Abstract
Results from earlier experiments in our laboratories revealed that both selective and nonselective inhibitors of cyclooxygenase-2 possess little potential for decreasing in vitro phagocytosis by rat macrophages or canine neutrophils and no potential for decreasing in vivo phagocytosis by the intact murine immune system. We now report the results of studies to assess in vitro and ex vivo effects of the drugs on 1) canine complement activation, 2) generation of superoxide anion and hydrogen peroxide (oxidative burst) by canine neutrophils, and 3) leukocytic chemotaxis and transmigration through endothelial cell monolayers. In vitro concentrations of naproxen sodium, SC-236, SC-245, and SC-791 ranging from 0.1 to 10 μM were tested for their abilities to inhibit canine complement-mediated hemolysis of opsonized sheep erythrocytes and to block phorbol myristate acetate-induced oxidative burst in canine neutrophils. Both models responded to known inhibitory agents, leupeptin in the complement activation test and staurosporine in the superoxide anion assay. In contrast, tested nonsteroidal anti-inflammatory drugs produced only trivial changes in complement activation and superoxide anion production. Experiments on plasma and neutrophils isolated from dogs administered an experimental selective COX-2 inhibitor during a 28-day toxicology study revealed no evidence of drug-associated changes in complement activation or formation of superoxide anion. SC-791 reduced chemotaxis of canine leukocytes toward zymosan-activated dog plasma, but not toward leukotriene B4. None of the other drugs tested significantly affected leukocytic chemotaxis. Ibuprofen, SC-245 and SC-791 but not SC-236, reduced transmigration of canine leukocytes through endothelial cell monolayers. Based on the results of these experiments and our earlier studies we have concluded that, although high (suprapharmacologic) concentrations of the drugs may induce in vitro evidence of apparent immunomodulation of the innate immune system, the findings are unlikely to represent a significant human health risk.
INTRODUCTION
Prostaglandins (PGs) are eicosanoid mediators generated through the cyclooxygenases (COX) that have been described as essential for modulating the immune response toward dietary antigens and localized bacterial infections (Tanaka et al., Citation1998; Newberry et al., Citation1999; Maloney et al., Citation2000). More specifically, PGE2 has been demonstrated to downregulate MHC Class II and IL-12 receptor expression, increase production of IL-10, and decrease production of TNF-alpha and IL-12 (Van der Pouw Kraan et al., Citation1995; Demeure et al., Citation1997; Kalinski et al., Citation1997; Wu et al., Citation1998), all important immunoregulatory cytokines for mediating protection to an infectious challenge. As COX is a key enzyme in the synthesis of PGE2, this provides a major target for the nonsteroidal anti-inflammatory drugs (NSAIDs) including both the conventional nonselective inhibitors such as ibuprofen and naproxen, as well as the new generation of selective COX-2 inhibitors, such as celecoxib and rofecoxib.
Inhibition of COX by NSAIDs has been clearly demonstrated to result in an anti-inflammatory response. COX-1 is a constitutively expressed isoform present in most tissues and COX-2 is a highly inducible isoform, expression of which is increased in inflammatory cells and tissues, including those surrounding localized bacterial infections such as macrophages and neutrophils (Khan et al., Citation2000). With the understanding that COX-1 and COX-2 are chronically expressed in tissues of the immune system, there has more recently been concern about the widespread use of NSAIDs and their potential adverse effects of COX inhibition on immune function (Colville-Nash et al., Citation2001). Although anecdotal in nature, there are reports in both human and veterinary literature suggestive of a direct association between susceptibility to soft tissue infections (especially gram positive cocci) and NSAID use (Stevens, Citation1995; Browne et al., Citation1996; Miller et al., Citation1996), including both the conventional nonselective inhibitors (Verfaillie et al., Citation2002) as well as the new generation of selective COX-2 inhibitors (Aronoff and Bloch, Citation2003). The worst-case clinical implications of drug-associated immunomodulation are sufficiently severe to prompt at least preliminary efforts to study possible interactions between COX-2 inhibitors and the mammalian immune system.
In investigating this issue, our initial focus was on the potential for NSAIDs to impair the natural host defense mechanisms or the innate immune response, as this is the first line of defense against many common pathogens and is essential to the control of common bacterial infections. We have previously reported the results of studies of the effects of NSAIDs on in vitro and in vivo phagocytic activity (Furst et al., Citation2004). In those experiments it was found that suprapharmacological concentrations of the drugs were required to cause only marginal inhibition of in vitro phagocytosis and that the oral administration of two selective COX-2 inhibitors did not compromise the phagocytic activity of the intact murine immune system.
We now report the effects of several selective and nonselective COX-2 inhibitors on additional parameters of innate immunity: (1) in vitro and ex vivo hemolytic complement activation in canine plasma, (2) in vitro and ex vivo oxidative burst activity by canine neutrophils, (3) in vitro leukocytic chemotaxis by canine leukocytes and (4) transmigration of canine leukocytes through a monolayer of endothelial cells. The results of these experiments continue to support our hypothesis that, if COX-2 inhibitors possess a potential for immunomodulation, it is small and of doubtful clinical significance.
METHODS
Drugs and Selectivity Ratios
Selective COX-2 inhibitors SC-236, SC-245, and SC-791, experimental compounds synthesized in our chemistry laboratories, and the conventional NSAIDs, naproxen and ibuprofen obtained from Sigma-Aldrich (St. Louis, MO) were used for these experiments. The pharmacologic properties of these drugs with respect to cyclooxygenase inhibition are described in . Ibuprofen and naproxen exhibit roughly 3- to 7-fold preference for COX-1 relative to COX-2. The selective COX-2 inhibitors exhibited unequivocal preference for COX-2 ranging from greater than 3700-fold (SC-236 and SC-245) to more than 16,000-fold (SC-791).
