Abstract
As the initiation step of bacterial infection or biofouling, bacterial adhesion on cells or substrates is generally an optimal target for antibacterial design. Phosphatidylethanolamine (PE) is the principal phospholipid in bacteria, and its function in bacterial adhesion remains unclear. In this study, four E. coli strains including two PE-deficient mutants (PE−PC− and PE−PC+ strains) and two PE-containing wild-type controls (PE + PC− strains) were recruited to investigate the influence of PE deficiency on bacterial adhesion. We found that PE deficiency could impair E. coli adhesion on macrophages (human THP-1-derived and mouse RAW264.7 macrophages) or glass coverslips by downregulating lipopolysaccharide (LPS) biosynthesis, which could be reversible by high galactose/lactose but not glucose cultivation. The data imply that PE play important role in bacterial adhesion probably via affecting LPS biosynthesis and suggest that targeting PE biosynthesis is also a potential antibacterial strategy.
Introduction
The adhesion of bacteria onto cellular surfaces is the early step of bacterial infection of plants and animals while the adhesion of bacteria onto substrate surfaces is closely related with biofilm formation, biofouling, bioenergy, among others. Understanding the factors and underlying mechanisms of causing/influencing bacterial adhesion on cells or substrates has important implications in the development of antibacterial drugs.
The principal phospholipid in bacteria is phosphatidylethanolamine (PE). In the Gram-negative bacterium Escherichia coli (E. coli), PE accounts for ∼70–80% of the total membrane lipids (the remaining lipids consist of ∼20% phosphatidylglycerol and 2–10% cardiolipin) (Shibuya Citation1992; Dowhan Citation1997). To investigate the functions of PE, a set of E. coli mutants in which PE is completely lacking or replaced by foreign lipids not found in wild-type E. coli have been constructed (DeChavigny et al. Citation1991; Xia & Dowhan Citation1995; Wikstrom et al. Citation2004, Citation2009; Dowhan Citation2013). These PE-deficient E. coli strains have been extensively applied to study the roles of PE in cell division (Mileykovskaya et al. Citation1998; Mileykovskaya & Dowhan Citation2005), motility and chemotaxis (Shi et al. Citation1993), stress response system activation (Mileykovskaya & Dowhan Citation1997; Keller et al. Citation2015), electron transfer chain (Mileykovskaya & Dowhan Citation1993), protein structure and function (Bogdanov et al. Citation1999; Mikhaleva et al. Citation2001; Bogdanov et al. Citation2010; Dowhan Citation2013), and others. However, the effect of PE deficiency on E. coli adhesion is little investigated.
In this study, the effects of PE on bacterial adhesion onto cells and substrates and the potential underlying mechanism were evaluated by using four E. coli strains including two PE-deficient mutants (PE−PC− and PE−PC+ strains) and two PE-containing wild-type controls (PE + PC− strains).
Materials and methods
E. coli strains, cultures and high sugar cultivation
Here, two E. coli mutants lacking phosphatidylethanolamine (PE) were recruited: (a) AD93 (pss93::kan recA srl::Tn10 nadB+), a well-established PE-deficient mutant (PE−PC− strain), constructed via inactivation of the phosphatidylserine synthase (pss) gene (DeChavigny et al. Citation1991); (b) AD93/ptac67 (pss93::kan recA srl::Tn10 nadB+ pcs+ amp), a PE−PC+ strain, established via expression of the phosphatidylcholine (PC) synthase (pcs) gene in AD93 (Chen et al. Citation2009). Moreover, two PE-containing E. coli strains were utilized as controls: (a) DH5α, a widely used PE + PC− strain; (b) AD93/pDD72 (pss93::kan recA srl::Tn10 nadB+ pss+ cam), a rescued PE + PC− wild type by introducing a functional copy of the pss gene into AD93 (DeChavigny et al. Citation1991).
All bacterial strains were grown in standard Luria–Bertani (LB) broth with respective supplements (50 μg/mL chloramphenical for DH5α and AD93/pDD72; 50 mM MgCl2 for AD93; 50 mM MgCl2 plus 1% choline and 50 μg/mL ampicillin for AD93/ptac67) at 37 °C (at 30 °C for the temperature-sensitive strain AD93/pDD72) as previously reported (Li et al. Citation2016). For high sugar cultivation, PE−PC− (AD93) or PE−PC+ (AD93/ptac67) bacteria were cultured in the corresponding complete medium but supplemented with high sugar (glucose, galactose, glucose-galactose mixture, or lactose; 0.5–2% for each one). All determinations involving bacteria were done on cultures that reached a final cell density with an absorbance value of ∼0.5 at 600 nm (A600 = ∼0.5).
