Abstract
Fluoride pollution in drinking water is an international problem as the fluoride present is often at levels above acceptable limits. In the studies reported here, sodium fluoride (NaF) treatment of rats by gavage for 28 days resulted in the induction of oxidative stress and immunotoxicity. It was shown here that NaF treatment lowered cellular immunity in the rats as illustrated by a significant diminution in peripheral blood lymphocyte, monocyte and neutrophil counts in conjunction with a reduction in splenocyte counts. Effects of NaF treatment on humoral immunity were reflected here in a lowering of the levels of plasma IgG specific to a test antigen (i.e., bovine serum albumin). Disorganization in the histoarchitecture was also noted in the host spleen and thymus after NaF treatment. To determine if oxidative stress was among the potential possible causes for the observed induced immunotoxicities, catalase and peroxidase activities along with malondialdehyde (MDA, product of free radical damage to cells) levels in the spleen and peripheral blood packed cells were also measured. The results indicated that there was a significant diminution in the activities of both the enzymes along with an elevation in MDA levels in both the tissues in treated rats. This report highlights the proposition that chronic exposure to fluoride contaminated drinking water is likely to result in immunotoxicity and, furthermore, that the damage to primary immune organs is due to an induction of oxidative stress.
INTRODUCTION
Currently, fluoride pollution is a worldwide problem as the fluoride present in the drinking water in South and North America, China, Sri Lanka, the West Indies, Holland, Italy, and Spain is often at levels above a tolerable limit (Suma Latha et al., Citation1999). In India, fluoride levels in drinking water are also above the acceptable limit in wide areas of the states of Andhra Pradesh, Tamil Nadu, Karnataka, Punjab, Bihar, Haryana, Kerala and West Bengal (Handa, Citation1975; Suma Latha et al., Citation1999). In West Bengal, six districts are affected by fluoride pollution and in most of the affected areas, fluoride present in drinking water is at a level of 10–20 ppm (Handa, Citation1975), and a maximum level of 38 ppm has been reported (Fazlul Hoque et al., Citation2003). The international standard for a permissible limit of fluoride in drinking water has been established at 1 ppm (Agrawal et al., Citation1997) and the safe level of fluoride via dietary intake in adults is 3-4 mg/d (Foulkes, Citation1997).
Though fluoride has an immense pharmaceutical importance (Robert, Citation1980), there are reports about fluoride-induced health disorders. The most common acute toxic effects that arise from fluoride intoxication are increased salivation, nausea, and abdominal pain (Robert, Citation1980). The most common toxicities that have been reported arising from chronic exposures are premature aging, skeletal and dental fluorosis, arthritic pain, osteosclerosis, and crippling fluorosis (Pak et al., Citation1989). Fluoride intoxication also results in neurologic damage, primarily in the impairment of both cognition and memory (Spithe, Citation1994). Fluoride intoxication also results in metabolic disorders (Bogin et al., Citation1976), hepatitis (Carlson and Suttle, Citation1996), renal toxicity (Suketa and Terui, Citation1980), muscular atrophy (Susheela and Kharp, Citation1990), decalcification and fragility of bone (Robert, Citation1980), and reproductive disorders (Ahotupa and Huhtaniemi, Citation1992). We have published results of studies that showed that sodium fluoride at a dose of 20 mg/kg body weight/d for chronic periods also resulted in an inhibition in testicular androgenesis and gametogenesis (Ghosh et al., Citation2002). Very recently, we reported that fluoride exposure at this dose resulted in the imposition of an oxidative stress in male reproductive organs (Das [Sarkar] et al., Citation2005).
There is some information about the toxic effects of fluoride on the immune system. Apart from a study that indicated that exposure to fluoride was correlated to an increase in the onset of autoimmunity (Gibson Sheim, Citation1992), there are very few publications along this in vivo line (Curnette et al., Citation1979). There are equally few papers in the scientific literature that report the results of studies to examine the immunotoxicologic effects of fluoride. Among these, one focused on select activities of immune cells after in vitro exposure and found that these were diminished by fluoride treatment (Yang et al., Citation2000). The studies being reported here sought to provide a somewhat clearer picture about the potential immunotoxicologic effects from exposure to fluoride ions. Utilizing an exposure scenario closer to what actually occurs among millions of people throughout the world each day, these studies focused on monitoring for specific effects of fluoride on cellular and humoral immunity as well as beginning to discern what might be the underlying mechanism(s)for the observed effects.