TABLE 1 Comparative cyclooxygenase inhibiting properties of ibuprofen, naproxen, SC-236, SC-791, and SC-245
Isolation of Biological Materials
Peripheral blood from healthy, untreated beagle dogs of either sex was used as the source of biological materials for the in vitro experiments. Blood samples for the conduct of ex vivo assessments of the effects of SC-791 on canine complement activation and generation of superoxide anion in response to phorbol myristate acetate (PMA) were obtained from female beagle dogs in a repeated dose toxicity study of the drug.
Toxicokinetic analyses of plasma samples revealed that, after 4 consecutive days of dosing, mean drug plasma concentrations had achieved steady state at approximately 12.5 μM. Blood samples for ex vivo assessments of effects on complement activity were collected both before and after initiation of daily dosing (13 and 4 days before and on drug days 14, 22, and 28). Samples collected for assessments of ex vivo effects of SC-791 on oxidative burst activity were drawn immediately prior to administration of the drug on drug days 1, 14, 22, and 28.
Serum for both in vitro and ex vivo assessments of complement activation was collected, without anticoagulant, and allowed to clot at room temperature for approximately 30 minutes. It was then centrifuged and the serum was stored at −20° or −70°C until use.
Blood for oxidative burst testing was collected over heparin. The method to isolate neutrophils employed three different densities (1.06, 1.07, and 1.09) of Percoll (Pharmacia Biotech Inc., Piscataway, NJ), a colloidal silica particle solution, layered on top of each other in a discontinuous gradient. Prior to the percoll isolation, samples were gently centrifuged and plasma removed and retained for later use in the assay. The approximate volume lost by removing the plasma was replaced with Hanks' Balanced Salt Solution (HBSS) without Ca+ + and Mg+ +, pH 7.4 (Gibco BRL, Grand Island, NY).
Cell suspensions were layered on the Percoll gradients and centrifuged for 50 minutes at 600 ×g. Neutrophils banded primarily at the 1.07/1.09 interface while the majority of the red blood cells migrated to and through the 1.09 solution and other cell types banded at various points in the 1.06 and 1.07 solutions. After lysis of residual red blood cells with buffered ammonium chloride/KHCO3, pH 7.4 solution (StemCell Technologies, Vancouver, BC), neutrophils were washed and resuspended in autologous serum to assess their oxidative burst response. Ex vivo assessment of the effects of SC-791 on oxidative burst generation were conducted on both isolated neutrophils and on whole blood.
Leukocytes for the in vitro chemotaxis assay were isolated from heparinized peripheral blood by a Ficoll density gradient procedure. Five mL of whole blood was mixed with 20 mL of HBSS supplemented with 10 mM HEPES buffer, pH 7.4 (Gibco BRL, Grand Island, NY). This mixture was layered over 10 mL of Histopaque 1077 (Sigma Chemicals, St. Louis, MO) and centrifuged for 25 minutes at 1200 × g. The leukocyte layer was collected and washed with HBSS supplemented with 10 mM HEPES.
EXPERIMENTAL PROCEDURES
Assessment of Total Complement Activity
Total complement activity was measured in vitro and ex vivo with a commercially available assay kit (EZ Complement CH50 Test Kit, Diamedix Corp., Miami, FL). The assay is based on complement-mediated hemolysis of opsonized sheep erythrocytes. The degree of complement activity present in serum is directly proportional to the amount of hemoglobin released during lysis of the erythrocytes. Sheep erythrocyte hemolysis was monitored by tracking changes in spectrophotometric absorbance at 540 nm.
Although the assay kit was intended for determination of human complement activity, aliquots of pooled canine serum were substituted for the reference human serum provided with the kit to meet our experimental needs.
In Vitro Assessment of Complement Activation Fresh stock solutions of naproxen, SC-236, SC-791, and SC-245 were prepared daily by dissolving the drugs in ethanol (SC-236 and SC-791), dimethyl sulfoxide (SC-245) or deionized water (naproxen sodium). Following addition of either drug or known inhibitory agent to the assay mixture, the concentration of the original solvent did not exceed 0.5%. The protease inhibitor, leupeptin hemisulfate (Calbiochem-Novabiochem Corp., La Jolla, CA), was employed as the known inhibitory agent for the complement activation test.
Tubes containing opsonized sheep erythrocytes were brought to room temperature, and then solutions of test or known inhibitory agents were added. Ten minutes later, reference serum (human or canine) was added and the mixture was allowed to stand at room temperature for 60 minutes. After centrifugation the absorbance of the supernatant was determined at 540 nm with a Diamedix EZ reader. Each set of incubations included tubes to control for spontaneous erythrocyte lysis and for changes in absorbance mediated by test or known inhibitory agents alone, and by untreated human or dog sera. Changes in absorbance attributable to experimental conditions were appropriately deducted from the absorbance values determined for each tube.
For in vitro experiments, the complement activity in the presence of test or known inhibitory agents was reported as a percentage of the untreated sera (negative control) absorbance. Incubations were conducted in triplicate and each drug was tested in 3 to 6 discrete experiments. Mean values (percent change from negative control) were computed for each triplicate and the data from the 3 to 6 experiments were subjected to one-way analysis of variance (ANOVA). When ANOVA indicated the presence of a treatment effect, Dunnett's test was applied to determine which treatment(s) elicited statistically significant changes.
