Abstract
Methanol (MeOH) toxicity, a potential problem from accidental, intentional, as well as occupational and daily ingestion of the agent, receives attention only after severe signs of intoxication have set in or death is imminent. While accidental and intentional exposures involve high doses, the occupational and ingestion forms more often reflect small daily intakes. Still, even at these low levels, little is known about the potential immunotoxic implications from these recurring exposures. As innate immunity confers a first-line of defense against infection, a study was designed to examine the effects of daily exposure to MeOH (at 1/4 LD50 level, for up to 15 or 30 days) on neutrophil (PMN) functions using rats that were (or were not) injected with sheep red blood cells (SRBC) during the course of exposures. Blood samples were analyzed for total (TLC) and differential leucocyte counts (DLC), and isolated neutrophils (PMN) were assessed for changes in function by monitoring phagocytic (PI) and avidity indices (AI), nitroblue tetrazolium (NBT) reduction, and adherence. Body weights were monitored during exposures and weights of major immune system organs (i.e., spleen, thymus, lymph nodes) were assessed at sacrifice. Body and organ weight, TLC, blood PMN levels, PMN PI, and adherence were all significantly decreased in SRBC-untreated rats that received MeOH, although these cells did also display significant increases in AI and NBT reduction. With SRBC-treated rats, though the percentage of PMN in the blood increased with ongoing MeOH exposure, all the other parameters were markedly decreased in comparison to their controls. Thus, this study showed that repeated exposures to MeOH modulates PMN functions, thereby potentially altering the first line of defense in a normal immune response in exposed hosts.
INTRODUCTION
Methyl alcohol (methanol; MeOH), first in the alcohol series, is normally used as an industrial solvent and cleanser. People handling products that contain MeOH may inhale the toxic vapor during its evaporation from the product surface. When MeOH was proposed for use as an alternate automotive fuel, criticisms were raised that this might result in an unacceptable increase in the risk of exposure to MeOH vapors and in the frequency of reported toxic effects (Maejima et al., Citation1993; CONCAWE, Citation1995).
Apart from the occupational or transportation-related routes, there are both direct and indirect means by which exposure to MeOH can commonly occur. Indirectly, aspartame artificial sweetener (used in various soft beverages) is metabolized to MeOH in the small intestine (Ranney and Oppermann, Citation1979; Stegink, Citation1984). Though the levels of aspartame intake might be relatively small in the context of a daily diet, these small doses still result in significantly increased levels of plasma MeOH (Davoli, Citation1986). Even if one avoids aspartame-bearing products, normal dietary exposure to MeOH may still occur from consumption of certain fruits, vegetables, and juices with high pectin content (i.e., direct ingestion; Butchko and Kotsonis, Citation1991; Frenkel et al., Citation1998). Furthermore, though some of the pectin content is converted directly to MeOH in these food products, unprocessed pectin can itself be converted in the gut to MeOH by the actions of local bacteria (i.e., another indirect form of ingestion; Siragusa et al., Citation1988; Lindenger et al., Citation1997), again resulting in substantive daily increases in blood MeOH content.
The two extreme forms of direct exposure to MeOH are via accidental or suicidal ingestion. In Third World nations, the use of MeOH as a common adulterant in country liquors increases the chances of accidental poisoning (Roldan et al., Citation2003). In either extreme case, the dose ingested invariably causes severe metabolic acidosis and critical clinical disturbances such as blindness, neuropathies, and in many cases, death (Kuteifan et al., Citation1998; Liu et al., Citation1998). The toxicity that arises in these situations is not only due to metabolites generated, but also to the MeOH per se. For example, the increased presence of MeOH in the blood can lead to severe shifts in brain monoamine levels (Jeganathan and Namasivayam, Citation1998, Citation1999). Methanol is also increasingly recognized as a hepatotoxin in that hepatocytes oxidize it first to formaldehyde and then to formate (Tephly, Citation1991). These processes are accompanied by elevations in NADH levels and increases in formation of superoxide anions that can contribute to lipid peroxidation events in the organ (Poli, Citation1993).