Monocytes/macrophages and cell cultures
Human THP-1 monocytic leukemia cells, purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were differentiated to macrophages by a phorbol ester (phorbo-12-myristate-13-acetate, PMA) as previously reported (Zhao et al. Citation2014). THP-1 cells were routinely cultured in DMEM media supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine. Approximately 1 × 105/ml THP-1 cells were seeded in petri dish, cultured at 37 °C in a 5% CO2 incubator for 2 days, and subsequently treated by PMA (100 ng/ml; Sigma, St. Louis, MO) for 2–3 days. After washing twice with PBS to remove free PMA and undifferentiated THP-1 cells, the THP-1-derived macrophages were cultured in growth medium without PMA. Mouse macrophage RAW264.7 cells, purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were routinely cultured in Gibco RPMI 1640 Media supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Glass modification and contact angle measurement
Normal glass coverslips are generally hydrophilic. To obtain a higher hydrophilicity, clean glass coverslips were soaked in a solution (H2SO4:H2O2 = 7:3) at 100 °C for 1 h. After washing with ultrapure water, the glass coverslips were sterilized by ultraviolet. The resulting coverslips were called “hydrophilic glass” in this study.
The hydrophobic glass coverslips were silanized using a self-assembling silane compound named octadecyltrichlorosilane (OTS). Clean glass coverslips were soaked in a solution (CrO3:H2SO4:H2O = 29:29:42) at room temperature for 48 h. After washing with ultra-pure water and dried with nitrogen gas, the glass coverslips were treated with 1% OTS (Sigma, St. Louis, MO) in chloroform for at least 3 h in a dessicator (air and moisture were removed by nitrogen gas). After rinsing with chloroform and dried with nitrogen gas, the resulting coverslips were called “OTS-glass” in this study.
Contact angles of water on glass with different modifications were measured with a DSA100 contact angle analyzer (Krüss GmbH, Hamburg, Germany). In general, a material with a higher hydrophilicity has a smaller contact angle (<90°) whereas the contact angle of a hydrophobic material is >90°. The average contact angles () confirmed the hydrophilicity or hydrophobicity of the three glass coverslips used in this study.
Table 1. Contact angles of water on glass with different modifications.
Measurement of E. coli adhesion on substrates and cells
For adhesion on substrates, bacteria (A600 = ∼0.5) fluorescently stained with or without SYBR Green II (Solarbio, Beijing, China) were incubated on the three types of substrates for 1 h. After gentle washing, the bacteria were observed under an LSM710 confocal microscopy (Carl Zeiss, Oberkochen, Germany) and the number of bacteria in each field was counted. For adhesion on cells, bacteria (A600 = ∼0.5) were incubated with SYBR Green II at 37 °C for 15 min. After washing with PBS, 50 μl of the bacteria were incubated with THP-1-derived macrophages or RAW264.7 cells (in petri dish) at 37 °C for 1 h. After gentle washing with PBS, the cells were fixed by 2% glutaraldehyde (Solarbio, Shanghai, China) for 10 min. After washing with PBS again, the samples were subjected to LSM710 or TCS STED (Leica, Solms, Germany) confocal microscopy. For quantification of average bacterial numbers per cell, approximately 100 THP-1-derived macrophages or 200 RAW264.7 cells were analyzed in each group.
Fluorescence detection of cell-surface CD14 and blocking experiments
For fluorescence detection of CD14 on cell surfaces, untreated THP-1 cells, THP-1-derived macrophages, and RAW264.7 cells in petri dishes were gently washed with PBS to remove culture medium, fixed with 4% paraformaldehyde for 20 min at room temperature, and rinsed three times with PBS to remove the fixative. Subsequently, the cells were incubated with biotinylated anti-CD14 mAb (BioLegend, San Diego, CA) at a final concentration of 1 μg/ml for 1 h at 37 °C, washed three times with PBS, and incubated with streptavidin-conjugated Alexa Fluor 555 (Life Technologies, Carlsbad, CA) at a final concentration of 1 μg/ml for 30 min at 37 °C. After washing thoroughly with distilled water, the cells were imaged with confocal microscopy.