METHODS
Experimental Design
Sixteen pathogen-free adult male albino Wistar rats (body weight of 120 ± 10 g, 90 days of age) were purchased from professional animals breeders (Joy Tara Traders, Kolkata, India). Upon arrival, all rats were acclimatized in controlled temperature and light-dark cycle facilities in our laboratory for 15 d prior to experimentation. Throughout this period, the animals were monitored for growth, health status, and food intake capacity to be certain that they were indeed healthy. For the experiments, animals were divided into two groups (n = 8) after matching body weights. One group was designated as the placebo control and the other the sodium fluoride (NaF)-treated group. Animals in both groups were first subjected to treatment with bovine serum albumin (BSA) along with complete and incomplete adjuvant for anti BSA development for a scheduled period (described in detail below). Twenty-eight d before completion of schedule period of adjuvant treatment, NaF treatment was started in one group while animals in the control group were subjected to placebo treatment. Specifically, animals in the NaF group were subjected to oral gavage with NaF at a dose of 20 mg/kg body weight (BW)/d for 28 consecutive days. This NaF dose is equivalent to 9 ppm of fluoride over the course of the exposures. Control animals were gavaged each day with an equal volume of distilled water. After completion of the treatment, the animals were sacrificed by light ether anaesthesia followed by decapitation. The body weights of all animals were recorded before decapitation. At sacrifice, a sample of each rat's blood was then collected from the femoral vein, and the spleen and thymus were aseptically removed and then weighed. Blood smears were prepared for differential counts. Plasma was isolated from the harvested blood for later use in assays of immunoglobulin levels. The cellular portion of the blood sample was used to prepare packed cell volumes for assays of various biochemical parameters. At collection, the packed cell volume weight was recorded. One part of each rat's spleen and thymus were designated for use in histologic studies. The remainder of each rat's spleen was used for determination of splenocyte counts and for oxidative stress assessment.
Splenocyte Counts and Peripheral Blood Lymphocyte, Neutrophil, Monocyte Counts
The duplicate blood smears prepared from each animal were processed with Leishman's stain. After drying, the slides were examined under light microscopy (Olympus-CH20i Germany) at 100X to determine the average peripheral blood counts of lymphocytes, neutrophils, and monocytes for each animal. A minimum of 100 leukocytes/slide were analysed from each smear. Results were subsequently expressed in terms of the percentages of each type among all leukocytes counted.
Splenocyte counts were performed by using forceps to tease apart the isolated sample of spleen in 5 ml of PBS at the tissue concentration of 50 mg/ml of PBS. Quantification of the splenocytes was then performed using a haemocytometer (Nandakar and Hegde, Citation1996). All results were expressed as total number of non-red blood cells/g splenic tissue.
Assessment of anti-BSA IgG Levels in Peripheral Blood
To assess plasma levels of IgG specific for BSA, induction of antibody was carried out by sensitization and boosting with BSA. For this purpose, 1 ml 1% BSA (Sigma, St. Louis, MO) in normal saline was injected subcutaneously (SC) with 1 ml of complete adjuvant. Two weeks later, 1 ml incomplete adjuvant was injected SC; this was followed by SC injections of incomplete adjuvant at 4, 6, and 8 weeks after the sensitizing injection. These final two boosters were provided to rats during the period of treatment with NaF or water vehicle. Ten to 12 d after completion of the boosting injections (which was also the terminal date of the NaF or vehicle treatments), blood samples from rats in each group were harvested and plasma levels of BSA-specific IgG were measured.