While immunotoxic activities of ethanol (EtOH) have been extensively studied, studies of the effects from exposures to MeOH have been lacking. Since PMN are professional phagocytes that constitute a prime part of the innate defense system used against microorganisms (Witko-Sarsat et al., Citation2000), the present study decided to analyze the effects from MeOH on the function of these cells to begin to clarify potential immunotoxicities that could arise during the course of continuous low-level human exposures to non lethal levels of this agent.
MATERIALS AND METHODS
Animals
Healthy inbred adult male Wistar rats weighing 180–200 grams were divided into 6 groups. Each group consisted of 6 animals that were maintained under standard laboratory conditions and given access to food (M/s. Hindustan Lever Ltd., India) and water ad libitum. Animal experiments were carried out upon obtaining clearance from the Institutional Animal Ethical Committee (IAEC NO: 08/010/03) and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Experimental Groups
The MeOH dose used was 1/4 LD50 (2.4 g/kg body weight [BW]; Roe, Citation1982) mixed 1:1 with saline. Group I control rats received daily intraperitoneal (IP) injections of saline only; Groups II and Group III rats were injected IP with MeOH for 15 and 30 days, respectively. Group IV rats received a single IP dose of 5 × 109 sheep red blood cells (SRBC) during the course of the saline injections. Group V rats received MeOH for 15 days, with SRBC being injected on day 10 of the regimen. Group VI rats received MeOH for 30 days and the SRBC on day 25. SRBC used for injections were collected in sterile Alsever's solution, washed 3× with pyrogen-free normal saline, adjusted to 5 × 109 cells/ml with saline, and injected in a 1-ml volume into Groups IV–VI rats. At the end of the exposures to MeOH or saline, jugular vein blood was drawn; to avoid variations in the measured results due to circadian rhythm, all blood collections were done at 8:00–10:00 AM.
Body and Organ Weights
Animal body weights were determined at 5-day intervals for up to 30 days. Weights of lymphoid organs (e.g., spleen, thymus, and groin lymph nodes) were analyzed at sacrifice (i.e., on the 15th and 30th days of exposure).
Total (TLC) and Differential Leukocyte (DLC) Counts
Blood samples were diluted with Turk's fluid in a WBC pipette so that all red cells were lysed without affecting the leukocytes present. Total leukocyte counts were then carried out in an improved Neubauer's counting chamber. To obtain the differential counts, blood smears were made on glass slides and treated with Leishman's stain. Under oil immersion, 100 leukocytes/slide were counted and cell types present identified based on size, granule presence, granule size and color, and shape of the nucleus.
Neutrophil (PMN) Adherence Test
Heparinized blood was analyzed for TLC and DLC by fixing blood smears and staining with Field's stain I & II-Leishman's stain. Blood samples were then loaded onto—and subsequently incubated on—nylon fiber columns (80 mg/ml, 15 min, 37°C) (Wilkonson, Citation1978). The incubated samples were then re-analyzed for TLC and DLC. The product of the TLC and the percentage of PMN yielded the neutrophil index (NI). Neutrophil adhesion (%) was calculated as 100 × (NIu – NIt)/NIu, where NIu = the NI of an untreated blood sample and NIt = NI of the sample after being on the column.
Phagocytosis
Phagocytic (PI) and avidity index (AI) were each determined using the protocols of Wilkonson (Citation1977). Briefly, the buffy coat isolated from a blood sample was incubated 10 min at 37°C in a solution containing 0.1 ml Hank's balanced salt solution [HBSS], 0.1 ml inactivated fetal calf serum [iFCS], and 0.1 ml of a suspension containing 2 × 108 heat-killed Candida albicans. The preparation was then centrifuged at 1000 rpm for 5 min. Smears were prepared, treated with Leishman's stain, and then examined under oil. One hundred PMN/slide were examined for uptake of the yeast particles. The number of positive cells/100 PMN on each slide was used to derive the PI. To estimate the AI, the total number of Candida albicans particles within 100 positive PMN (a value then divided by 100 to yield mean particle number per cell) was determined.