For blocking experiments, living THP-1-derived macrophages or RAW264.7 cells in petri dishes were incubated with anti-CD14 antibody (BioLegend, San Diego, CA) at 37 °C for 1 h. After washing with PBS, the cells were incubated with living PE+ bacteria fluorescently stained with SYBR Green II, washed with PBS, fixed by 2% glutaraldehyde for 10 min. After washing with PBS again, the samples were subjected to confocal microscopy for imaging and quantification (bacteria on approximately 100 THP-1-derived macrophages or 200 RAW264.7 cells were analyzed in each group).
LPS extraction
Lipopolysaccharide (LPS) was extracted from E. coli using LPS extraction kit (iNtRON Biotechnology, Inc., Gyeonggi-do, Korea) according to the manufactory provided protocol. Briefly, E. coli bacteria (A600 = ∼0.5) were harvested via centrifugation at 13,000 rpm at room temperature and mixed with Lysis Buffer, followed by addition of chloroform (at room temperature for 5 min) to separate the phenol layer from aqueous layer. After centrifugation at 13,000 rpm for 10 min at 4 °C, the supernatants were incubated with Purification Buffer at −20 °C for 10 min. After centrifuging the solution at 13,000 rpm for 15 min at 4 °C, LPS pellet was collected and washed with 70% EtOH. After centrifuging again for 3 min, the LPS pellet was collected, dried at room temperature, and dissolved in 10 mM Tris-HCl buffer (pH 8.0). To get higher purity, LPS was incubated with 30 mg/ml proteinase K at 50 °C for 30 min. Purchased LPS of E. coli (Sigma, St. Louis, MO) was used as a standard LPS (1 mg/ml).
SDS-PAGE
All LPS preparations were treated at 100 °C for 5 min in an aliquot of 0.1 mol/L Tris-HCl buffer (pH 6.8) containing 20 g/L SDS, 200 g/L sucrose, 1% β-mercaptoethanol and 100 mg/L bromphenol blue, and fractionated on an SDS-polyacrylamide gel containing 5% and 12% acrylamide in the stacking and separating gels, respectively. Electrophoresis was performed in 5 × Tris-glycine buffer containing 0.125 M Tris, 1.25 M glycine, and 0.5% (w/v) SDS at 12 mA in the stacking gel and 25 mA in the separating gel until the tracking dye had run to the bottom of the separating gel.
Silver staining
SDS-PAGE-fractionated LPS preparations were stained by the conventional silver-staining method. LPS was oxidized in the gel with 7 g/L periodic acid in 30% ethanol-10% acetic acid at 22 °C for 20 min. After washing three times with distilled water for 5 min, the gel was stained at 30 °C for 30 min with freshly prepared staining solution (1 g/L AgNO3). After washing once with distilled water for 10 s, the gel was soaked for 20 min in a freshly prepared solution containing 30 g/L Na2CO3 and 0.02% formaldehyde. Then, the color reaction was stopped by exposure to 10% acetic acid followed by repeated washings in distilled water. The gel was photographed after color reaction.
Statistical analysis
All values are expressed as mean ± SEM from at least three independent experiments. Statistical analyses were performed using One-way ANOVA or standard t-test to determine the significant difference (*p < 0.05; **p < 0.01; ***p < 0.001).
Results and discussion
PE-deficient E. coli mutants have been constructed for almost three decades and applied for studies on PE functions (DeChavigny et al. Citation1991; Dowhan Citation2013). For the first time, the PE-deficient strains were utilized here to investigate the influence of PE on bacterial adhesion. In this study, four E. coli strains including two PE-lacking mutants (AD93 and an AD93-derived PC-containing strain which were designated here as PE−PC− and PE−PC+, respectively), one wild-type PE + PC− control (DH5a) and one AD93-derived rescued wild-type control (designated as PE + PC− in figures) were recruited.
First of all, the adhesion of bacteria onto cells was evaluated. In order to readily identify individual bacteria on cell surfaces, living bacteria of the four types (i.e. DH5α, PE + PC−, PE−PC−, and PE−PC+ E. coli strains, respectively) were stained with a bright fluorescent dye (SYBR Green II) and washed to remove excess fluorescent molecules prior to incubation with human THP-1-derived macrophages or mouse RAW264.7 macrophages. Single fluorescently stained bacteria on individual cells could be clearly observed by confocal microscopy (). The statistical analysis shows that the average number of PE+ bacteria per cell is significantly higher than that of PE− bacteria (). Subsequently, the adhesion of bacteria on substrate (glass coverslip) was evaluated. Confocal images (upper panel of ) and statistical analysis (glass groups of ) obviously show that the average number of PE+ bacteria on glass coverslips is significantly higher than that of PE− bacteria. These data imply that PE deficiency impairs E. coli adhesion onto cells and substrates.