Antibody determination was carried out using an enzyme-linked immunosorbent assay (ELISA). In the ELISA protocol, Highbond plates (Costar 3590) were first coated overnight at 4°C with 1 μg/ml BSA antigen in 0.05 M carbonate buffer (pH 9.6), washed, and then blocked with PBS-Tween/3% milk powder for 4 hrs at room temperature. After dilution to 1:100, each plasma sample was then added to designated wells and the plate was incubated at 4°C for 1 hr. After washing away any unbound plasma components, secondary antibody (mouse anti-rat IgG conjugated with horseradish peroxidase; Sigma) was added to the wells and the plate incubated at 37°C for a further 1 hr. Following a further set of washes and addition of substrate (one tablet of ABTS [Sigma], added to 50 ml of phosphate-citrate buffer with one tablet of urea hydroxide peroxide [Sigma], in 100 ml of distilled water), the plates were incubated a final 45 min at room temperature. The color reaction was then stopped with addition of 20 μl 2 M H2SO4 and the optical density in each well was then measured at 450 nm in an ELISA strip reader spectrophotometer (Merck-MIOS mini) (Engvall and Perlmann, Citation1972).
Assessment of Oxidative Stress
Oxidative stress within splenic tissue as well as within packed cells prepared from peripheral blood samples was measured biochemically. As an indicator of endogenous antioxidant enzyme activities, peroxidase and catalase activities were measured using the modified procedure of Sadasivam and Manickam (Citation1996). Both the spleen and packed cell samples were homogenized separately in 0.1 M phosphate buffered saline (PBS, pH 7.4) to yield a final tissue concentration of 100 mg/ml. The homogenates were then centrifuged at 10,000 × g for 15 min at 4°C and supernatants were recovered. In a spectrophotometer cuvette, 0.1 ml supernatant was then mixed with 2.85 ml phosphate buffer (pH 7.4, 25°C) and 0.05 ml of 20 mM guaiacol solution (Loba Chemie Pvt, Ltd, Mumbai, India). After allowing for an initial change in absorbance of 0.005/min at 436 nm, 0.3 ml of 0.042% H2O2 solution (Merck, Mumbai, India) was added to the cuvette. After gentle mixing by pipette, the absorbance was then allowed to reach 0.050. The time required (Δt, in minutes) for there to be an increase in the absorbance of 0.100 was then measured. All data were then expressed in terms of units of activity/mg tissue after extrapolation from a standard curve generated using varying levels (i.e., units) of commercially-purchased horseradish peroxidase (Sigma).
The catalase activity in each sample was measured biochemically (Beers and Sizer Citation1952). Spleen and packed cell samples were each homogenized in 0.05 M Tris-HCl buffer solution (pH 7.0; SRL, Mumbai, India) at the tissue concentration of 50 mg/ml. Each homogenate was then centrifuged at 10,000 × g at 4°C for 10 min and the supernatants recovered. In a cuvette, 0.5 ml of H2O2 (5 mM) and 2.5 ml distilled water were mixed and the absorbance at 240 nm was noted. The H2O2 used was prepared by dilution of 0.15 ml superoxol (Merck) with 25 ml of 0.05 M phosphate buffer (pH 7.0). After addition of 40 μl of test supernatant, the absorbance was monitored at 30 sec intervals for 3 min. As the optical density measured reflects the peroxide concentration in the cuvette, the activity of catalase in a 3 min period was deduced and then expressed as mM H2O2 consumed/mg tissue/min.
As a final marker of the potential oxidative stress that was present in each tissue, the levels of malondialdehye (MDA, reflective of peroxidative damage to cell membranes) were measured (Ohkawa et al., Citation1979). Spleen and packed cell samples were each homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4) at a tissue concentration of 50 mg/ml. The homogenates were then centrifuged at 10,000 g at 4°C for 5 min and the supernatants recovered for estimation of MDA. For this, 0.5 ml supernatant was mixed with 0.5 ml normal saline and 2 ml of TBA-TCA mixture (0.39 g thiobarbituric acid [TBA; Merck] in 75 ml 0.25 N HCl containing 15 g trichloroacetic acid [TCA; Merck]). The volume of the mixture was then brought up to 100 ml with 95% ethanol, and heated at 100°C for 10 minutes. The mixture was then cooled to room temperature, centrifuged at 4000 rpm for 10 min, and the absorbance of supernatant was measured spectrophotometriaclly at 535 nm. After accounting for background absorbance using buffer blanks, the total TBARS (TBA-reactive substrate) concentration in each sample was derived from the TBA extinction coefficient ε = 1.56 × 105 M−1 cm−1. After accounting for the dilution steps inherent to the protocol, the original level of MDA in each sample was ultimately calculated. All data was then expressed in terms of nM MDA/mg tissue sample.