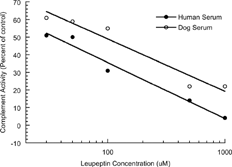
A preliminary experiment was conducted to ascertain the appropriate concentrations of leupeptin to use as the known inhibitory agent and to ensure that dog serum was an appropriate substitute for the human serum provided with the assay kit. Concentrations of 0, 30, 50, 100, 500, and 1000 μ M of leupeptin hemisulfate were tested, side-by-side, on samples of human and canine sera. The results of that experiment are summarized in the Results and .
FIG. 1 Inhibitory effects of leupeptin on human and canine complement activities. Human and canine complement were incubated with opsonized sheep erythrocytes in the presence of leupeptin (30, 50, 100, 500, or 1000 μM) for 60 minutes. Each data point represents the mean of 6 to 9 experiments, each consisting of 3 replicate incubations. Optical density was measured at 540 nm. Data points represent absorbance expressed as percent of control (0 μ M leupeptin). Leupeptin IC50 concentrations were 38.2 and 106.8 μM for human and dog sera, respectively.
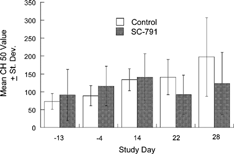
Ex Vivo Assessment of Effects of SC-791 on Total Complement Activity For ex vivo assessments, aliquots (7 μL) of canine serum obtained from SC-791-dosed dogs were added to tubes containing opsonized sheep erythrocytes and incubated and monitored as described above. Sheep erythrocyte lysis was monitored spectrophotometrically and the results were expressed as CH50 values. CH50 values describe the amount of complement required to achieve 50% lysis of the opsonized sheep erythrocytes present. Thus, inhibition of complement activation is reflected by elevations in CH50 values.
Assessment of Superoxide Anion Production by Neutrophils
In Vitro Assessment of Superoxide Anion Production Superoxide anion generation was monitored by the microtiter plate method described by Bjorquist et al. (Citation1994). Oxidative burst activity of canine neutrophils was induced by the method of Tan et al. (Citation1998) in which neutrophils were incubated in the presence of phorbol myristate acetate (PMA, Sigma). In vivo, superoxide production in lysosomes is mediated by NADPH-oxidase (Segal and Abo, Citation1993), which is activated by protein kinase C (Korchak et al., Citation1984). PMA, a known activator of protein kinase C, is a potent indirect stimulant of superoxide anion generation by neutrophils.
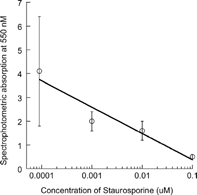
Staurosporine, a protein kinase C inhibitor, was used as a known inhibitiory agent for inhibition of oxidative burst activity. A preliminary experiment was conducted to verify the inhibitory effects of staurosporine on PMA-induced superoxide anion formation in our laboratory. Staurosporine was tested at concentrations of 0, 0.001, 0.01, and 0.1 μM. The results of that experiment are summarized in the Results and . PMA-induced superoxide production by neutrophils was monitored by spectrophotometic detection of ferricytochrome c reduction in the presence of staurosporine or the test compounds.
FIG. 2 Inhibitory effect of staurosporine on PMA (100 ng/mL) induced superoxide anion formation by canine neutrophils. Neutrophils were exposed to 100 ng/mL PMA in the presence of 0, 0.001, 0.01, or 0.1 μM of staurosporine and optical density at 550 nm was determined. Data points represent the mean ± SD of 4 incubations, each conducted in triplicate.
Neutrophils were incubated (1 hour at 37°C in O2 with 5% CO2,) in 96-well microtiter plates in Dulbecco's phosphate-buffered saline (DPBS)/glucose, pH 7.4, with ferricytochrome c. Test compounds, dissolved in DPBS/glucose, were added to incubation wells and the reaction was started by addition of PMA. The final volume of incubation medium was 300 μL/well containing 3 × 105 neutrophils, 375 μg of ferricytochrome c, 30 ng of PMA, 0.1, 1, or 10 μM of test agent or 0.01 or 0.1 μM staurosporine. After 30 minutes, absorbance at 550 nm was determined with a Power Wave 200 scanning spectrophotometer (Biotek Industries, Winooski, VT). To ensure that the increased absorbance observed was due entirely to superoxide production, additional cells were incubated with 20 μg/mL superoxide dismutase (SOD; Sigma) Baseline superoxide production (in the absence of PMA) for each animal was determined. The same end point was determined in neutrophils stimulated with PMA alone and PMA in the presence of SOD.
Wells containing only neutrophils, ferrichrome c, and PMA served as negative controls. Absorbance in wells containing neutrophils, ferrichrome c, PMA, and test (or known inhibitory) articles were compared to that in the negative control wells. Effects of test agents on PMA-induced superoxide anion formation were expressed as a percentage of superoxide anion formed in negative control wells.
Naproxen and the selective COX-2 inhibitors were each tested, always in triplicate, 3 to 6 times. Neutrophils from each dog were used only once for each drug. The effects of test agents on superoxide anion production were expressed as a percentage of control value and data were analyzed by ANOVA and Dunnett's test.
Ex Vivo Assessment of Effects of SC-791 on Oxidative Burst Ex vivo effects of SC-791 on the ability of canine neutrophils to respond to PMA with the generation of superoxide anion and hydrogen peroxide were assessed by the method of Himmelfarb et al. (Citation1992). Dihydrorhodamine (DHR), a dye that easily permeates most cell membranes, in the presence of reactive oxygen species such as those generated during an oxidative burst, is oxidized to a fluorescent compound, rhodamine-123. Rhodamine-123 fluorescence can be monitored by flow cytometry. Intensity of fluorescence is directly proportional to the number of neutrophils exhibiting intracellular oxidative burst activity.