Figure 1. PE deficiency impairs E. coli adhesion onto cells. (A) Representative confocal images showing the adhesion of four fluorescently-stained (SYBR Green II) E. coli strains on human THP-1-derived macrophages. Upper panel (from left to right): DH5α, PE + PC− strain, PE−PC+ strain, and PE−PC− strain, respectively; bottom panel: enlarged images (the fluorescently stained bacteria are clearly evident) from the boxed areas in upper panel. (B) Representative confocal images showing the adhesion of the fluorescently-stained (SYBR Green II) E. coli strains on mouse RAW264.7 macrophages. (C, D) Statistical analysis of the bacteria adhered on THP-1-derived and RAW264.7 macrophages, respectively (the number of bacteria per cell. ***, p < 0.001 compared with DH5α/PE + PC−).
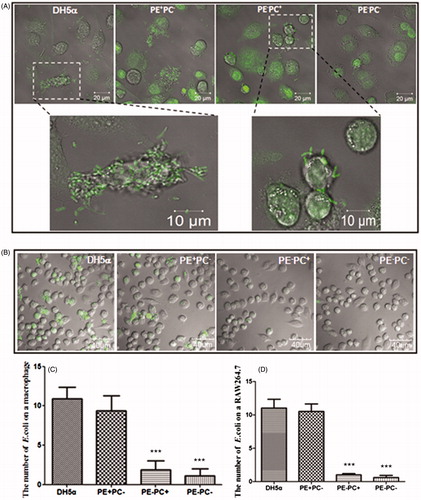
Figure 2. PE deficiency impairs E. coli adhesion onto substrates which is not related to hydrophobicity. (A) Representative images showing the adhesion of the four bacterial types on different substrates (panels from top to bottom: glass, hydrophilic glass, and OTS-glass, respectively). (B) Statistical analysis of the bacteria adhered on the substrates (the number of bacteria per field as a representative image showed in A. *, p < 0.05; ***, p < 0.001 compared with the glass groups; #, p < 0.05 compared with PE+ strains).
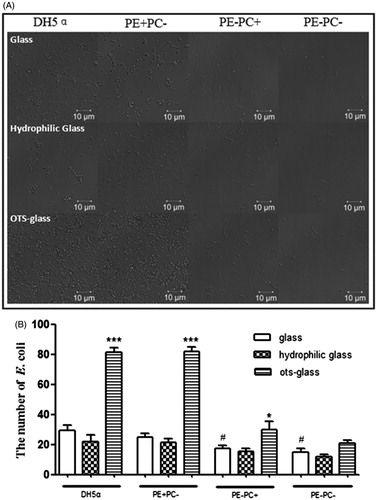
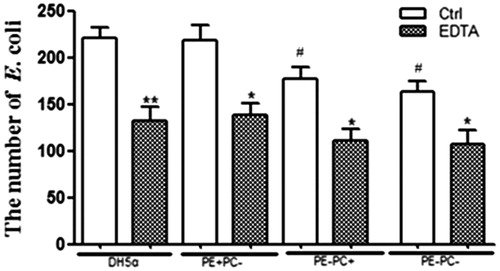
Many factors may influence E. coli adhesion on cells/substrates among which hydrophobicity is generally the main one. To investigate the potential contribution of hydrophobicity, hydrophilic and hydrophobic (i.e. OTS-glass) glass coverslips chemically modified from normal glass coverslips () were used for bacterial adhesion. Confocal images () and statistical analysis () clearly show that increase in hydrophobicity of substrate dramatically promotes the adhesion of PE+ bacteria rather than that of PE− bacteria. Lipopolysaccharide (LPS) on bacterial surface is also reported to mediate bacterial adhesion on substrates/cells potentially through hydrogen binding (Tomme et al. Citation1996; Abu-Lail & Camesano Citation2003; Strauss et al. Citation2009). To study the potential contribution of LPS, the bacteria were treated with EDTA to deplete LPS on bacterial surfaces prior to adhesion on glass coverslips. Confocal images (data not shown) and statistical analysis () show that LPS depletion significantly impairs the adhesion of both PE+ and PE− bacteria. These preliminary data imply that the PE deficiency-induced decrease of E. coli adhesion on cells/substrates was probably related to LPS instead of hydrophobicity.
