electrical properties. Pflugers Arch 2004; 448: 363–375
- Dhein S. Gap junction channels in the cardiovascular system: Pharmacological and physiological modulation. Trends Pharmacol Sci 1998; 19: 229–241, Review
- Dhein S. Pharmacology of gap junctions in the cardiovascular system. Cardiovasc Res 2004; 62: 287–298, Review
- Duffy H S, Fort A G, Spray D C. Cardiac connexins: Genes to nexus. Adv Cardiol 2006; 42: 1–17
- Ebihara L. Expression of dog connexin 40 and 45 in paired Xenopus oocytes. Intercellular Communication Through Gap Junctions. Progress in Cell Research, Vol. 4, Y Kanno, K Kataoka, Y Shiba, Y Shibata, T Shimazu. Elesvier Science, Amsterdam 1995; 395–398
- Elenes S, Rubart M, Moreno A P. Junctional communication between isolated pairs of canine atrial cells is mediated by homogeneous and heterogeneous gap junction channels. J Cardiovasc Electrophysiol 1999; 10: 990–1004
- Elenes S, Martinez A D, Delmar M, Beyer E C, Moreno A P. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys J 2001; 81: 1406–1418
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein R A, Hülser D F, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 1995; 129: 805–817
- Jongsma H J, Wilders R, Takens-Kwak B R, Rook M B. Are cardiac gap junctions voltage sensitive?. Progress in Cell Research, Volume 3: Gap Junctions, J E Hall, G A Zampighi, R M Davis. Elsevier Science Publishers, Amsterdam 1993; 187–192
- Metzger P, Weingart R. Electric current flow in cell pairs isolated from adult rat hearts. J Physiol 1985; 366: 177–195
- Miquerol L, Dupays L, Theveniau-Ruissy M, Alcolea S, Jarry-Guichard T, Abran P, Gros D. Gap junctional connexins in the developing mouse cardiac conduction system. Novartis Found Symp 2003; 250: 80–98, discussion 98–109, 276–279. Review
- Moreno A P, Laing J G, Beyer E C, Spray D C. Properties of gap junction channels formed of connexin 45 endogenously expressed in human hepatoma (SKHep1) cells. Am J Physiol 1995; 268: C356–C365, (2 Pt 1)
- Moreno A P, Rook M B, Fishman G I, Spray D C. Gap junction channels: Distinct voltage-sensitive and-insensitive conductance states. Biophys J 1994; 67: 113–119
- Muller A, Lauven M, Berkels R, Dhein S, Polder H R, Klaus W. Switched single-electrode voltage-clamp amplifiers allow precise measurement of gap junction conductance. Am J Physiol 1999; 276: C980–C987, (4 Pt 1)
- Nielsen P A, Kumar N M. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett 2003; 540: 151–156
- Polontchouk L O, Valiunas V, Haefliger J A, Eppenberger H M, Weingart R. Expression and regulation of connexins in cultured ventricular myocytes isolated from adult rat hearts. Pflugers Arch 2002; 443: 676–689
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods 1991; 37: 15–26
- Salameh A, Frenzel C, Boldt A, Rassler B, Glawe I, Schulte J, Muhlberg K, Zimmer H G, Pfeiffer D, Dhein S. Subchronic alpha-and beta-adrenergic regulation of cardiac gap junction protein expression. FASEB J 2006; 20: 365–367
- Salameh A, Schneider P, Muhlberg K, Hagendorff A, Dhein S, Pfeiffer D. Chronic regulation of the expression of gap junction proteins connexin40, connexin43, and connexin45 in neonatal rat cardiomyocytes. Eur J Pharmacol 2004; 503: 9–16
- Severs N J, Rothery S, Dupont E, Coppen S R, Yeh H I, Ko Y S, et al. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech 2001; 52: 301–322, Review
- Traub O, Eckert R, Lichtenberg-Fraté H, Elfgang C, Bastide B, Scheidtmann K H, Hülser D F, Willecke K. Immunochemical and electrophysiological characterization of murine connexin40 and-43 in mouse tissues and transfected human cells. Eur J Cell Biol 1994; 64: 101–112
- Valiunas V, Bukauskas F F, Weingart R. Conductances and selective permeability of connexin43 gap junction channels examined in neonatal rat heart cells. Circ Res 1997; 80: 708–719
- Valiunas V, Weingart R, Brink P R. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res 2000; 86: E42–E49
- van Veen T A, van Rijen H V, Jongsma H J. Physiology of cardiovascular gap junctions. Adv Cardiol 2006; 42: 18–40, Review
- Veenstra R D. Voltage clamp limitations of dual whole-cell gap junction current and voltage recordings. I. Conductance measurements. Biophys J 2001; 80: 2231–2247
- Weng S, Lauven M, Schaefer T, Polontchouk L, Grover R, Dhein S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J 2002; 16: 1114–1116
- Wilders R, Jongsma H J. Limitations of the dual voltage clamp method in assaying conductance and kinetics of gap junction channels. Biophys J 1992; 63: 942–953