Samples of 100 μL of heparinized whole blood were incubated with DHR (25 μ g/mL, Molecular Probes, Eugene, OR) at 37°C for 15 minutes. After incubation, 100 μL aliquots of either phosphate buffered saline (Ca++- and Mg++-free, PBS, pH 7.4, Gibco BRL, Grand Island, NY) or PMA (1.0 μ g/mL or 0.1 μ g/mL in PBS with 10 mM glucose) were added to tubes containing the whole blood. PBS served as a negative control treatment to determine nonspecific fluorescence. Nonspecific fluorescence was subtracted from fluorescence detected in the PMA-stimulated mixtures. After addition of PBS or PMA, tubes were incubated at 37°C for 15 minutes followed by lysis of red blood cells using the Q-Prep (Coulter Corporation, Miami, FL).
Samples were analyzed for fluorescence with a Coulter XL-MCL flow cytometer equipped with an argon laser. Cell populations were enumerated by light-scatter gating measuring linear forward light scatter (FSC) and linear side scatter at an approximate 90° angle. Fluorescence was derived from a photomultiplier tube with a 525 nm band pass filter using four-decade log amplification. Data are presented as a percentage of SC-791-exposed neutrophils yielding positive fluorescence in response to PMA treatment relative to fluorescence in neutrophils from the respective PBS control treatment.
In Vitro Assessment of Leukocyte Chemotaxis
General Experimental Procedures and Conditions Chemotaxis toward either zymosan-activated dog plasma (ZAS) or leukotriene B4 (LTB4, Sigma) was determined using the ChemoTx microplate system (Neuroprobe, Inc., Bethesda, MD). The chemoattractants, diluted in RPMI medium were pipetted into microplate wells and covered with an 8-μm pore-size filter. Leukocytes (105) in RPMI medium were pipetted onto the filter and the plates were incubated at 37°C for 30 minutes. Filters were then fixed with methanol, stained with Diff-Quick stain (Dade International, Inc., Miami, FL) and examined microscopically (at 400× magnification) to determine the number of cells that had migrated to the underside of the filter. Twenty microscopic fields were counted on each filter. Cytochalasin B was used as the known inhibitory agent of chemotaxis.
Leukocytes from some, but not all, dogs exhibit a small amount of migration despite the absence of attractant. For this reason a “background” leukocyte migration was determined for each experimental condition tested. The leukocyte background migration was subtracted from that elicited by the chemoattractant. The adjusted number of leukocytes exhibiting migration was termed “specific migration.” Mean specific migrations were computed from quadruplicate incubations and data were analyzed by analysis of variance.
On each experimental day all drug and control conditions were tested on leukocytes from a single dog. Four concentrations of each drug (0, 0.1, 1, or 10 μM) were tested, in quadruplicate, in the presence and absence of chemoattractant.
Selection of Attractant Concentrations Preliminary incubations were conducted to determine the optimal concentrations of chemoattractants for use in these experiments. Canine leukocytes were incubated over concentrations of ZAS (ranging from 0 to 10%) or LTB4 (ranging from 0.1 to 1000 nM) for 30 minutes. Mean numbers of migrating cells were determined from duplicate or triplicate incubations. The objective of the preliminary experiment was to determine attractant concentrations that would elicit specific migration by roughly 1/3 to 2/3 of the tested leukocytes. The results of these experiments are summarized in the Results and .
TABLE 2 Responsiveness of canine leukocytes to chemoattractantsFootnote1
Testing Drugs on Leukocytic Chemotaxis Naproxen was initially dissolved in water, SC 791, and cytochalasin B in ethanol and SC-245 and SC-236 in DMSO. Solvent concentrations in final incubations did not exceed 0.5%. Cytochalasin B was tested at 0.01, 0.1, 0.5, or 1 μM and drugs were tested at 0.1, 1, or 10 μM.
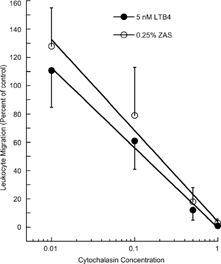
Leukocytes were not pooled as in vitro assessment of chemotaxis exhibited substantial between-animal variability. Consequently, all drugs were tested against leukocytes from the same four animals. Assays were conducted in triplicate and raw data (i.e., the number of cells exhibiting specific migration) were analyzed by analysis of variance. The consistent and dose-related inhibitory effect of cytochalasin B on chemotaxis of canine leukocytes is demonstrated in .
FIG. 3 Inhibitory effects of cytochalasin B on chemotaxis of canine leukocytes toward LTB4 and ZAS. Canine leukocytes were exposed to 0.01, 0.1, 0.5, or 1.0 μM cytochalasin B then exposed to either 5 nM LTB4 or 0.25% ZAS. Leukocytes migrating through filters were counted. Extent of migration in the absence of cytochalasin was considered to be 100% of control and effects of cytochalasin were expressed as percent of control.
Assessment of Canine Leukocyte Migration Through Endothelial Cell Monolayers
In vitro effects of the drugs on the ability of leukocytes to transmigrate endothelial cell monolayers were assessed according to the method of Hofbauer et al. (Citation1998). Canine leukocyte migration through homologous endothelial cells was induced by stimulation of monolayers of cultured endothelial cells with recombinant human tumor necrosis factor-alpha (TNFα) (Invitrogen Corporation, Carlsbad, CA).