Figure 3. LPS depletion by EDTA significantly impairs the adhesion of the four E. coli strains onto glass coverslips. *, p < 0.05; **, p < 0.01 compared with the corresponding controls; #, p < 0.05 compared with PE + strains (i.e. DH5α and PE + PC− groups).
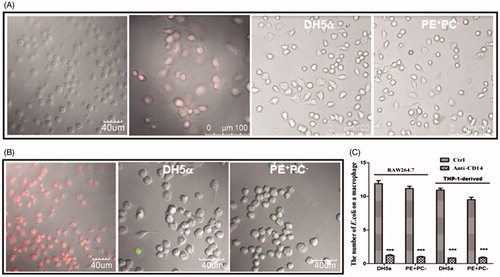
Toll-like receptor-4 (TLR4) and CD14 have been identified as recognition receptor and co-receptor, respectively, on cell surfaces that respond to LPS, and CD14 has been reported to have the role of binding LPS and subsequently presenting it to TLR4 (Muroi et al. Citation2002; Palsson-McDermott & O'Neill Citation2004; Wu et al. Citation2012; Lee et al. Citation2013). Generally, CD14 expression is very low in untreated THP-1 cells but relatively high in PMA-activated THP-1-derived macrophages (Daigneault et al. Citation2010) and RAW264.7 macrophages which are confirmed by our fluorescence confocal images (left two panels of and left panel of ). To test whether E. coli adhesion on cells is correlated with LPS/CD14, blocking experiments were performed using anti-CD14 mAb. The data show that the average number of PE+ E. coli bacteria per cell decreased dramatically when CD14 on living cells was blocked by antibody prior to bacterial adhesion (right two panels of and ), confirming the contribution of LPS to bacterial adhesion on cells.
Figure 4. The antibody blocking of cell-surface CD14 (a putative co-receptor for LPS recognition) inhibits E. coli adhesion onto cells. (A) Left two panels: CD14 was fluorescently stained on fixed THP-1 cells and THP-1-derived macrophages, respectively; right two panels: the adhesion of fluorescently-stained (SYBR Green II) PE+ E. coli strains on THP-1-derived macrophages pre-treated with anti-CD14 mAb. (B) Left panel: CD14 was fluorescently stained on fixed RAW264.7 cells; right two panels: the adhesion of fluorescently-stained (SYBR Green II) PE+ E. coli strains on RAW264.7 cells pre-treated with anti-CD14 mAb. (C) Statistical analysis of the bacteria adhered on macrophages pre-treated with or without anti-CD14 mAb (the number of bacteria per cell. ***, p < 0.001 compared with the corresponding controls).
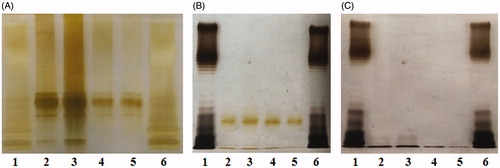
To further elucidate its contribution, LPS was extracted from each of the four E. coli strains and then detected via SDS-PAGE and silver staining. As expected, the LPS content of PE− E. coli was much lower than that of PE+ E. coli (). After LPS depletion by EDTA, it was found that the LPS contents of all four E. coli strains decreased (lanes 2–5 in ) and that the LPS content in supernatant of PE− E. coli (lanes 4 and 5 in ) was much lower than that of PE+ E. coli (lanes 2 and 3 in ). The data imply that PE deficiency probably downregulates LPS biosynthesis of E. coli finally causing impaired bacterial adhesion on cells/substrates. Although the underlying mechanisms are unclear, the implication is supported by previous studies reporting that some residues in the outer 3-deoxy-d-manno-octulosonic acid (Kdo) moiety of E. coli LPS are derived from PE (Hasin & Kennedy Citation1982; Kanipes et al. Citation2001).
Figure 5. PE deficiency downregulates LPS biosynthesis of E. coli. (A) Silver-stained SDS-PAGE pattern of LPS from the four E. coli strains. (B) Silver-stained SDS-PAGE pattern of LPS from the bacteria treated with EDTA or (C) from the supernatants. Lanes 1 and 6: standard LPS; lanes 2–5: DH5α, PE+PC−, PE−PC+, and PE−PC− strains, respectively. The origin of electrophoretic mobility is on the top.
