Abstract
Intermittent administration stimulates bone formation, whereas sustained elevation of parathyroid hormone (PTH) as in hyperparathyroidism stimulates bone resorption. Even though PTH(1-34) is the only anabolic agent clinically approved for the treatment of osteoporosis, the molecular mechanism whereby PTH mediates these opposing effects depending on timing of administration is not well understood. In this study, we sought to determine the involvement of gap junctions and hemichannels, and the protein that forms them, connexin 43 (Cx43), in the effect of PTH(1-34) on osteoblast mineralization. The osteoblast-like cell line MLO-A5 that rapidly mineralizes in culture was used. Intermittent PTH enhances mineralization, whereas continuous PTH inhibits this process. The mineralization was significantly inhibited by 18 β-glycyrrhetinic acid, an inhibitor known to block gap junctions and hemichannels. When the cells were treated with PTH(1-34), gap junctional coupling was increased; however, the degree of stimulation was similar between intermittent and continuous treatment. The permeabilization to dye was not detected under various intermittent or continuous PTH treatments. On the other hand, the overall level of Cx43 protein increased in response to continuous PTH treatment. In contrast, when the cells were subjected to intermittent treatment overall level of Cx43 was unchanged, but there was an increase of connexons associated with an increase in Cx43 expression on the cell surface. Our results suggest that Cx43 overall expression, connexon formation and cell surface expression are differentially regulated by intermittent and continuous PTH(1-34), implying the involvement of Cx43 and Cx43-forming channels in mediating the effects of PTH on bone formation.
INTRODUCTION
PTH and its active peptide, PTH(1-34), have been known to play important roles in regulating bone remodeling through initiation of bone formation as well as activation of bone resorption (Fitzpatrick and Bilezikian Citation1996). Intermittent administration of PTH has been reported to stimulate bone formation; in contrast, continuous treatment promotes bone resorption (Hock et al. Citation2002; Rubin et al. Citation2002). The anabolic function of PTH is reported to occur through the activation of survival signaling in osteoblasts by delaying osteoblast osteoporosis, which contributes to an increase in cell numbers (Jilka, Citation2007). PTH has been approved and used clinically as the only anabolic agent for the treatment of osteoporosis and fractures (Neer et al. Citation2001); however, the molecular mechanism, especially, the opposite effects exerted by intermittent versus continuous regimens of PTH treatment remain largely uncharacterized.
Gap junctions are transmembrane channels which connect the cytoplasm of two adjacent cells. These channels permit small molecules, such as small metabolites, ions, and intracellular signaling molecules (i.e. calcium, cAMP, inositol triphosphate) to pass between cells and have been demonstrated to be important in modulating cell and tissue functions in many organs (Goodenough et al. Citation1996). Gap junction channels are formed by members of a family of related proteins known as connexins. Six connexin monomers assemble into a connexon in one cell and connect to a connexon from another cell to form a whole gap junction channel. The morphological proof of the existence of gap junction structures has been obtained for osteoblasts and osteocytes (Palumbo et al. Citation1990; Doty, Citation1981). Cx43 is the major connexin expressed in cultured primary osteoblasts (Schirrmacher et al. Citation1992), primary osteocytes in vivo (Edelson, Citation1990) and in the osteoblastic and osteocytic cell lines (Chiba et al. Citation1993; Steinberg et al. Citation1994; Cheng et al. Citation2001; Thi et al. Citation2003). It has been found that treatment with PTH increased gap junction mediated cell coupling and Cx43 expression in several osteoblastic cell lines (Schiller et al. Citation1992; Van der Molen et al. Citation1996b; Donahue et al. Citation1995). The increase of Cx43 expression by PTH was a result of upregulation through activation of Cx43 DNA promoter (Civitelli et al. Citation1998), and a PTH responsive element was identified on Cx43 mRNA (Mitchell et al. Citation2001). The increase of Cx43 expression by PTH is dependent upon cell type and developmental state of the cell (Schiller et al. Citation1997). More importantly, a recent study showed that mice with an osteoblast-specific deletion of Cx43 attenuated anabolic effect of intermittent PTH on bone formation (Chung et al. Citation2006), suggesting an important role of Cx43 in mediating the anabolic action of intermittent PTH.
To investigate the molecular mechanism of gap junctions/hemichannels and Cx43 in bone mineralization and formation in response to intermittent and continuous treatment of PTH, we adopted an osteoblast-like MLO-A5 cell line as our model. This cell line displays features of spontaneous, rapid mineralization, which forms bone-like structures and chemical components remarkably similar to bone tissues in vivo (Kato et al., Citation2001). Consistently, we found that the different effects of intermittent and continuous PTH treatment on mineralization observed in vivo were also reproduced in this cell line. The mineralization process shown with intermittent PTH treatment and in control cultures was inhibited by a gap junction/hemichannel inhibitor. Moreover, we show that gap junctions, but not hemichannel opening, were increased by both intermittent and continuous PTH treatment. Importantly, Cx43 expression and connexon formation were differentially regulated by intermittent and continuous PTH.