Histoarchitecture Analyses
Portions of the spleen and thymus from each animal were fixed in Carnoy's solution and paraffin blocks were then prepared. All samples from each rat were recovered from the same zone within the given organ. Using a rotary microtome, 5 μm thick sections of the organs were then prepared and hematoxylin-eosin (H&E) staining performed. Isolated tissue sections were analysed under a light microscope (Olympus-CH20i Germany) (100X) with special attention being paid to the architecture of splenic nodules and the arrangement of the cortex and medulla in thymic lobules (Nandakar and Hegde, Citation1996).
Statistical Methods
All data reflecting quantitative parameters were analyzed using the Student's two-tail t-test. A level of significance was considered when p < 0.05 was obtained (Das and Das, Citation1998).
RESULTS
Body Weight and Organ Weights and Packed Cell Volume Weight
There was no significant change in the body weights of the fluoride-treated animals as compared to the controls (). A significant 48.44% elevation in relative weight of spleen in terms of body weight (i.e., splenic index) was noted in the fluoride-treated hosts compared to the control (). Similarly, the relative weight of thymus (i.e., thymic index) was increased by 30.76% in the fluoride-treated rats. The packed cell volume weight was decreased in fluoride-treated animals in respect to placebo control and the diminution was 26% from their control counterparts ().
TABLE 1 Effect of NaF on body weight, and on splenic and thymic indices and on packed cell volume weight in male albino rats
Neutrophil, Lymphocyte, Monocyte and Splenocyte Count
Lymphocytes, monocytes, and neutrophils in the peripheral blood were counted and expressed in terms of relative percentages of all leukocytes counted. The relative percentages of lymphocytes was decreased by 9.97%, monocytes by 42.08%, and neutrophils by 18.39% after the 28 d of NaF treatment when compared to the percentages of each cell type in the blood of the placebo controls (). Splenocyte counts in the treated rats were also decreased significantly (by 53.07%) compared to the levels in control rats' organs ().
TABLE 2 Effect of NaF on peripheral blood immune cell profiles, blood anti-albumin IgG levels, and splenocyte numbers
Plasma IgG Level
The level of plasma IgG specific for BSA in the NaF-treated rats was decreased significantly (i.e., by 52%) as compared to the placebo controls ().
Oxidative Stress Analysis
Treatment of the rats with NaF resulted in the activities of both catalase and peroxidase being decreased significantly in their spleen and peripheral blood packed cells (). In the splenic tissue, the change in catalase activity was 52.08%, and that of peroxidase was 48.28%, compared to levels in tissues from their control counterparts. Similarly, in the packed blood cells, the fluoride treatment resulted in a 43.12% and 19.23% change in the activities of the catalase and peroxidase, respectively.
TABLE 3 Effect of NaF on catalase and peroxidase activity and formation of malondialdahyde (MDA) in rat samples
When MDA values were analysed, it was seen that the level of MDA was significantly elevated in both the splenic tissue and packed blood cells of the fluoride-treated rats (). The 28 d of treatment resulted in an increase of 35.60% and 53.13% in splenic and in packed blood cell MDA levels, respectively.
Histoarchitecture Study
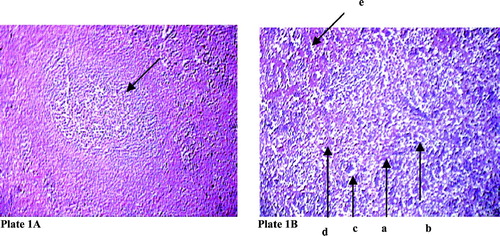
In the control group, normal histoarchitecture was noted within the splenic nodules. However, after NaF treatment, disorganization of splenic nodules along with vacuolation in the splenic nodules was evident ( and ). Sodium fluoride treatment also resulted in an elevation in the proportion of reticulin and collagen structures in the spleen, along with an elevation in the degree of hemosiderosis.