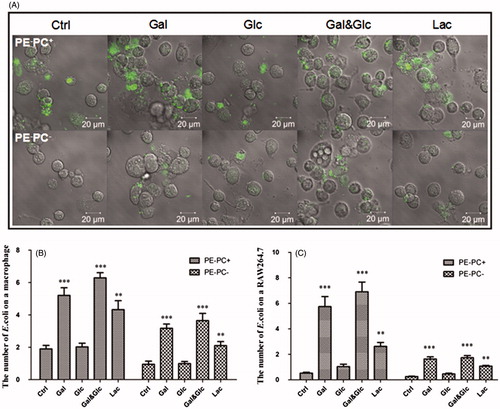
It has been reported that PE is required for the proper topology/conformation and active transport functions of bacterial transporters/permeases (Zhang et al. Citation2005; Gbaguidi et al. Citation2007) including lactose permease (LacY) of E. coli (Bogdanov & Dowhan Citation1995; Bogdanov et al. Citation2002; Wang et al. Citation2002; Bogdanov et al. Citation2010; Dowhan & Bogdanov Citation2011). Therefore, when PE is absent the transport proteins have incorrect tertiary structures and do not function correctly (e.g. LacY is unable to transport lactose into E. coli). So, we hypothesized that high-lactose cultivation will not alter LPS synthesis of PE-lacking E. coli since lactose is unable to be transported into bacteria. To test the hypothesis, LPS contents from PE-deficient strains (PE−PC− and PE−PC+ strains) cultivated in normal medium but supplemented with high sugar (glucose, galactose, glucose-galactose mixture, and lactose, respectively) were detected and compared. Surprisingly, the data () indicate that high galactose or lactose cultivation dramatically enhanced LPS biosynthesis of PE− bacteria whereas high glucose cultivation had no effects. Moreover, high galactose or lactose but not glucose cultivation significantly increased the adhesion of PE− bacteria on substrates () or cells (). The data imply that high galactose/lactose but not glucose cultivation can reverse the impaired adhesion ability of PE-deficient E. coli on cells/substrates via upregulating LPS synthesis.
Figure 6. High galactose/lactose cultivation enhances LPS synthesis of PE-deficient E. coli strains. PE−PC− (A) and PE−PC+ (B) bacteria were cultivated in the corresponding medium but supplemented with high sugar (glucose, galactose, glucose-galactose mixture, and lactose, respectively) at different concentrations (0.5%, 1%, and 2%, respectively). Showed are the silver-stained SDS-PAGE patterns of LPS from these bacteria. The first and last lanes are standard LPS; the second lanes are PE- bacteria without high sugar cultivation.
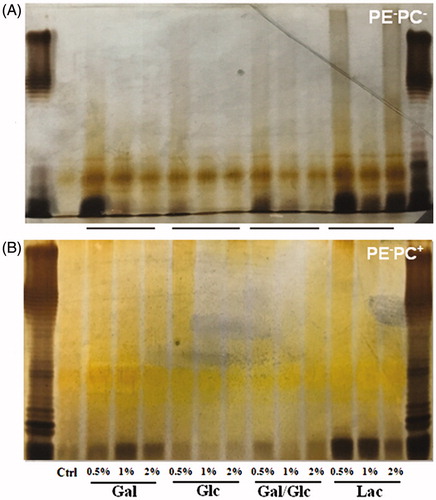
Figure 7. High galactose/lactose cultivation promotes the adhesion of PE-deficient E. coli strains onto substrates. (A) Representative images showing the substrate adhesion of PE−PC+ (upper panel) and PE−PC− (lower panel) bacteria cultivated with high sugar (panels from left to right: no sugar, 1% galactose, 1% glucose, 1% galactose-glucose mixture (1:1), and 1% lactose, respectively). The bacteria were fluorescently stained with SYBR Green II. (B) Statistical analysis of the bacteria adhered on substrates (the number of bacteria per field as a representative image showed in A. **, p < 0.01; ***, p < 0.001 compared with the corresponding controls).
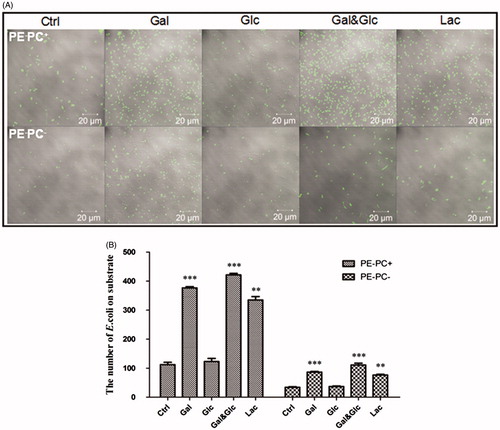
Figure 8. High galactose/lactose cultivation increases the adhesion of PE-deficient E. coli strains onto macrophages. (A) Representative images showing the adhesion of PE−PC+ (upper panel) and PE−PC− (lower panel) bacteria cultivated with high sugar onto macrophages. The bacteria were fluorescently stained with SYBR Green II. (B) Statistical analysis of the PE-deficient bacteria adhered on THP-1-derived macrophages (the number of bacteria per cell. **, p < 0.01; ***, p < 0.001 compared with the corresponding controls). (C) Statistical analysis of the PE-deficient bacteria adhered on RAW264.7 macrophages (**, p < 0.01; ***, p < 0.001 compared with the corresponding controls).