MATERIALS AND METHODS
Materials
Tissue culture medium and protein standards were purchased from Invitrogen (Carlsbad, CA); fetal bovine serum (FBS) and calf serum (CS) from Hyclone Laboratories (Logan, UT); rhodamine dextran (RD) (Mr: 10 kDa) and LY (Mr: 547 Da) from Molecular Probes (Eugene, OR); paraformaldehyde (16% stock solution) from Electron Microscopy Science (Fort Washington, PA); nitrocellulose membrane from Schleicher & Schuell (Keene, NH); rat tail collagen type I, 99% pure from Becton Dickinson Laboratories (Bedford, MA); polyester sheets from Regal Plastics (San Antonio, TX); rabbit anti-C/EBP homologous protein (CHOP) antibody, Santa Cruz Biotechnology (Santa Cruz, CA); thapsigargin from CalBiochemical (La Jolla, CA); EZ-link™ Sulfo-NHS-LC-Biotin, avidin bead and BCA micro-protein assay kit, Pierce Chemical (Rockford, IL); Chemiluminescence kit, ECL, Amersham (Piscataway, NJ); X-OMAT AR film, Eastman Kodak (Rochester, NY). Rabbit anti-Cx43 antiserum was generated and affinity purified based on our previously published protocol (He et al. Citation1999). MLO-A5 cell line was a generous gift from Dr. Lynda Bonewald at the University of Missouri, School of Dentistry. All other reagents were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Cell Culture, von Kossa Staining and Quantitation
MLO-A5 cells were cultured on collagen-coated (rat tail collagen type I, 0.15 mg/ml) surfaces. Cells were grown in alpha modified essential medium (α-MEM) supplemented with 5% FBS and 5% CS, and incubated in 5% CO2 incubator at 37°C as previously described (Kato et al., Citation2001).
For von Kossa staining, the cells, 5 × 104, were cultured in 12-well tissue culture plates (2.8 × 104/cm2) for up to 14 days with α-MEM supplemented with 5% FCS/5% CS, 5 mM β-glycerol phosphate and 100 μ g/ml ascorbic acid. At the third day after plating of the cell, for continuous treatment, cells were treated with PTH(1-34) continuously and then media were changed every 48 h containing fresh PTH(1-34). For intermittent treatment, the cells were treated with PTH(1-34) for 4 h every 48 h. At the end of the required days in culture, the cells were rinsed with PBS and fixed with 10% buffered formalin for 10 min and then von Kossa stained as described (Harris et al., Citation1994; Kato et al., Citation2001). The intensity of von Kossa staining was quantified using ImageJ software (National Institute of Health (NIH)).
Dye-Transfer Assay for Gap Junction Intercellular Communication
The “scrape-loading” dye-transfer assay was modified based on a published procedure (El-Fouly et al., Citation1987; Rubin et al., Citation2002). In this method, cells were scratched in the presence of two fluorescence dyes: rhodamine dextran (RD, Mr:10 kDa) and LY (Mr: 372 Da). RD is too large to pass through gap junction channels, therefore serving as a tracer dye for the cells originally receiving the dye. MLO-A5 cells were washed 3 times with Hank's Balanced Salt Solution (HBSS) plus 1% BSA for 5 min each. Then 1% RD and 1% LY dissolved in PBS were applied to the cells that were subsequently scraped lightly with a 26 gauge needle. After incubation for 10 min, cells were washed with HBSS 3 times, then twice with PBS, and finally fixed in fresh 2% paraformaldehyde (from 16% stock) for 20 min. The dye-transfer results were examined using a fluorescence microscope in which LY could be detected using the filter set for fluorescein, and RD could be detected using the filter set for rhodamine. The number was determined by manually counting the numbers of RD-receiving cells (> 500), which are connected to their neighboring cells, as monitored by rhodamine fluorescence divided by number of non-disrupted primary cells receiving LY. The percentage of dye-transfer was calculated.
Dye Uptake Assay
MLO-A5 cells were grown to ensure that the majority of the cells were not physically in contact. LY was used as a tracer for hemichannel activity, and rhodamine dextran (RD) (Mr 10 kDa) was used as a negative control. Cells maintained in normal or Ca2 +-free MEM were exposed to 10− 8M PTH(1-34) intermittently or continuously for 48 h as described for dye transfer assay or treated for short periods of time including 1 min, 5 min, 15 min, 30 min, 2 h, 4 h or concurrent PTH treatment in the absence or presence of 5 mM EGTA. For control of dye uptake, LY/RD was directly dropped at the top of the cell by pipetting. Cells were washed with medium containing 1.8 mM Ca2 + and then fixed with 1% paraformaldehyde. Similar fields were observed under the fluorescence microscope and over 500 cells were counted for each experiment. Dye uptake was presented as a percentage of LY fluorescent cells.