Preparation of Endothelial Cell Monolayers Canine umbilical vein endothelial cells (CUVEC) obtained from Cambrex Corp., East Rutherford, NJ, were seeded in 500 μl EGM2 media (Clonetics, Cambrex Corp.) using fibronectin-coated 3 μm microporous membrane inserts for 24-well culture plates (3–4 × 105 cells per insert). On the day of cultured monolayer utilization, cells were incubated with TNFα-containing RPMI medium (Clonetics, Cambrex Corp.) for a final presence of 500 ng of TNFα in the inserts. After 6 hours, the monolayers were washed with fresh RPMI medium and transferred to fresh culture plate wells, each of which contained 600 μl RPMI medium.
Leukocyte Migration Assays Freshly isolated leukocytes (1 × 106 cells) were exposed to test agents then added to the cultured endothelial cell monolayers, which had been previously exposed to TNFα. The monolayers, leukocytes, and drugs were incubated 16 hours at 37°C in O2, with 5% CO2. All incubations were conducted in triplicate.
Drug concentrations tested were predicated on observed in vivo plasma concentrations of drugs in dogs during the conduct of earlier repeated dose toxicity studies. The SC-compounds were tested at 0.1-, 1-, and 10-fold multiples of a no-observable-toxic-effect plasma concentration. Ibuprofen was tested at 0.5-, 5-, and 25-fold increments of estimated Cmax therapeutic value for humans. Stock solutions of test compounds were prepared in DMSO then diluted to final incubation concentrations in RPMI media.
At completion of the incubation phase, aliquots of the RPMI media that had surrounded inserts were removed from the well and the number of cells that had migrated through the endothelial cells and fibronectin membrane was determined with a hemocytometer. All assays were conducted on leukocytes obtained from 5 dogs. Triplicate incubations were run and each drug was tested only once with cells from each dog. Cell migration in the presence of TNFα alone served as the control during this exercise.
The results of assays were analyzed by analysis of variance coupled with Dunnett's test. For purposes of tabular presentation of data, normalizing data to percent of control responses minimized interday variability.
RESULTS
Effects of COX Inhibitors on Complement Activation
In Vitro Experiments Leupeptin consistently reduced both human and canine complement activation as evidenced by concentration dependent reductions in absorbance at 540 nm, . Human serum was more sensitive to leupeptin than was dog serum having IC50 values of 38.2 and 106.8 μ M in human and dog sera, respectively. Despite the difference in susceptibility of human and canine sera to leupeptin, the concentration response curves for the two complement sources were parallel and it was concluded that canine serum was a suitable source of complement for the purposes of these assays and that leupeptin was a suitable known inhibitory agent with which to monitor the activity of the assay.
The effects of leupeptin, naproxen, and the selective COX-2 inhibitors on canine complement activity are summarized in . Both concentrations of leupeptin, consistently and significantly (p < 0.0001) reduced the extent of complement-mediated lysis of opsonized sheep erythrocytes by canine neutrophils. The magnitudes of reductions (up to 57% and 28% of controls, by 100 and 1000 μM of leupeptin, respectively) were dose-related.
TABLE 3 Effects of leupeptin, naproxen, and selective cox-2 inhibitors on canine complement activity in vitro
All concentrations of SC-245 tested also reduced complement activity (p = 0.0022) but there was no evidence of a dose-response relationship and the magnitude of effect was essentially the same (22 to 26%) regardless of concentration tested, possibly as a result of saturation. No concentration of either SC-236 or SC-791 significantly reduced complement activity (p = 0.3112 and 0.4405, respectively). All concentrations of naproxen slightly reduced the activity of canine complement, however only the lowest concentration of naproxen at 0.1 μ M, was statistically significant (p < 0.05).
Ex Vivo Experiment Hemolytic complement levels, expressed as CH50 values, determined in dogs before and after administration of SC-791 for up to 28 consecutive days are shown in . Daily administration of 14 mg/kg/day of SC-791 for as long as 28 consecutive days was without apparent effect on the complement system of female beagle dogs. Mean CH50 values for control and SC-791-dosed animals were not significantly different at each of the observation periods.
Effects of COX Inhibitors on Neutrophil Oxidative Burst
Reviews of the literature present several methods that yield very pure neutrophil populations (Coligan et al., Citation1993; Sugawara et al., Citation1995), however, the efficiency of the recovery methods were poor, recovering approximately 10–25% of the starting neutrophil number in peripheral blood. Most neutrophil isolation methods were developed for human peripheral blood and do not take into account that canine neutrophils differ from human neutrophils in that their densities are quite variable, and that the densities of some canine lymphocyte populations are very similar to those of some neutrophil populations. We developed a novel method that combines a primary density gradient centrifugation with ammonium chloride lysis of remaining red blood cells that provided a > 75% recovery of starting neutrophil numbers and a cell purity of > 95% neutrophils.
The in vitro effects of the staurosporine, naproxen, and the selective COX-2 inhibitors on PMA-induced oxidative burst (superoxide anion production) by canine neutrophils are summarized in .
TABLE 4 Effects of staurosporine, naproxen, and selective COX-2 inhibitors on PMA-induced superoxide anion production by canine neutrophils
Staurosporine caused a consistent and concentration-related reduction in superoxide anion production by canine neutrophils in response to PMA stimulation, . Based on the results of this preliminary experiment concentrations of 0.01 and 0.1 μM of staurosporine were selected for use as the known inhibitory agent and both concentrations were tested during each set of experimental replicates.