Taken together, our data reveal that PE deficiency can impair E. coli adhesion on cells or substrates by downregulating LPS synthesis, which is reversible by high galactose/lactose but not glucose cultivation. Although the underlying molecular mechanisms are presently unknown, the results suggest two possible antibacterial strategies aiming at bacterial adhesion: directly by inhibiting LPS biosynthesis (Cipolla et al. Citation2011) or indirectly by targeting PE biosynthesis (e.g. inhibiting enzymes involved in PE biosynthesis).
Acknowledgments
We would like to thank Prof. Xingguo Wang for kindly providing the three E. coli mutants (i.e. AD93, AD93/pDD72, and AD93/ptac67 strains). This study was supported by the National Natural Science Foundation of China (grant #: 81560083) and the Natural Science Foundation of Jiangxi Province (grant #: 20151BAB205005 and 20151BBG70035).
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Abu-Lail NI, Camesano TA. 2003. Role of lipopolysaccharides in the adhesion, retention, and transport of Escherichia coli JM109. Environ Sci Technol. 37:2173–2183.
- Bogdanov M, Dowhan W. 1995. Phosphatidylethanolamine is required for in vivo function of the membrane-associated lactose permease of Escherichia coli. J Biol Chem. 270:732–739.
- Bogdanov M, Heacock P, Guan Z, Dowhan W. 2010. Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc Natl Acad Sci USA. 107:15057–15062.
- Bogdanov M, Heacock PN, Dowhan W. 2002. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 21:2107–2116.
- Bogdanov M, Umeda M, Dowhan W. 1999. Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J Biol Chem. 274:12339–12345.
- Chen F, Zhao Q, Cai X, Lv L, Lin W, Yu X, Li C, Li Y, Xiong M, Wang XG. 2009. Phosphatidylcholine in membrane of Escherichia coli changes bacterial antigenicity. Can J Microbiol. 55:1328–1334.
- Cipolla L, Polissi A, Airoldi C, Gabrielli L, Merlo S, Nicotra F. 2011. New targets for antibacterial design: Kdo biosynthesis and LPS machinery transport to the cell surface. Curr Med Chem. 18:830–852.
- Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. 2010. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5:e8668.
- DeChavigny A, Heacock PN, Dowhan W. 1991. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 266:5323–5332.
- Dowhan W, Bogdanov M. 2011. Lipid-protein interactions as determinants of membrane protein structure and function. Biochem Soc Trans. 39:767–774.
- Dowhan W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem. 66:199–232.
- Dowhan W. 2013. A retrospective: use of Escherichia coli as a vehicle to study phospholipid synthesis and function. Biochim Biophys Acta. 1831:471–494.
- Gbaguidi B, Hakizimana P, Vandenbussche G, Ruysschaert JM. 2007. Conformational changes in a bacterial multidrug transporter are phosphatidylethanolamine-dependent. Cell Mol Life Sci. 64:1571–1582.
- Hasin M, Kennedy EP. 1982. Role of phosphatidylethanolamine in the biosynthesis of pyrophosphoethanolamine residues in the lipopolysaccharide of Escherichia coli. J Biol Chem. 257:12475–12477.
- Kanipes MI, Lin S, Cotter RJ, Raetz CR. 2001. Ca2+-induced phosphoethanolamine transfer to the outer 3-deoxy-D-manno-octulosonic acid moiety of Escherichia coli lipopolysaccharide. A novel membrane enzyme dependent upon phosphatidylethanolamine. J Biol Chem. 276:1156–1163.
- Keller R, Arioz C, Hansmeier N, Stenberg-Bruzell F, Burstedt M, Vikstrom D, Kelly A, Wieslander A, Daley DO, Hunke S. 2015. The Escherichia coli envelope stress sensor CpxA responds to changes in lipid bilayer properties. Biochemistry. 54:3670–3676.