Nitroblue Tetrazolium (NBT) Reduction Test
Nitroblue tetrazolium reduction was performed to evaluate the potential killing abilities of PMN from MeOH-treated and control rats (Gifford and Malawista, Citation1970). Briefly, 0.5 ml of heparinized blood was incubated at 37°C for (30 min) on a glass slide. The slide was then washed gently with cold saline, and 0.6 ml NBT medium (0.2 ml of 0.34% sucrose solution, 0.2 ml NBT [1 mg/ml], and 0.2 ml iFCS) was added. After incubating 30 min at 37°C, the slide was washed with cold saline, air dried, and counterstained with 0.7% [w/v] safranin (1 g in solution of 100 ml water + 40 ml glycerine). When exposed to NBT dye, activated PMN convert it to formazan crystals within their cytoplasm. The change in presence of these crystals was used as an indicator of potential to perform intracellular killing. After staining was completed, each slide was examined under oil; 100 PMN/slide were counted to determine the total and relative percentage of formazan-positive cells in each blood specimen.
Statistical Analysis
Data were expressed as mean ± SD. Statistical significance between groups were analyzed by a 1-way analysis of variance (ANOVA). When a difference was noted, Tukey's multiple comparison was also performed. Values were deemed significantly different from one another at p < 0.05.
RESULTS
Body weights of rats over the entire experimental period are shown in . A significant loss in body weight was observed in all MeOH-exposed rats from day 15 onwards as compared to their respective SRBC-injected or -uninjected controls. indicates the organ weights of the rats during the study period. A significant decrease in the weights of the spleen, thymus, and lymph node was noted in all rats treated with MeOH as compared to their respective controls. As a function of exposure, the weights of these organs were significantly decreased as length of exposure increased.
FIG. 1 Body weights of rats that did not receive SRBC treatment. Values are expressed as Mean ± SD from 6 animals. *p < 0.05 significance vs. control groups.
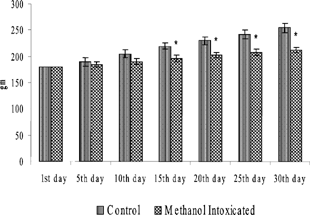
FIG. 2 Body weights of rats that received SRBC injection. Values are expressed as Mean ± SD from 6 animals. *p < 0.05 significance vs. control groups.
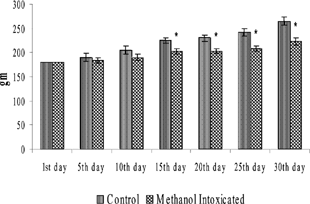
TABLE 1 Lymphoid organ weights in rats from the various treatment groups
The results of the TLC and DLC in rats that had not received SRBC are reported in . Fifteen- and 30-day-treatments caused significant decreases in TLC and the percentage of PMN in the blood as compared to levels in control rats. Marked decreases in TLC were also seen in 30-day-treated rats as compared to their 15-day counterparts. However, no significant alterations in the percentage of blood lymphocytes were noted in any MeOH-exposed host. A similar lack of effect on percentages of blood eosinophils, basophils, and monocytes was evident (data not shown).
TABLE 2 MeOH effects on total and differential leukocyte counts of SRBC-untreated rats
The effects from MeOH treatment on the functions of PMN from these rats are listed in . A significant decrease in the PI of isolated PMN was recorded in both 15- and 30-day MeOH-exposed rats as compared to that in cells from their controls. However, the AI and NBT reduction parameters were significantly elevated in both exposure groups. Though not affected in the 15-day treatment group, adherence by PMN from rats receiving MeOH for 30 days was significantly decreased compared to their controls. When comparing effects between the two MeOH groups, both PMN NBT reduction and adherence were significantly decreased by the longer regimen.