Plate 1 Effect of sodium fluoride on histoarchitecture of splenic nodule in rat. Hematoxylin and eosin stain (100 X) (arrow in indicates splenic nodule. In arrow indicates derangement of splenic nodule (a) with collagen fibre infiltration (b), vaculation in organ structure (c) along with reticulin (d) and hyperpigmentation (e).
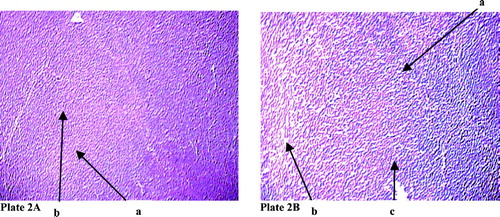
The thymic section of the normal rats displayed expected distinct cortical and medullary parts. However, after NaF treatment, there was remarkable disorganization in the cortical and medullary zones of the thymic lobules, along with vacuolation both in the cortex as well as in the medulla ( and ). There were also clear indications of hyperpigmentation in these affected regions.
Plate 2 (A) Changes in the arrangement of cortex and medulla in thymic lobule in rat after sodium fluoride treatment. Hematoxylin and eosin stain (100 X). Arrow denoted by ‘a’ mark indicates the medullary part of the thymic lobule ‘b’ mark cortical part of the thymic lobule. (B) Arrow with ‘a’ mark indicates derangement in the cortex and medullary part of the thymic lobule, ‘b’ mark arrow indicates vaculation, ‘c’ mark arrow indicates pigmentation.
DISCUSSION
There is a very little information about the immunotoxic effects of fluoride in the scientific literature. Among the limited numbers of publications on the topic, it had been proposed that fluoride could induce autoimmunity by denaturing endogenous proteins (Curnette et al., Citation1979). One in vitro study focussed that cellular immunity is also likely to be affected by sodium fluoride (Gibson Sheim, Citation1992). To date, there is a near complete lack of information about the in vivo immunotoxic effects of sodium fluoride. In that connection, the experiments reported here were designed to determine the specific effects—and the possible cause(s)—for any fluoride induced immunotoxic effects. In particular, these studies sought to determine if there might be any role for oxidative stress in any observed immunomodulation.
The differential counts of lymphocytes, monocytes, and neutrophils were decreased significantly in fluoride-treated rats that are consistent with an earlier report (Gabler and Leong, Citation1979). Because sodium fluoride can cause reactive oxygen species generation, it has been suggested that these effects on leukocytes could be due to an induced oxidative stress (Hartfield and Rabinson, Citation1990). Because of the impact on lymphocytes, effects from fluoride on humoral immunity have also been suggested (Jain and Susheela, Citation1987). Some of the results here support this hypothesis; the significantly lower levels of BSA-specific plasma IgG are a perfect marker for the likely effects of this halide on the host humoral immune system (Vailes et al., Citation2001).
A derangement in the histoarchitecture of the thymus and spleen after sodium fluoride treatment may be due to an oxidative stress imposition. This effect is likely similar to that observed in other organs as was noted in previous experiments from our laboratory (Das [Sarkar] et al., Citation2005). This alteration in the histology of either organ is due to an induced oxidative stress is confirmed in part here by the results of the studies that indicated that the activities of critical antioxidant enzymes were significantly decreased and concomitantly, that the levels of a cellular by-product reflecting progressive free radical damage to organ cells were significantly elevated.
This functional derangement in these primary immune organs (especially the spleen) is also manifested in the low splenocyte counts in the fluoride-treated hosts. This conclusion can be reached as earlier studies have repeatedly shown the correlation between splenic derangement and changes in splenocyte count (Singh and Haldar, Citation2005). Interestingly, the potential for an immunotoxic effect from sodium fluoride is also supported here by the observed elevations in the relative weight of spleen and thymus (i.e., splenic and thymic indices). In fact, this finding is consistent with that in other studies that examined the effects of fluoride-containing insecticides on primary lymphoid organs (Bely, Citation2000).