- Lee H-S, Bilehal D, Lee G-S, Ryu D-S, Kim H-K, Suk D-H, Lee D-S. 2013. Anti-inflammatory effect of the hexane fraction from Orostachys japonicus in RAW 264.7 cells by suppression of NF-κB and PI3K-Akt signaling. J Funct Foods. 5:1217–1225.
- Li M, Gan C, Shao W, Yu C, Wang X, Chen Y. 2016. Effects of membrane lipid composition and antibacterial drugs on the rigidity of Escherichia coli: different contributions of various bacterial substructures. Scanning. 38:70–79.
- Mikhaleva NI, Golovastov VV, Zolov SN, Bogdanov MV, Dowhan W, Nesmeyanova MA. 2001. Depletion of phosphatidylethanolamine affects secretion of Escherichia coli alkaline phosphatase and its transcriptional expression. FEBS Lett. 493:85–90.
- Mileykovskaya E, Dowhan W. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J Bacteriol. 179:1029–1034.
- Mileykovskaya E, Dowhan W. 2005. Role of membrane lipids in bacterial division-site selection. Curr Opin Microbiol. 8:135–142.
- Mileykovskaya E, Sun Q, Margolin W, Dowhan W. 1998. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J Bacteriol. 180:4252–4257.
- Mileykovskaya EI, Dowhan W. 1993. Alterations in the electron transfer chain in mutant strains of Escherichia coli lacking phosphatidylethanolamine. J Biol Chem. 268:24824–24831.
- Muroi M, Ohnishi T, Tanamoto K. 2002. Regions of the mouse CD14 molecule required for toll-like receptor 2- and 4-mediated activation of NF-kappa B. J Biol Chem. 277:42372–42379.
- Palsson-McDermott EM, O'Neill LAJ. 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 113:153–162.
- Shi W, Bogdanov M, Dowhan W, Zusman DR. 1993. The pss and psd genes are required for motility and chemotaxis in Escherichia coli. J Bacteriol. 175:7711–7714.
- Shibuya I. 1992. Metabolic regulations and biological functions of phospholipids in Escherichia coli. Prog Lipid Res. 31:245–299.
- Strauss J, Burnham NA, Camesano TA. 2009. Atomic force microscopy study of the role of LPS O-antigen on adhesion of E. coli. J Mol Recognit. 22:347–355.
- Tomme P, Creagh AL, Kilburn DG, Haynes CA. 1996. Interaction of polysaccharides with the N-terminal cellulose-binding domain of Cellulomonas fimi CenC. 1. Binding specificity and calorimetric analysis. Biochemistry. 35:13885–13894.
- Wang X, Bogdanov M, Dowhan W. 2002. Topology of polytopic membrane protein subdomains is dictated by membrane phospholipid composition. EMBO J. 21:5673–5681.
- Wikstrom M, Kelly AA, Georgiev A, Eriksson HM, Klement MR, Bogdanov M, Dowhan W, Wieslander A. 2009. Lipid-engineered Escherichia coli membranes reveal critical lipid headgroup size for protein function. J Biol Chem. 284:954–965.
- Wikstrom M, Xie J, Bogdanov M, Mileykovskaya E, Heacock P, Wieslander A, Dowhan W. 2004. Monoglucosyldiacylglycerol, a foreign lipid, can substitute for phosphatidylethanolamine in essential membrane-associated functions in Escherichia coli. J Biol Chem. 279:10484–10493.
- Wu JF, Zhou JM, Chen XG, Fortenbery N, Eksioglu EA, Wei S, Dong JC. 2012. Attenuation of LPS-induced inflammation by ICT, a derivate of icariin, via inhibition of the CD14/TLR4 signaling pathway in human monocytes. Int Immunopharmacol. 12:74–79.
- Xia W, Dowhan W. 1995. Phosphatidylinositol cannot substitute for phosphatidylglycerol in supporting cell growth of Escherichia coli. J Bacteriol. 177:2926–2928.
- Zhang W, Campbell HA, King SC, Dowhan W. 2005. Phospholipids as determinants of membrane protein topology. Phosphatidylethanolamine is required for the proper topological organization of the gamma-aminobutyric acid permease (GabP) of Escherichia coli. J Biol Chem. 280:26032–26038.
- Zhao S, Liao H, Ao M, Wu L, Zhang X, Chen Y. 2014. Fixation-induced cell blebbing on spread cells inversely correlates with phosphatidylinositol 4,5-bisphosphate level in the plasma membrane. FEBS Open Bio. 4:190–199.