TABLE 3 Effect of MeOH on ex vivo neutrophil functions of SRBC-untreated rats
The results of effects from MeOH treatment on TLC and DLC levels in SRBC-treated rats are shown in . Compared to their control counterparts, while both 15- and 30-day MeOH-treated SRBC-treated rats had significant decreases in their TLC, each group also evidenced significant increases in the percentage of PMN in their blood. Interestingly, only the SRBC-treated rats that received MeOH for the shorter (15-day) period had a significant decrease in the percentage of blood lymphocytes compared to SRBC-treated controls. While the percentages of lymphocytes in the 30-day MeOH rats were also lower than the controls, these differences were not significant and fell midway between control and shorter regimen-rat levels. This differs from the other parameters in the table in that when the two treatment groups were compared, both the TLC and percentages of blood PMN were significantly decreased in rats in the longer regimen. No discernible changes in percentages of blood eosinophils, basophils, or monocytes were seen after MeOH exposures in conjunction with SRBC treatment (data not shown).
TABLE 4 MeOH effects on total and differential counts of SRBC-treated rats
The effects from MeOH exposure on PMN functions in SRBC-treated rats are listed in . The PI, AI, NBT reduction, and adherence of PMN obtained from SRBC-treated rats that underwent either MeOH treatment were all significantly reduced compared to control counterparts. In comparing the effects from MeOH between the two treatment groups, only PMN adherence was seen to be markedly further decreased as the SRBC-treated rats continued to receive the MeOH.
TABLE 5 Effect of MeOH on ex vivo neutrophil functions in SRBC-treated rats
DISCUSSION AND CONCLUSIONS
To best determine the potential impact of daily low-level MeOH intake on an immune response, an examination of effects on the ability of critical immune cells to phagocytize and, in turn, become activated (as would occur during the process of an actual infection) was a critical starting point. For these studies, SRBC would be used as stimulating agents since phagocytes readily engulf these particulate exogenous non-infectious antigens and because the SRBC are highly immunogenic (Mathieu et al., Citation2002). Specifically, this study evaluated potential changes in some select functions of PMN (both unactivated and SRBC-activated) as indicators of immunotoxicity that could arise during the course of ongoing low-level daily exposure to MeOH. As the effects of MeOH on PMN functions have never been documented, all results were ultimately analyzed in the context of those that have been noted with EtOH, the next member in the alcohol series. This was done because EtOH has been studied extensively with regard to its immunotoxicity in many laboratory animals and with respect to PMN specifically (Patel et al., Citation1996; Szabo, Citation1997; Zhang et al., Citation1998; Greenberg et al., Citation1999a, Citation1999b; Boe et al., Citation2003; Chetty-Raju et al., Citation2004).
The results indicate that repeated exposure of rats to MeOH at a sublethal dose affected PMN levels and functions, as well as other immune system-related endpoints. Because circulation of immune cells is essential for maintaining an effective immune defense network (Sprent and Tough, Citation1994), the effects on TLC and PMN levels suggest that repeated exposure to MeOH could affect normal cell trafficking. These studies also showed corresponding significant falls in major immune system organ weights in these hosts. Whether these effects were due to toxicities on the cells while in the bloodstream or while residing in the organs is uncertain. However, Liesivuori and Savolainen (Citation1991) report that MeOH distribution to body organs and tissues is usually in proportion to their water content. Thus, while local concentrations of MeOH in each immune system organ could be roughly equivalent, the total burden of MeOH could be higher in one vs. another, and so the magnitude of effect on residing cells could correspondingly differ.
While the presence of MeOH in these organs or the bloodstream could impact on the survival of many immune cells (including PMN), it could also affect their normal functions before death occurred. For example, migration of PMN from the bloodstream requires firm adhesion to blood vessel surfaces, a process mediated through interactions of cell surface β2-integrins (Springer, Citation1995). The results of the present study suggested that MeOH exposure significantly reduced PMN adherence in a manner similar to that reported with EtOH. Specifically, incubation of whole blood with EtOH also caused a dose-dependent inhibition of PMN adherence to nylon fiber columns (MacGregor et al., Citation1974) while chronic EtOH intoxication of hosts has been shown to result in granulocytes with decreased adherence properties (Rajkovic et al., Citation1984).