Western Blotting
Lysates of MLO-A5 cells from various treatments were prepared and crude membranes were prepared as previously described (Musil et al., Citation1990). The protein concentration of crude membrane samples of MLO-A5 cells was determined using the Micro-BCA assay according to the manufacturer's instruction. Membranes were incubated with our affinity-purified anti-Cx43 antibody (1:250 dilution), monoclonal anti-β-actin antibody (1:5000 dilution) or anti-CHOP antibody (1:1000 dilution) and Western blotting was performed. The primary antibody was detected using peroxidase-conjugated secondary anti-rabbit or anti-mouse IgG followed by use of a chemiluminescence reagent kit (ECL). The intensity of Cx43 bands was quantified by densitometry (NIH imageJ).
Immunofluorescence Labeling and Fluorescence Microscopy
The cells cultured on the cover slides were washed three times with PBS, each wash of 5 min duration. This was followed by fixation in 2% paraformaldehyde in PBS for 30 min at room temperature after which the cells were washed three times with PBS for 5 min each, followed by incubation for 30 min in blocking solution containing 2% normal goat serum, 2% fish skin gelatin, and 1% BSA in PBS, and then incubated for 1 h at room temperature with an affinity-purified anti-Cx43 antibody diluted 1:500 in blocking solution. Cells were washed three times; 5 min for each wash with PBS and then incubated for 1 h with fluorescein-conjugated goat anti-rabbit IgG diluted 1:250 in blocking solution. Fluorescence microscopy was performed using an Olympus B-MAX microscope (Olympus, Tokyo, Japan) and recorded on a “Spot II” digital camera (Diagnostic Instruments, Tokyo, Japan). For dye uptake results, LY was detected using the filter set for fluorescein, and RD using the filter set for rhodamine. To reach statistical significance, over 500 cells were counted for each assay.
Sucrose Gradient Sedimentation
Isolated crude membranes were solubilized in 1% Triton-X-100 and fractioned on a 5–20% linear sucrose gradient as previous described (Jiang and Goodenough, Citation1996). Centrifugation was performed in a Beckman SW60 Ti rotor at 49,000 rpm for 12 h at 4°C, after which 200-μ l fractions were collected. Each fraction loaded on SDS-PAGE was immunoblotted with anti-Cx43 antibody and band intensity was quantified using densitometry (ImageJ, NIH).
Cell Surface Biotinylation
Biotinylation of cell monolayers was performed based on a modification of a procedure that was described previously (Daniels and Amara, Citation1998). MLO-A5 cells were labeled with 1 mg/ml EZ-link™ Sulfo-NHS-LC-Biotin in PBS at 4°C for 20 min. The cells were washed 3 times with PBS containing 100 mM glycine and lysed in lysis buffer (133 mM NaCl, 5 mM KCl, 1% dextrose, 21 mM HEPES) plus 1% Na deoxycholate, 1% Triton-X-100, 0.1% SDS, and proteases inhibitors. Cell lysates were mixed with equal volumes of monomeric avidin beads and incubated for 60 min at room temperature. The beads were then washed several times with PBS until no proteins could be detected by measurement of spectrometric absorbance at 280 nm. The biotinlyated proteins were eluted by boiling for 5 min in sample loading buffer containing 1% SDS and 2% 2-mercaptoethanol and equal volumes of each sample was loaded on SDS-PAGE and analyzed by Western blotting using affinity-purified antibody Cx43 antibody.
Statistical Analysis
Data was analyzed using the one-way ANOVA and Student-Newman-Keuls multiple comparison test with the Instat biostatistic program (GraphPad software) and presented as the mean ± SEM of three determinations. In the figures, asterisks indicate the degree of significant differences (**, ρ < 0.01 and ***, ρ < 0.001).