Exposure of canine neutrophils to either 0.01 or 0.1 μM staurosporine consistently and significantly (p ≤ 0.001) caused dose-related reductions (by 51 and 87% relative to controls, respectively) in superoxide anion production in response to PMA stimulation. Neither naproxen nor the selective COX-2 inhibitors, caused reductions in superoxide anion formation. The only statistically significant change in superoxide anion production was a greater than 2-fold increase after exposure of neutrophils to the highest concentration of SC-245 (10 μM).
The results of the ex vivo assessment of the effects of SC-791 on PMA-induced oxidative burst (H2O2 production) by canine neutrophils in whole blood are summarized in . The ability of neutrophils from SC-791-dosed dogs to respond to PMA was assessed in isolated neutrophils as well as in samples of whole blood. The results of experiments conducted with whole blood and with isolated neutrophils were essentially identical, so only the results obtained in experiments with whole blood are presented.
TABLE 5 Oxidative burst assessed through hydrogen peroxide production in SC-791-exposed canine neutrophils challenged with PMAFootnote1
Regardless of their source (SC-791-dosed or control dogs), the percent of neutrophils that exhibited fluorescence was positively related to the concentration of PMA with which they were stimulated. Treatment of dogs with SC-791 was without effect on responsiveness of neutrophils to in vitro PMA stimulation. The mean percentages of cells from dosed- and control dogs that exhibited fluorescence after 14, 22, or 28 consecutive days of treatment were statistically identical.
Effects of COX Inhibitors on Canine Leukocyte Chemotaxis
Both ZAS and LTB4 produced “inverted U” shaped dose-response curves with maximum responses being produced by approximately 5% ZAS and 50 nM LTB4. Higher concentrations of the chemoattractants consistently elicited less than maximal responses.
Considerable dog-to-dog variability in responsiveness to either attractant was observed when data were expressed as specific migration as in . Maximal responses of canine leukocytes to 5% ZAS ranged from 386 to 1171 cells and ranged from 300 to 1,032 migratory cells to 50 nM LTB4. Despite this between dog variability the minimal to maximal migratory responses were clearly and consistently dose related. Regression analyses revealed that the correlation coefficients for leukocytes obtained from the five discrete dogs were consistently ≥ 0.8 (data not shown).
Based on the data summarized in , we selected 5 nM of LTB4 and 0.25% ZAS as optimal concentrations of attractants against which to test potential inhibitors of leukocytic chemotaxis. These concentrations of attractants were anticipated to induce specific migration of approximately 30% to 60% of leukocytes.
The results of assays of naproxen, SC-236, SC-791, and SC-245 on canine leukocyte chemotaxis, expressed as specific migration, are summarized in . Naproxen inhibited canine leukocyte chemotaxis induced by LTB4 in a concentration-related manner (40% at 10 μ M) but this reduction was not statistically significant due to a high degree of variability. Naproxen was not tested against ZAS.
TABLE 6 In vitro effects (specific migration) of naproxen and selective COX-2 inhibitors on canine leukocyte chemotaxis
SC-791 caused a slight but concentration-related inhibition of chemotaxis in response to both attractants and the inhibition of the response to ZAS was statistically significant (p = 0.0023) although inhibition of the LTB4 response was not (p = 0.1708). SC-245 caused a slight (approximately 24%) decrease in LTB4-induced migration but migration in response to ZAS was actually somewhat increased (23%).
SC-236 inhibited neither ZAS- nor LTB4-induced chemotaxis. In fact, SC-236 exposed leukocytes actually exhibited a 32–33% increase in specific migration in response to both attractants.
In Vitro Assessment of Leukocyte Migration Through Endothelial Cells
Ibuprofen has been previously shown to reduce migration of human leukocytes through human endothelial cell monolayers (Hofbauer et al., Citation1998) and thus was used as a point of reference. The effects of ibuprofen on TNFα-mediated leukocyte transmigration by canine leukocytes are summarized in . Transmigration was significantly reduced in a dose-related fashion.
TABLE 7 Effects of selective COX-2 inhibitors on canine and human leukocyte transmigration through endothelial monolayers
The effects of SC-236, SC-791, and SC-245 on transmigration of canine leukocytes through endothelial cell monolayers are summarized in . Exposure of canine leukocytes to either 1.3 or 13 μM of SC-236 was without statistically significant effect. In the presence of 13 μM SC-236, the extent of transmigration of canine leukocytes was approximately 12% less than that of controls but this difference was not statistically significant. Higher concentrations of SC-236 were not tested due to cytotoxicity as evidenced by lysis of leukocytes.
SC-791 caused dose-related and statistically significant decreases in transmigration of canine leukocytes. Although not significantly affected, canine cells exposed to the lowest concentration of SC-791 (3.9 μM) exhibited a 19% lower rate of transmigration than control.
The mid- and high concentrations of SC-245 also caused statistically significant decreases in transmigration by leukocytes. Again, the lowest concentration was associated with a numerical, but not statistically significant (18%) reduction.
DISCUSSION AND CONCLUSIONS
Our assessments of selective and nonselective COX-2 inhibitors for immunotoxicologic potential continue to yield less than persuasive evidence of a likely significant interaction between either class of drugs and the immune system. In earlier studies (Furst et al., Citation2004) it was observed that naproxen, indomethacin, and experimental selective COX-2 inhibitors, SC-236, SC-245, and SC-791, caused marginal reductions in phagocytic activity of resident rat peritoneal macrophages. Those inhibitory activities were consistently small, generally requiring suprapharmacological concentrations. Similarly, suprapharmacological concentrations (10 μM) of either SC-236 or SC-791 were required to decrease phagocytosis by canine neutrophils. Those in vitro results were corroborated by in vivo experiments during which neither naproxen nor SC-236 affected the intact murine phagocytic system.