The studies here also indicated that MeOH exposure led to decreased PMN phagocytic ability and avidity, parameters dependent largely on proper expression of adhesion molecules. Studies have shown that this expression is very susceptible to effects from free radical-induced oxidative stress (Knight, Citation2000). The potential for this stress is high here; Skrzydlewska and Farbiszewski (Citation1999) reported increases in cell lipid peroxidation and decreases in antioxidant levels during MeOH intoxication. The analyses of EtOH effects on PMN also implied that decreases in phagocytosis might be due to defective adhesion molecule expression. Specifically, Zhang et al. (Citation1998) noted that acute EtOH intoxication inhibited inducible PMN β2-integrin (CD11b/c, CD18) expression in conjunction with decreased phagocytosis. Moreover, when these PMN were stimulated by N-formyl-L-methionyl-L-leucyl-L-phenylalanine to induce hyperadherence, cells from rats that received EtOH displayed dose-dependent inhibition of this endpoint (MacGregor et al., Citation1988). As such, it can be inferred that the decreases in PMN phagocytosis in the present study may also have been due to decreases in adhesiveness.
Despite the observations of decreased phagocytic indices in both SRBC-treated and -untreated rats exposed to MeOH, there was a divergence in their respective trends with regard to avidity. The PMN of SRBC-untreated rats given MeOH were found to be enormous in comparison to those of their SRBC-injected counterparts. Whether increase in size led to increased avidity (or vice-versa) is unclear at this time. However, what is apparent is that in SRBC-untreated rats, MeOH exposure led to significant increases in avidity as compared to control rats while in the SRBC-treated rats, the effect of MeOH was the opposite. Follow-up studies in regard to this finding are underway.
That SRBC-treated rats that received MeOH showed a marked decrease in PMN NBT reduction might possibly be due again to effects on the cell membrane. Studies have shown that EtOH intake results in inhibition of PMN delivery to sites of infection or inflammation (MacGregor et al., Citation1988; Nilsson et al., Citation1996). For those cells that reach these sites, active oxygen radical and oxidative burst product generation is essential for optimal microbial killing. It has been suggested that decreased oxyradical, superoxide anion, and hydrogen peroxide synthesis after exposure could be a mechanism underlying EtOH-induced changes in antibacterial immune defenses (Sczabo, Citation1999). Interestingly, in the study here, there was a marked decrease in NBT reduction in PMN from SRBC-injected rats that received MeOH, but increases in this parameter in PMN from SRBC-untreated rats. While increasing the period of MeOH exposure caused a slight worsening of this effect in SRBC-injected rats, the shorter exposure actually had the maximal effect in their counterparts. In either case, this suggests that duration of MeOH exposure likely plays a role in a potential worsening of immune functions.
In conclusion, this study has reported significantly altered PMN functions (e.g., modified phagocytic and avidity indices, NBT reduction, and adherence) following repeated daily exposures of rat hosts to MeOH for periods up to 30 days. These results are in general agreement with those few reports that have examined if there were any immunotoxic effects at all from intentional or accidental MeOH exposure. Further studies still need to be carried out to analyze at the molecular and cellular levels how MeOH is causing these alterations, as well as to better characterize other potential effects that could impact on the immunocompetence of an exposed host.
ACKNOWLEDGMENTS
We are grateful to the late Dr. A. Namasivayam for his advice. This work was supported by a grant from the University Grant Commission (UGC), Government of India, New Delhi.