RESULTS
The in vivo Bone Formation in Response to PTH(1-34) was Reproduced in MLO-A5 Cells, and Gap Junction and Hemichannel Inhibitor Attenuated the Bone Nodule Formation
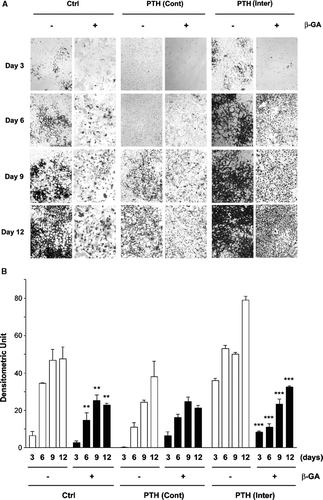
Previous reports from in vivo studies have shown opposite effects of intermittent and continuous effects of PTH on bone formation. To establish MLO-A5 cells as a cell model for studying the underlying molecular mechanism of the effects of PTH on bone mineralization, we treated MLO-A5 cells with PTH(1-34) intermittently (4 h exposure for every 48 h) or continuously for up to 12 days and mineralization was assayed by von Kossa staining (). Starting from day 3 and up to day 12, MLO-A5 cells consistently stained most strongly for the cells under intermittent PTH treatment (PTH (inter)) exhibiting typical “honeycomb”-like morphology (Kato et al., Citation2001), and similar increase over time, though to lesser extent was seen in non-treatment control (Ctrl) cultures (). In contrast, continuous PTH (PTH (Cont)) treatment showed the weakest and dystrophic staining. 18 β-glycyrrhetinic acid (β-GA) treatment inhibited the mineralization process. The degrees of mineralization and inhibition by β-GA were quantified by densitometry (ImageJ software, NIH) (). β-GA significantly reduced mineralization in control and intermittent PTH(1-34) treated cells, although the extent of the decrease appeared to be even greater in intermittent PTH treatment. We also attempted another gap junction/hemichannel inhibitor, carbenoxolone, but this inhibitor was somehow toxic to the cell when cultured for a long time required for the mineralization assay (data not shown). Together, these results suggest that similar to the in vivo observations, intermittent PTH(1-34) treatment promotes mineralization whereas continuous application attenuates this process. Moreover, treatment of cultures with a gap junction and hemichannel inhibitor reduces mineralization under control as well as PTH treatment.
Figure 1 Effect of intermittent and continuous PTH(1-34), and/or β-GA on MLO-A5 cell mineralization. (A) At the third day after cell plating, MLO-A5 cells were treated with 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h every 48 h in the presence (+) or absence (−) of β-GA. After 3, 6, 9, and 12 days of culturing, the cells were fixed and mineralization was analyzed by von Kossa staining. (B) The degree of mineralization was quantified by densitometry (ImageJ, NIH). Non-β-GA (-) verse β-GA (+) samples with corresponding treatment regimes: **, ρ < 0.01 and ***, ρ < 0.001. The data are presented as the mean ± SEM and n = 3.
Stimulation of Gap Junction Intercellular Coupling either with Intermittent or Continuous Treatment with PTH(1-34)
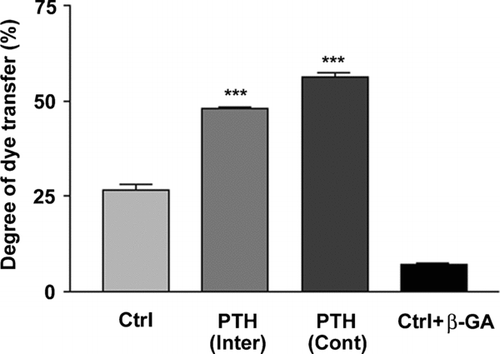
“Scrape loading” dye transfer analysis was conducted to examine the effects of PTH(1-34) on gap junction intercellular coupling. Both intermittent and continuous PTH(1-34) treatments significantly stimulated intercellular coupling detected by LY dye transfer (). Although the transfer level was slightly higher in continuous PTH-treated cells, there was no significant difference as compared to intermittent PTH treatment. These results suggest that both intermittent and continuous PTH(1-34) treatments exert similar stimulatory effect on gap junction coupling in MLO-A5 cells while these treatments have profoundly different effects on mineralization, implying that there is no tight control of mineralization by junctional coupling.
Figure 2 Upregulation of intercellular coupling observed in both intermittent and continuous treatments with PTH(1-34). MLO-A5 cells were treated in the absence or presence of 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h for a total of 48 h or were incubated with gap junction inhibitor β-GA. Intercellular coupling between cells was analyzed using the scrape-loading dye transfer assay. Lucifer yellow (LY) was used as a fluorescent tracing molecule for gap junctional coupling and rhodamine dextran (RD) serves as a marker for dye-loaded cells. The percentage of dye transfer was referred to as the percentage of RD-receiving cells that form gap junctions. The degree of dye transfer in intermittent (Inter) or continuous (Cont) PTH treated versus non-treated (Ctrl) or β-GA treated cells, ***ρ < 0.001. The data are presented as mean ± SEM and n = 3.