Although the studies here have shown that there are likely immunomodulatory effects from exposure to sodium fluoride, the data for an increase in splenic index and concurrent decreases in splenocyte numbers does present a quandary. That the splenic weight in each exposed rat was increased in spite of low splenocyte counts may be explained by the deposition of reticulin and collagen in their spleen as well as by hyperplasia of the cells of the local reticuloendothelial system (Knyash et al., Citation1971a, Citation1971b). Similarly, these apparent conflicting outcomes might be the result of the increases in hemosiderosis or pigment deposition (Knyash et al., Citation1971a, Citation1971b) as was observed here. Moreover, inhibition in testicular activity may lead to splenic enlargement (Viselli et al., Citation1997) and fluoride intoxication results diminution in testicular steroidogenesis (Ghosh et al., Citation2002). As there is no significant changes in general body weight, it may be deduced that these above changes were not due to general toxicity induction by the fluoride ion but, rather, due to its specific effect on the immune system organ itself.
From this experiment, it may be concluded that sodium fluoride can impose toxic effects upon both the cellular and humoral arms of the immune system. Furthermore, it is likely that these effects arise out of the induction of an oxidative stress. Ongoing research in our laboratory should help to clarify more precisely the immunomodulatory mechanisms by which this halide may be affecting the health of so many inhabitants of South Asia that are routinely exposed to fluoride through drinking water in each day.
Note: S. Das (Sarkar) is also affiliated with Vidyasagar University.
REFERENCES
- Agrawal V., Vaish A. K., Vaish P. Groundwater quality: Focus on fluoride and fluorosis in Rajasthan. Curr. Sci. 1997; 73: 743–746, [CSA]
- Ahotupa M., Huhtaniemi I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally-cryptorchid rat testis. Biol. Reprod. 1992; 46: 1114–1118, [CSA]
- Beers R. F., Sizer I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952; 195: 133–140, [CSA]
- Bely M. Lymphoid depletion of spleen due to experimental fluorosis in rats. Fluoride 2000; 33: 81–82, [CSA]
- Bogin E., Abrams M., Avida Y., Israeli B. Effects of fluoride on enzymes from serum, liver, kidney, skeletal and heart muscles of mice. Fluoride 1976; 9: 42–46, [CSA]
- Carlson J. R., Suttle J. W. Pentose phosphate pathway enzymes and glucose oxidation in fluoride fed rats. Am. J. Physiol. 1996; 210: 79–83, [CSA]
- Curnette J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. J. Clin. Invest. 1979; 63: 637–647, [CSA]
- Das (Sarkar) S., Maiti R., Ghosh D. Induction of oxidative stress on reproductive and metabolic organs in sodium fluoride-treated male albino rats: Protective effect of testosterone and vitamin E co-administration. Tox. Mech. Methods 2005; 15: 271–277, [CSA]
- Das D., Das A. Testing of hypothesis. Statistics in Biology and Psychology. 7th Edition. Academic Press, KolkataIndia 1998; 113–117
- Engvall E., Perlmann P. Enzyme linked immunosorvent assay (ELISA) III: Quantification of specific antibodies enzyme labelled anti-immunoglobulin in antigen coated tubes. J. Immunol. 1972; 109: 129–135, [CSA]
- Fazlul Hoque A. K. M., Khaliquzzaman M., Hossain M. D., Khan A. H. Fluoride levels in different drinking water sources in Bangladesh. Fluoride 2003; 36: 38–44, [CSA]
- Foulkes R. G. Dietary reference intakes: Calcium, phosphorous, magnesium, vitamin D and fluoride. Fluoride 1997; 30: 4, [CSA]