REFERENCES
- Boe D. M., Nelson S., Zhang P., Quinton L., Bagby G. J. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 2003; 27: 1838–1845, [PUBMED], [INFOTRIEVE], [CSA]
- Butchko H. H., Kotsonis F. N. Acceptable daily intake vs. actual intake: The aspartame example. J. Am. Coll. Nutr. 1991; 10: 258–266, [PUBMED], [INFOTRIEVE]
- Chetty-Raju N., Cook R., Erber W. N. Vacuolated neutrophils in ethanol toxicity. Br. J. Haematol. 2004; 127: 478, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- CONCAWE. Alternative Fuels in the Automotive Market. (Report No. 2/95, prepared for the CONCAWE Automotive Emission Management Group by its Technical Coordinator, R. C. Hutcheson), CONCAWE, Brussels 1995; 67
- Davoli E. Serum methanol concentrations in rats and in men after a single dose of aspartame. Food Chem. Toxicol. 1986; 24: 187–189, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Frenkel C., Peters J. S., Tieman D. M., Tiznado M. E., Handa A. K. Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (Lycopersicon esculentum) fruit. J. Biol. Chem. 1998; 273: 4293–4295, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gifford H. R., Malawista S. E. A simple rapid micromethod for detecting chronic granulomatous disease of childhood. J. Lab. Clin. Med. 1970; 75: 511–571, [PUBMED], [INFOTRIEVE]
- Greenberg S., Ouyang J., Zhao X., Parrish C., Nelson S., Giles T. D. Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice. Alcohol. Clin. Exp. Res. 1999a; 23: 1435–1445, [PUBMED], [INFOTRIEVE], [CSA]
- Greenberg S., Zhao X., Hua L., Wang J. F., Nelson S., Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcohol. Clin. Exp. Res. 1999b; 23: 735–744, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jeganathan P. S., Namasivayam A. Methanol induced biogenic amine changes in discrete areas of rat brain: Role of simultaneous ethanol administration. Indian J. Physiol. Pharmacol. 1998; 32: 1–10
- Jeganathan P. S., Namasivayam A. Brain biogenic amine levels of methanol administration: Possible mechanism of action on central monoaminergic neurons on discrete areas of brain Wistar rat. Indian J. Physiol. Pharmacol. 1999; 33: 151–156
- Knight J. A. Review: Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000; 30: 145–158, [PUBMED], [INFOTRIEVE]
- Kuteifan K., Oesterle H., Tajahmady T., Gutbub A. M., Laplatte G. Necrosis and haemorrhage of the putamen in methanol poisoning shown on MRI. Neuroradiology 1998; 40: 158–160, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Liesivuori J., Savolainen H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 1991; 69: 157–163, [PUBMED], [INFOTRIEVE]
- Lindinger W., Taucher J., Jordan A., Hansel A., Vogel W. Endogenous production of methanol after the consumption of fruit. Alcohol. Clin. Exp. Res. 1997; 21: 939–943, [PUBMED], [INFOTRIEVE], [CSA]
- Liu J. J., Daya M. R., Carrasquillo O., Kales S. N. Prognostic factors on patients with methanol poisoning. J. Toxicol. Clin. Toxicol. 1998; 36: 175–181, [PUBMED], [INFOTRIEVE], [CSA]
- MacGregor R. R., Safford M., Shalit M. Effect of ethanol on functions required for the delivery of neutrophils to sites of inflammation. J. Infect. Dis. 1988; 157: 682–689, [PUBMED], [INFOTRIEVE]
- MacGregor R. R., Spagnuolo P. J., Lentnek A., 1. Inhibition of granulocyte adherence by ethanol, prednisone and aspirin measured with assay system. N. Engl. J. Med. 1974; 291: 642–645, [PUBMED], [INFOTRIEVE]
- Maejima K., Suzuki T., Numata H., Maekawa A., Nagase S., Ishinishi N. Recovery from changes in blood, nasal cavity and/or lungs of rats caused by expo-sure to methanol-fueled engine exhaust. J. Toxicol. Environ. Health 1993; 39: 323–340, [PUBMED], [INFOTRIEVE]