No Induction of the Opening of Hemichannels by PTH(1-34)
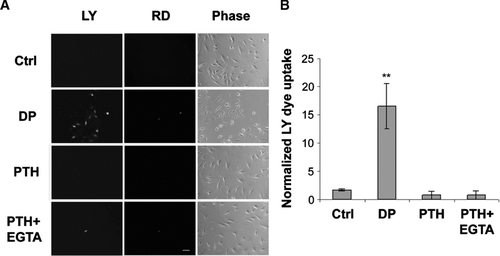
In addition to gap junctions, Cx43 also forms hemichannels in bone cells. To determine if connexin-forming hemichannel function is responsive to PTH(1-34) treatment, a dye uptake assay was conducted (). Similar to osteocyte-like cells (Cherian et al., Citation2005), mechanical stimulation by dye dropping (DP) induced the opening of hemichannels. Application of PTH(1-34) failed to induce the opening of hemichannels detected by LY dye uptake analysis. Treatment with EGTA, a Ca2 + chelator known to induce hemichannel opening in some cells, plus PTH(1-34) (PTH + EGTA) also did not result in the opening of hemichannels. Quantitative analysis further confirmed the dye uptake observation (). We also did not detect hemichannel activities using the calcein dye release assay with live cell imaging (data not shown). In addition, PTH(1-34) exposure under various time regimes including the same intermittent and continuous treatment used in the mineralization assay, short treatment for 1 min, 5 min, 15 min, 30 min, 2 h, 4 h or concurrent treatment with PTH failed to induce dye uptake (data not shown). Moreover, continuous or intermittent treatment had no additive effect on dye uptake induced by the “dye dropping” assay. Together, these data suggest that application of PTH(1-34) did not lead to hemichannel opening detected by dye uptake and release analyses.
Figure 3 No effect of PTH(1-34) on hemichannel dye uptake. (A) MLO-A5 cells cultured at low cell density without cell-cell contact were treated in the absence (Ctrl) or presence of 1 × 10− 8 M PTH(1-34) (PTH) or PTH(1-34) plus 5 mM EGTA (PTH + EGTA) for 30 min and dye uptake was conducted with LY/RD. For dropping (DP) analysis, LY/RD dyes were directly delivered to the cell by pipetting. bar: 100 μ m. (B) The numbers of MLO-A5 cells with dye uptake in the absence or presence of PTH(1-34), PTH(1-34) plus EGTA or dropping were counted and quantified. Ctrl, PTH or PTH+EGTA versus DP: **ρ < 0.01. The data are presented as mean ± SEM and n = 3.
Continuous, but not Intermittent, Treatment with PTH(1-34) Increased Overall Cx43 Levels and Retention of Cx43 Inside the Cell
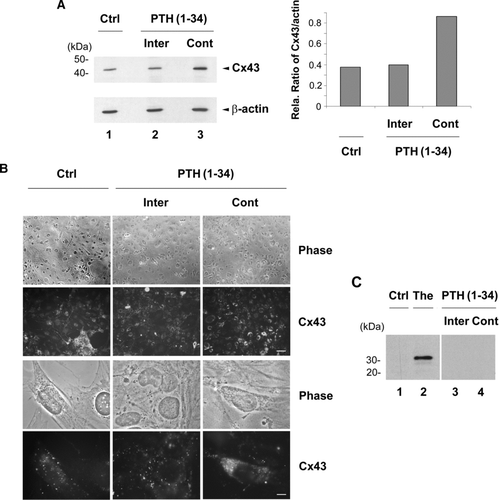
The expression and cellular distribution of Cx43 were examined in response to intermittent and continuous PTH(1-34) treatment (, lanes 2 and 3 respectively). As compared to non-treated control (Ctrl) (, lane 1) and the levels of β-actin, continuous treatment enhanced expression of Cx43 levels relative to β-actin (lane 3), while no increase was detected in cells treated with intermittent PTH(1-34) (lane 2). This observation was further confirmed by densitometry and the ratio of Cx43 vs β-actin (, right panel). The increased expression of Cx43 by continuous PTH(1-34) (Cont) was also consistently observed by immunofluorescence analysis (). Unlike punctate expression pattern of untreated control and intermittent (Inter) PTH treated cells, continuous PTH treatment resulted in the retention of the majority of Cx43 protein inside the cell. To explore the possibly that continuous PTH leads to ER stress which subsequently resulted in accumulation of Cx43 inside the cell, we examined the levels of ER stress marker protein, transcription factor CHOP, by Western blots (). CHOP protein was detected in thapsigargin (The), the ER stress inducer, treated cells (, lane 2). However, no expression of CHOP was detected in non-treated (lane 1), intermittent (lane 3) or continuous (lane 4) treated MLO-A5 cells, suggesting that continuous treatment of PTH(1-34) does not lead to the elevation with ER stress, and thereby the accumulation of Cx43 inside the cell was not a consequence of ER stress.
Figure 4 Cx43 expression and intracellular distribution in MLO-A5 cells in response to intermittent and continuous treatment with PTH(1-34). MLO-A5 cells were treated with 1 × 10− 8M PTH(1-34) either continuously (Cont) for 48 h or intermittently (Inter) for 4 h per 48 h culture or not treated for 48 h (Ctrl). (A) The above treated cells were lysed, and crude membranes were isolated and immunoblotted with affinity-purified anti-Cx43 or anti-β-actin antibody. The protein band intensity was measured by densitometry and the intensity ratio of Cx43 versus β-actin was calculated (right panel). (B) The above treated cells were immunolabeled with affinity purified anti-Cx43 antibody followed by FITC-conjugated goat anti-rabbit secondary antibody. Upper panels, low magnification; bar: 100 μ m. Lower panels, high magnification; bar: 10 μ m. (C) Cells were treated in the absence (Ctrl, lane 1) or presence of PTH(1-34) intermittently (lane 3) or continuously (lane 4) or with 1 μ M thapsigargin (The) (lane 2). The cell lysates were immunoblotted with anti-CHOP antibody.