- Gabler W. L., Leong P. A. Fluoride inhibition of polymorphonuclear leukocytes. J. Dent. Res. 1979; 48: 1933–1939, [CSA]
- Ghosh D., Das (Sarkar) S., Maiti R., Jana D., Das U. B. Testicular toxicity in sodium fluoride treated rats: Association with oxidative stress. Reprod. Toxicol. 2002; 16: 385–390, [CSA]
- Gibson Sheim L. M. Effects of fluoride on immune system function. Complete. Med. Res. 1992; 6: 111–113, [CSA]
- Handa B. K. Geochemistry and genesis of fluoride-containing in groundwater in India. Ground Water 1975; 13: 275–281, [CSA]
- Hartfield P. J., Rabinson J. M. Fluoride-mediated activation of the respiratory burst in electropermeabilized neutrophils. Biochem. Biophys. Acta. 1990; 54: 176–180, [CSA]
- Jain S. K., Susheela A. K. Effect of sodium fluoride on antibody formation in rabbits. Environ. Res. 1987; 44: 117–125, [CSA]
- Knyash V. S., Tkach N. Z., Tsarevskii L. P., Milovanova V. I. Pathomorphological changes in internal organs of white rats under the inhalation effect of gliftor. Tr. Inst. Krave. Patol. Akad. Nauk. Kaz. Ssr. 1971a; 22: 23–28, [CSA]
- Knyash V. S., Tkach N. Z., Tsarevskii L. P. Morphological changes of experimental animals after oral administration of gliftor. Tr. Inst. Krave. Patol. Akad. Nauk. Kaz. Ssr. 1971b; 22: 28–30, [CSA]
- Nandakar C. T., Hegde U. C. Immuno-endocrine aspects of ovarian function. Cellular and Molecular Signalling of Reproduction, J. Sengupta, D. Ghosh. New Age International Publishers, New DelhiIndia 1996; 33–47
- Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxidation in animal tissues by thioberbituric acid reaction. Anal. Biochem. 1979; 95: 351–358, [CSA]
- Pak C. V., Sakhu K., Zerwekh J. E., Parcel C., Peterson R., Johnson K. Safe and effective treatment of osteoporosis with intermittent slow release of sodium fluoride: Augmentation of vertebral bone mass and inhibition of fractures. J. Clin. Endocrinol. Metab. 1989; 68: 150–159, [CSA]
- Robert C. H., Jr. Agents affecting calcification: Calcium, parathyroid hormone, calcitonin, vitamin D and other compounds. Goodman Gillman's the Pharmacological Basis of Therapeutics, 7th Edition, L. S. Goodman, T. W. Rall, F. Murad. Macmillan Publishing Co., New York 1980; 1496–1522
- Peroxidase. Methods in Biochemistry, S. Sadasivam, A. Manickam. New Age International Publisher, New DelhiIndia 1996; 108–110
- Singh S. S., Haldar C. Melatonin prevents testosterone-induced suppression on immune parameters and splenocyte proliferation in Indian tropical jungle bush quail, Perdicula asiatica. Genetic Comp. Endocrinol 2005; 141: 226–232, [CSA]
- Spithe B. Psychopharmacology of fluoride: A review. Int. Psychopharmacol. 1994; 9: 79–82, [CSA]
- Suketa Y., Terui Y. Adrenal function and changes of sodium and potassium in serum and urine in fluoride intoxicated rat. Fluoride 1980; 13: 4–9, [CSA]
- Suma Latha S., Ambika S. R., Prasad S. J. Fluoride contamination status of groundwater in Karnataka. Curr. Sci. 1999; 6: 730–734, [CSA]
- Susheela A. K., Kharp P. Aortic calcification in chronic fluoride poisoning: Biochemical electron microscopic evidence. Exp. Mol. Pathol. 1990; 53: 72–80, [CSA]
- Vailes L. D., Perzanowski M. S., Wheatley L. M., Platts-Mills T. A., Chapman M. D. IgE and IgG antibody responses to recombinant Alt a1 as a marker of sensitization to Alternaria in asthma and atopic dermatitis. Clin. Exp. Allergy 2001; 31: 1891–1895, [CSA]
- Viselli S. M., Reese K. R., Far J., Kovacs wjans Olsen N. J. Androgen alter B-cell development in normal male mice. Cell. Immunol. 1997; 182: 99–104, [CSA]
- Yang Q., Xie Y., Depierre J. W. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 2000; 122: 219–226, [CSA]