- Mathieu P., Guillot C., Gerdes C., Buzelin F., Lowenstein P., Castro M., Soulillou J. P., Anegon I. Adenovirus-mediated CD40Ig expression attenuates chronic vascular rejection lesions in an aorta allotransplantation model. Trans. Proc. 2002; 34: 743–744, [CSA], [CROSSREF]
- Nilsson E., Hallden G., Magnusson K. E., Hed J., Palmblad J. In vitro effects of ethanol on polymorphonuclear leukocyte membrane receptor expression and mobility. Biochem. Pharmacol. 1996; 51: 225–231, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Patel M., Keshavarzian A., Kottapalli V., Badie B., Winship D., Fields J. Z. Human neutrophil functions are inhibited in vitro by clinically-relevant ethanol concentration. Alcohol. Clin. Exp. Res. 1996; 20: 275–283, [PUBMED], [INFOTRIEVE], [CSA]
- Poli G. Liver damage due to free radicals. Br. Med. Bull. 1993; 49: 604–609, [PUBMED], [INFOTRIEVE]
- Rajkovic I. A., Yousif-Kadara A. G., Wyke R. J., Williams R. Polymorpho-nuclear leukocyte locomotion and aggregation in patients with alcoholic liver disease. Clin. Exp. Immunol. 1984; 58: 654–662, [PUBMED], [INFOTRIEVE], [CSA]
- Ranney R. E., Oppermann J. A. A review of the metabolism of the aspartyl moiety of aspartame in experimental animals and man. J. Environ. Pathol. Toxicol. 1979; 2: 979–985, [PUBMED], [INFOTRIEVE]
- Roe O. Species differences in methanol poisoning. CRC. Crit. Rev. Toxicol 1982; 10: 275–286
- Roldan J., Frauca C., Duenas A. Alcohol intoxication. An. Sist. Sanit. Navar. 2003; 26: 129–139, (Suppl 1)[PUBMED], [INFOTRIEVE]
- Sczabo G. Consequence of alcohol consumption's on host defense. Alcohol 1999; 34: 830–841
- Siragusa R. J., Cerda J. J., Baig M. M., Burgin C. W., Robbins F. L. Methanol production from the degradation of pectin by human colonic bacteria. Am. J. Clin. Nutr. 1988; 47: 848–851, [PUBMED], [INFOTRIEVE]
- Skrzydlewska E., Farbiszewski R. Protective effect of N-acetylcysteine on reduced glutathione, reduced glutathione related enzymes and lipid peroxidation in methanol intoxication. Drug Alcohol Depend. 1999; 57: 61–67, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sprent J., Tough D. F. Lymphocyte life-span and memory. Science 1994; 265: 1395–1400, [PUBMED], [INFOTRIEVE]
- Springer T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann. Rev. Physiol. 1995; 57: 827–872, [CROSSREF]
- Stegink L. Aspartame metabolism in humans: Acute dosing studies. Aspartame: Physiology and Biochemistry, L. Stegink, L. Filer. Marcel Dekker, Inc., New York 1984; 143
- Szabo G. Alcohol's contribution to compromised immunity. Alcohol Health Res. World 1997; 21: 30–41, [PUBMED], [INFOTRIEVE], [CSA]
- Tephly T. R. Mini review—The toxicity of methanol. Life Sci. 1991; 48: 1031–1041, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wilkonson P. C. Phagocytosis of killed Candida albicans. Techniques in Clinical Immunology, R. A. Thompson. Blackwell Publication, Oxford 1977; 212
- Wilkonson P. C. Neutrophil adhesion test. Handbook of Experimental Pharmacology, J. K. Vane, S. H. Ferreria. Springer-Verlag, Berlin 1978; Vol. I: 109
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions, and pathophysiological aspects. Lab. Invest. 2000; 80: 617–653, [PUBMED], [INFOTRIEVE], [CSA]
- Zhang P., Bagby G. J., Xie M., Stoltz D. A., Summer W. R., Nelson S. Acute ethanol intoxication inhibits neutrophil β 2 integrin expressions in rat during endotoxemia. Alcohol. Clin. Exp. Res. 1998; 22: 135–141, [PUBMED], [INFOTRIEVE], [CSA]