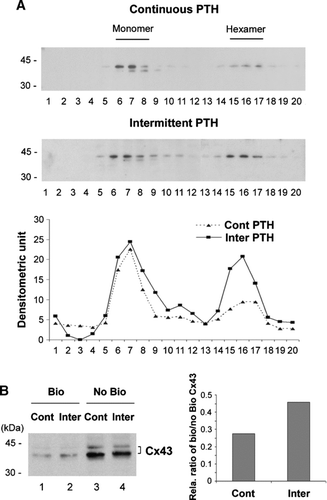
Intermittent Treatment with PTH(1-34) Stimulated Cx43 Connexon Formation
Prior to formation of gap junction and/or hemichannels, Cx43 protein monomers have to be assembled into hexamers, called connexons. Cx43 connexons can be separated from monomers by sucrose sedimentation gradient (Musil and Goodenough, Citation1991). Compared to continuous PTH(1-34), intermittent treatment increased connexon formation with the migration coefficient close to 9S, corresponding to the size of hexamer connexon (, upper panel). The densitometric measurement of band intensity further confirmed the observation of Western blots (, lower panel). Cell surface expression of Cx43 was assessed by determining the amount of Cx43 that can be biotinylated due to exposure on the cell surface (). Intermittent exposure of PTH(1-34) (, lane 2) in cells increased surface expressed Cx43 as compared to continuous treatment (lane 1), which was confirmed by densitometry and the ratio of biotinylated verse non-biotinylated Cx43 (, right panel). This observation is correlated with increased formation of connexons in response to intermittent PTH(1-34).
Figure 5 Intermittent PTH promoted connexon formation and surface expression of Cx43. (A) Sucrose gradient analysis of Cx43 connexons isolated from PTH(1-34) treated cells. MLO-A5 cells were treated with PTH(1-34) either continuously or intermittently for 48 h. The crude membranes were solubilized by 1% Triton-X-100 and separated by centrifugation, and supernatants were fractionated on a linear gradient of 5–20% sucrose. The collected fractions were immunoblotted with affinity purified anti-Cx43 antibody. The protein band intensity from sucrose gradient fractions of continuous and intermittent PTH treated samples was measured by densitometry (lower panel) (n = 3). (B) After the treatment with continuous (lanes 1 and 3) or intermittent (Inter) (lanes 2 and 4) PTH(1-34), cells were treated with biotin. The cells were lysed and equal amounts of total protein were applied to avidin-conjugated beads to isolate biotin-labeled proteins. The biotinylated (Bio) Cx43 that bound to avidin beads (lanes 1 and 2) and non-biotinylated (No Bio) Cx43 that flew through the beads (lanes 3 and 4) were detected by Western blots by using anti-Cx43 antibody. The protein band intensity was measured by densitometry and the intensity ratio of biotinylated (Bio) verse non-biotinylated (No Bio) Cx43 was calculated (right panel).
DISCUSSION
In this study, we show that intermittent application of PTH(1-34) promoted bone mineralization in MLO-A5 cells, whereas continuous exposure attenuated this process. Gap junction intercellular coupling was significantly increased in response to both intermittent and continuous PTH; however, hemichannel activity was not detected by either treatment. Interestingly, continuous but not intermittent, PTH stimulated overall Cx43 levels with the retention of Cx43 inside the cell. In contrast, intermittent PTH promoted formation of Cx43 connexons, which was correlated with the increased cell surface expression of Cx43. These data suggest that Cx43 is differentially regulated by treatment regimens of PTH, providing further implication of the involvement of Cx43 and its forming channels in the effects of PTH on bone mineralization and formation.
Consistent with the observation in vivo, the mineralization in response to intermittent and continuous regimens was closely reproduced in cultured MLO-A5 cells. Intermittent application of PTH has a strong anabolic effect on bone nodule formation, whereas continuous exposure had a catabolic effect. These data further support the establishment of MLO-A5 cells as a potential cell model for studying mechanisms of bone mineralization and PTH action. The impaired effect of continuous PTH(1-34) on mineralization has been proposed to be caused by the desensitization of cAMP-mediated cell signaling activated by PTH (Bergwitz et al., Citation1994). However, in our study, we found that Cx43 expression was elevated by continuous PTH treatment, implying the persistent activation of the signaling cascade since Cx43 like other connexins has a very short half life (Koval, Citation2006) and activation of cAMP pathway leads to an increase in Cx43 expression and gap junction communication in bone cells (Civitelli et al. Citation1998; Cherian et al. Citation2003). It is true that unlike the in vivo situation, PTH may not be dramatically degraded after addition to cultured cells, which may remain active for a relatively longer period of time (Jilka, Citation2007). However, here we show similarities in the mineralization process by these two regimens of PTH administration as seen in vivo, implying that comparable mechanisms exist in the cultured MLO-A5 cells.