In the current experiment the results of our leukocyte migration assay revealed inhibitory effects of both nonselective and selective COX-2 inhibitors on in vitro canine leukocyte migration through endothelial cell monolayers. Ibuprofen had been previously reported to inhibit human leukocyte migration through human endothelial cell monolayers (Hofbauer et al., Citation1998) and in our hands it caused an unequivocal and dose-related reduction in migration of canine leukocytes. Both SC-791 and SC-245 also caused dose-related and statistically significant reductions in transmigration of canine leukocytes. Concentrations of ibuprofen and the selective COX-2 inhibitors required to significantly inhibit leukocyte transmigration were generally in the toxicological range.
Exposure of canine leukocytes to SC-791 also caused a statistically significant reduction in chemotaxis toward 0.25% ZAS although a significant reduction toward LTB4 was not observed. Naproxen, SC-236 and SC-245 were all without statistically significant effects on leukocytic chemotaxis.
The results of these in vitro experiments on the leukocytic chemotaxis and transmigration models suggest some potential for interaction between SC-791 and SC-245 and the immune system. If these selective COX-2 inhibitors do possess a potential for immunosuppression, their clinical liability appears to be similar to that of ibuprofen. And, as was the case during our studies on phagocytosis, these in vitro models required suprapharmacological concentrations of the drugs to exhibit even marginal inhibitory activity.
Assessments of the effects of selective and nonselective COX-2 inhibitors on other immunological functions afforded no evidence of immunotoxicological potential for the drugs. The lowest concentration of naproxen tested was associated with a 21% reduction in complement activation but higher concentrations were without effect. The absence of a dose-response relationship supports the conclusion that the “low-dose effect” observed was spurious.
Earlier investigators (Minta et al., Citation1983; Auteri et al., Citation1988) reported that several NSAIDs caused in vitro inhibition of human complement activation but concentrations in the millimolar range were required. Since the concentrations tested by those workers far exceeded clinical relevance, we are unable to contrast our naproxen results with those of their earlier study.
Neither SC-236 nor SC-791 was associated with reductions in complement activation but every concentration of SC-245 tested resulted in 22% to 26% reductions. Although statistically significant, interpretation of the data is necessarily equivocal. Despite a 100-fold differential, there was no differentiation between magnitudes of reductions associated with the low- and high-concentrations. The marginal, albeit statistically significant, inhibition of complement activation at supratoxicological concentrations cannot be considered meaningful evidence of an immunotoxicological threat.
Finally, the results of the ex vivo assessment of effects of SC-791 on complement activation corroborate our interpretation of the in vitro results. Administration of 14 mg/kg/day of SC-791 for up to 28-consecutive days resulted in steady-state plasma concentrations of approximately 12.5 μM of the parent drug and its active metabolite. That suprapharmacological dose was without effect on the functional hemolytic complement levels in dogs.
Our in vitro canine neutrophil model for assessing oxidative burst activity responded to stimulation by PMA with a prompt increase in superoxide anion production and the protein kinase C inhibitor, staurosporine, caused reproducible and concentration-related decreases in the response. But there was no evidence that any of the drugs tested in the model, naproxen, SC-236, SC-791, or SC-245, reduced superoxide anion generation. The highest concentration of SC-245 (10 μM) was actually associated with a statistically significant increase in superoxide generation. Also, the concentration of SC-245 required to cause the effect was so high relative to anticipated clinical concentrations the findings are of doubtful significance.
Our in vivo assessment of the potential for SC-791 to affect oxidative burst activity through hydrogen peroxide production yielded results that confirmed those obtained by use of the in vitro model. Exposure of neutrophils isolated from SC-791-dosed dogs to either 0.1 or 1 μg/mL of PMA elicited the anticipated oxidative burst. Steady-state plasma concentrations of SC-791 and its metabolite (which totaled approximately 12.5 μM) caused no reduction in PMA-induced oxidative burst activity.
The results of these and our earlier studies continue to support a working hypothesis that like nonselective COX inhibitors, at least some selective COX-2 inhibitors may possess some ability to interact with the immune system. However, their potential to do so appears to be slight. Although suprapharmacologic or toxicologic concentrations in our models may induce evidence of immunomodulation, these findings are of questionable predictive validity for a meaningful human health risk. We believe the findings indicate, at most, a weak potential for clinical effects but because of that, warrant mechanistic investigations to probe for clinically relevant information regarding the ultimate predictive utility of the original observation.
The models used for these investigations provided reproducible results and were consistently sensitive to known inhibitory agents. Although they are somewhat labor intensive, we believe they are well suited for incorporation into batteries of tests that might find utility in the assessment of immunotoxicological potential of chemicals with suspected therapeutic utility.
ACKNOWLEDGMENTS
The authors express their appreciation to Dale Morris, Anita White, Sandra Curtiss, Amy Cohen, Jeanne Stapleton, Elsa Blomquist, Kassandra McGehee, and Cindy Gross for their technical expertise and assistance.