The disruption of the mineralization by a gap junction and hemichannel inhibitor implies the involvement of these channels in bone formation. This is consistent with previous reports showing the crucial roles of Cx43 in mediating bone formation mineralization, and the anabolic effects of PTH in osteoblast-like cells and mouse models in vivo (Van der Molen et al., Citation1996a; Lecanda et al., Citation2000). Interestingly, we did not observe a significant difference in levels of gap junction coupling between intermittent and continuous treatment. One of the possible explanations might be the sensitivity of the dye transfer approach, such that small differences might not be readily detectable by this method. Since scrape loading dye transfer approach is invasive involving certain cell damages, we attempted to use less invasive “parachuting” dye transfer approach. However, MLO-A5 cells could not be cultured at such high cell density suitable for this assay. Surprisingly, as compared to intermittent, we observed a slightly higher cell coupling, though not significant, by continuous PTH treatment, which reduces the possibility of the involvement of increased gap junction coupling in promoting bone mineralization.
We observed that PTH differentially regulates Cx43 depending upon the treatment regimes; continuous PTH increases total Cx43 protein levels, but the majority of Cx43 appear to be retained inside the cell. In contrast, intermittent PTH enhanced connexon formation correlated with increased cell surface expression. Immunofluorescence imaging showed that a large amount of Cx43 is accumulated inside the cell in response to continuous PTH treatment. One possible explanation could be that continuous PTH exposure dramatically increases Cx43 and possibly other protein expression, which leads to “clogging” of protein secretory pathways, albeit we failed to detect any visible ER stress. Surprisingly, continuous PTH did not compromise gap junctional coupling since continuous and intermittent PTH treatments have similar stimulatory effects. This could be partially explained by a relatively small portion of the total Cx43 protein pool forming gap junctions.
The possible model derived from this study is that the stimulatory effect of intermittent PTH on bone mineralization is likely to be mediated through the increased cell surface expression of Cx43 protein that may form functional hemichannels, but unlikely through gap junctions. The biochemical evidence prompted us to examine the functional involvement of Cx43-hemichannels. Intriguingly, we did not observe any activities of hemichannels. To capture the transient opening of hemichannels, we also conducted dye release analysis using a live cell imaging approach, which also failed to identify any hemichannel activities. One possible interpretation could be that only small numbers of hemichannels are open, which makes it barely detectable with dye uptake method. Electrophysiological measurements might be a sensitive, alternative approach. One of the possible technical challenges for conducting this assay could be that as we have shown hemichannels are sensitive to mechanical stress and manipulation with electrodes might provoke hemichannel opening even in the absence of PTH treatment. The possible roles of hemichannels and Cx43 in mediating the effects of intermittent and continuous PTH are currently under further investigation in our laboratory. Alternatively, Cx43 functions differently in growth versus resorption and yet PTH has effects on Cx43 activity in both PTH treatment regimes, albeit different effects under the two conditions. Another unlikely possibility is that functional channels formed by Cx43 are not required for bone mineralization associated with PTH treatment, and uncharacterized mechanism(s) may be involved. Ultimately, understanding of the molecular mechanisms behind these PTH treatment regimens will provide invaluable clues for therapeutic application and drug development.
ACKNOWLEDGEMENTS
We thank Dr. Lynda Bonewald from University of Missouri, School of Density for generously providing MLO-A5 cell line. This work was supported by NIH Grant AR46798 and Welch Foundation Grant AQ-1507.
REFERENCES
- Bergwitz C, Abou-Samra A B, Hesch R D, Juppner H. Rapid desensitization of prarathyroid homrone dependent adenylate cyclase in perfused human osteosarcoma cells (SaOS-2). Biochim Biophys Acta 1994; 1222: 456
- Cheng B, Zhao S, Luo J, Sprague E, Bonewald L F, Jiang J X. Expression of functional gap junctions and regulation by fluid flow shear stress in osteocyte-like MLO-Y4 cells. J Bone Miner Res 2001; 16: 249–259
- Cherian P P, Cheng B, Gu S, Sprague E, Bonewald L F, Jiang J X. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem 2003; 278: 43146–43156
- Cherian P P, Siller-Jackson A J, Gu S, Wang X, Bonewald L F, Sprague E, Jiang J X. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 2005; 16: 3100–3106
- Chiba H, Sawada N, Oyamada M, Kojima T, Nomura S, Ishii S, Mori M. Relationship between the expression of the gap junction protein and osteoblast phenotype in a human osteoblastic cell line during cell proliferation. Cell Structure & Function 1993; 18: 419–426
- Chung D J, Castro C H, Watkins M, Stains J P, Chung M Y, Szejnfeld V L, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 2006; 119: 4187–4198
- Civitelli R, Ziambaras K, Warlow P M, Lecanda F, Nelson T, Harley J, Atal N, Beyer E C, Steinberg T H. Regulation of connexin43 expression and function by prostaglandin E2 (PGE2) and parathyroid hormone (PTH) in osteoblastic cells. J Cell Biochem 1998; 68: 8–21
- Daniels G M, Amara S G. Selective labeling of neurotransmitter transporters at the cell surface. Methods Enzymol 1998; 296: 307–318
- Donahue H J, Mcleod K J, Rubin C T, Andersen J, Grine E A, Hertzberg E L, Brink P R. Cell-to-cell communication in osteoblastic networks: cell-line-dependent hormonal regulation of gap junction function. J Bone Miner Res 1995; 10: 881–889
- Doty S B. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 1981; 33: 509–512
- Edelson E. Conduits for cell/cell communication. MOSAIC 1990; 21: 48–56
- El-Fouly M H, Trosko J E, Chang C C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res 1987; 168: 422–430
- Fitzpatrick L. A., Bilezikian J. P. Actions of parathyroid homrone, J. P. Bilezikian, L. G. Raisz, G. A. Rodan. Academic Press, New York 1996; 339–346
- Goodenough D A, Goliger J A, Paul D L. Connexins, connexons, and intercellular communication. Annu Rev Biochem 1996; 65: 475–502
- Harris S E, Bonewald L F, Harris M A, Sabatini M, Dallas S, Feng J Q, Ghosh Choudhury N, Wozney J, Mundy G R. Effects of transforming growth factor on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and typeI collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 1994; 9: 855–863
- He D S, Jiang J X, Taffet S M, Burt J M. Formation of heteromeric gap junction channels by connexin 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci USA 1999; 96: 6495–6500
- Hock J M, Fitzpatrick L A, Bilezikian J P. Actions of parathyroid hormone. Principle of Bone Biology, J P Bilezikian, L G Raisz, G A Rodan. Academic Press, New York 2002; 463–481
- Jiang J X, Goodenough D A. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA 1996; 93: 1287–1291
- Jilka R L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 2007; 40: 1434–1446
- Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald L F. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralized in culture. J Bone Miner Res 2001; 16: 1622–1633
- Koval M. Pathway and control of connexin oligomerization. Trend Cell Biol 2006; 16: 159–166
- Lecanda F, Warlow P M, Sheikh S, Furlan F, Steinberg T H, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 2000; 151: 931–943
- Mitchell J A, Ou C, Chen Z, Nishimura T, Lye S J. Parathyroid hormone-induced up-regulation of connexin-43 mesenger ribonucleic acid (mRNA) is mediated by sequences within both the promoter and the 3′untranslated region of the mRNA. Endocrinology 2001; 142: 907–915
- Musil L S, Beyer E C, Goodenough D A. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 1990; 116: 163–175
- Musil L S, Goodenough D A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 1991; 115: 1357–1374
- Neer R M, Arnaud C D, Zanchetta J R, Prince R, Gaich G A, Reginster J Y, Hodsman A B, Eriksen E F, Ish-Shalom S, Genant H K, Wang O, Mitlak B H. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441
- Palumbo C, Palazzini S, Marotti G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990; 11: 401–406
- Rubin M R, Cosman F, Lindsay R, Bilezikian J P. The anabolic effect of parathyroid hormone. Osteoporos. Int. 2002; 13: 267–277
- Schiller P C, Mehta P P, Roos B A, Howard G A. Hormonal regulation of intercellular communication: parathyroid hormone increases connexin 43 gene expression and gap-junctional communication in osteoblastic cells. Molecular Endocrinology 1992; 6: 1433–1440
- Schiller P C, Roos B A, Howard G A. Parathyroid hormone up-regulation of connexin 43 gene expression in osteoblasts depends on cell phenotype. J Bone Miner Res 1997; 12: 2005–2013
- Schirrmacher K, Schmiz I, Winterhager E, Traub O, Bremmer F, Jones D, Binbmann D. Characterization of gap junctions between osteoblast-like cells in culture. Calcif Tissue Int 1992; 51: 285–290
- Steinberg T H, Civitelli R, Geist S T, Robertson A J, Hick E, Veenstra R D, Wang H Z, Warlow P M, Westphale E M, Laing J G, et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J 1994; 13: 744–750
- Thi M M, Kojima T, Cowin S C, Weinbaum S, Spray D C. Fluid flow stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol (Cell Physiol) 2003; 284: C389–C403
- Van d er, Molen M A, Rubin C T, Mcleod K J, Mccauley L K, Donahue H J. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem 1996b; 271: 12165–12171
- Van d er, Molen M A, Rubin C T, Mcleod K J, Mccauley L K, Donahue H J. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J. Biol. Chem. 1996a; 271: 12165–12171