REFERENCES
- Aronoff D. M., Bloch K. C. Assessing the relationship between the use of nonsteroidal anti-inflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine 2003; 82: 225–235, [PUBMED], [INFOTRIEVE]
- Auteri A., Saletti M., Blardi P., DiPerri T. Action of a new non-steroidal anti-inflammatory drug, nimesulide, on activation of the complement system: An in vitro study. Int. J. Tissue React. 1988; 4: 217–221
- Bjorquist P., Palmer M., Bengt E. K. Measurement of superoxide anion production using maximal rate of cytochrome (III) C reduction in phorbol ester stimulated neutrophils, immobilized to microtiter plates. Biochem. Pharmacol. 1994; 48: 1967–1972, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Browne B. A., Holder E. P., Rupnick L. A. Nonsteroidal anti-inflammatory drugs and necrotizing faciitis. Am. J. Health-Syst. Pharm. 1996; 53: 265–269, [PUBMED], [INFOTRIEVE], [CSA]
- Coligan J. E., Kruisbeek A. M., Margulies D. H., Shevach E. M., Strober W. Preparation and functional analysis of human nonlymphoid cells. Current Protocols in Immunology, J. E. Coligan, B. E. Bierer, D. H. Margulies, E. M. Shevach, W. Strober. John Wiley & Sons, New York 1993; 7.23.1–7.23.16
- Colville-Nash P. R., Gilroy D. W. Potential adverse effects of cyclooxygenase-2 inhibition: Evidence from animal models of inflammation. BioDrugs 2001; 15: 1–9, [PUBMED], [INFOTRIEVE], [CSA]
- Demeure C. E., Yang L. P., Desjardins C., Raynauld P., Delespesses G. Prostaglandin E primes naïve T-cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 1997; 27: 3526–3532, [PUBMED], [INFOTRIEVE], [CSA]
- Furst S. M., Khan K. N., Komocsar W. J., White K. L., Peachee V. L., Mennear J. H. Screening new drugs for immunotoxic potential: I. Assessment of the effects of conventional and selective COX-2-inhibiting NSAIDs on in vitro and in vivo phagocytic activity. J. Immunotoxicol. 2004; 1: 149–158, [CROSSREF]
- Himmelfarb J., Hakim R. M., Holbrook D. G., Leeber D. A., Ault K. A. Detection of granulocyte reactive oxygen species formation in whole blood using flow cytometry. Cytometry 1992; 13: 83–89, [PUBMED], [INFOTRIEVE]
- Hofbauer R., Speiser W., Kapiotis S. Ibuprofen inhibits leukocyte migration through endothelial cell monolayers. Life Sci. 1998; 62: 1775–1781, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kalinski P., Hilkens C. M., Snijders A., Snijdewint F. G., Kapsenberg M. L. IL-12-deficient dendritic cells, generated the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naïve T helper cells. J. Immunol. 1997; 159: 28–35, [PUBMED], [INFOTRIEVE]
- Khan K. N., Stanfield K. M., Trajkovic D. T., Harris R. K. Cyclooxygenase-2 expression in inflammatory lung lesions of nonhuman primates. Vet. Pathol. 2000; 37: 512–516, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus-response coupling in the human neutrophil, II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J. Biol. Chem. 1984; 259: 4076–4082, [PUBMED], [INFOTRIEVE]
- Maloney C. G., Thompson S. D., Hill H. R., Bohnsack J. F., McIntyre J. F., Zimmerman G. A. Induction of cyclooxygenase-2 by human monocytes exposed to group B streptococci. J. Leukocyte Biol. 2000; 67: 615–621, [PUBMED], [INFOTRIEVE], [CSA]
- Maziasz T., Khan K. N., Talley J., Gierse J., Seibert K. The development of drugs that target cyclooxygenase-2. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press Inc., Totowa, NJ 2003
- Miller C. W., Prescott J. F., Mathews K. A., Betschel S. D., Yager J. A., Guru V., DeWinter L., Low D. E. Streptococcal toxic shock syndrome in dogs. JAVMA 1996; 209: 1421–1426, [PUBMED], [INFOTRIEVE], [CSA]
- Minta J. O., Urowitz M. B., Smythe H. A., Isenman D. E. Effect on the human complement system of the major nonsteroidal anti-inflammatory drugs: Aspirin, indomethacin, phenylbutazone, oxyphenbutazone and sulindac. Clin. Exp. Immunol. 1983; 53: 555–561, [PUBMED], [INFOTRIEVE], [CSA]
- Newberry R. D., Stenson W. F., Lorenz R. G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat. Med. 1999; 5: 900–906, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Segal A. W., Abo A. The biochemical basis of the NAPH oxidase of phagocytes. Trends Biochem. Sci. 1993; 18: 43–47, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Stevens D. L. Could nonsteroidal anti-inflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome?. Clin. Infect. Dis. 1995; 21: 977–980, [PUBMED], [INFOTRIEVE], [CSA]
- Sugawara T., Miyamoto M., Takayama S., Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J. Pharm. Tox Meth. 1995; 33: 91–100, [CROSSREF]
- Tan A. S., Ahmed N., Berridge M. V. Acute regulation of glucose transport after activation of human peripheral blood neutrophils by phorbol myristate acetate, fMLP, and granulocyte-macrophage colony-stimulating factor. Blood 1998; 91: 549–655, [CSA]
- Tanaka K., Tanaka H., Kanemoto Y., Tsuboi I. The effects of non-steroidal anti-inflammatory drugs on immune functions of human peripheral blood mononuclear cells. Immunopharmacology 1998; 40: 209–217, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Van d er, Pouw Kraan T. C., Boeije L. C., Smeenk R. J., Wijdenes J., Aarden L. A. Prostaglandin E2 is a potent inhibitor of human interleukin-12 production. J. Exp. Med. 1995; 181: 775–779, [CROSSREF]
- Verfaillie G., Knape S., Corne L. A case of fatal necrotizing fasciitis after intramuscular administration of diclofenac. Eur. J. Emerg. Med. 2002; 9: 270–273, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wu C. Y., Wang K., McDyer J. F., Seder R. A. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J. Immunol. 1998; 161: 2723–2730, [PUBMED], [INFOTRIEVE]