Abstract
In building upon the increasing utility of avian immunology as an alternative to the use of mammalian systems for assessing immunotoxic potentials of environmentally and/or occupationally encountered chemicals, in vitro studies were performed to determine if easily obtainable freshly harvested cells from White Leghorn chickens could be used to detect effects on a critical immune cell type by any given toxicant. These studies also sought to determine if these cells could also potentially provide a vehicle to elucidate potential mechanisms underlying effects previously described for the test agent in in vivo investigations. Here, specifically, the toxic effect (and thus, ultimately, the potential to be an immunotoxicant in situ) of a commonly used commercially available form of deltamethrin on Leghorn lymphocytes was examined. The toxicity of this synthetic pyrethroid was evaluated by assaying both lymphoproliferation and apoptosis after exposure. A significant (p < 0.05) inhibitory effect of deltamethrin on mitogen-stimulated lymphocytes was observed at 10− 5 M. Apoptosis was detected using DNA ladder analyses; annexin-V binding assays incorporating an immunoperoxidase technique were used to identify translocated phosphatidylserine; and, electron microscopy, including both scanning and transmission types, were used to detect ultrastructural changes (e.g., chromatin condensation, membrane blebbing and phagocytosis) in deltamethrin-treated cells. Our results indicated that deltamethrin, even at a very low dose, could give rise to potential immunotoxicities in situ by inducing toxicity (i.e., apoptosis) in immunogenic cells. Our results also show that this particular in vitro system may be used as an alternative to standard laboratory animals for the performance of immunotoxicity experiments involving pesticides.
INTRODUCTION
Synthetic pyrethroids are extensively used insecticides. Deltamethrin, a synthetic insecticide based structurally on natural pyrethrins, rapidly paralyzes the insect nervous system thereby giving a quick knockdown effect (Haug and Hoffman, Citation1990). Deltamethrin has very good residual activity for outdoor uses (e.g., field crops, cattle dip, tsetse flies) and for indoor uses (e.g., against mosquitoes, stable flies, horseflies, fleas, cockroaches, and stored product insects) (Worthing, Citation1987). Deltamethrin also has a very broad-spectrum control. While deltamethrin is considered the most powerful of the synthetic pyrethroids, it is an agent of moderate toxicity to mammals. However, extensive and unmethodical uses of one or more of its various formulations (i.e., as Decis, Kordon, K-Othrine, Butox, Delta, Deltamix, or Sadethrin) for crop protection and/or for protecting environmental health consistently pose a threat to the health of livestock, birds, fishes, and even humans (Casida et al., Citation1983).
The effect of pesticide chemicals on the integrity of the immune system has recently drawn considerable interest as an additional indicator of potential problems from persistent inadvertent exposure. Immunotoxicants are a class of factors present in the external environment that cause significant changes in the immune mechanisms in humans and animals (CitationDietert et al., 1996). The initial references to the susceptibility of the immune system—and its potential use for the detection of sub-clinical toxic states—were reported in the 1970s and early 1980s (Vos and Van Genderen, Citation1973; Loose et al., Citation1978; Faith et al., Citation1980). The work of Descotes was one of the first to indicate that it was important to focus on the vertebrate immune system as a critical target for the toxic actions of pesticides (Descotes, Citation1988). Subsequent studies showed that in fact, the immune system is more sensitive and reacts more rapidly than other organ systems to the effects of pesticides, even if concentrations of these chemicals are lower than those necessary for inducing clear acute systemic toxicoses (Black et al., Citation1992; Raszyk et al., Citation1997).
With respect to deltamethrin, very little is known about its effects (as either a pure form or in one of its various formulations) on the immune system of exposed hosts. In fact, a review of the literature indicates that the majority of studies of toxicity from this agent have focused almost exclusively on effects upon the central nervous system/brain (Aziz et al., Citation2001; Wu and Liu, Citation2003; Patro and Patro, Citation2005). One study to specifically examine effects from host exposure to this pesticide showed that oral dosing of mice for 14 (at 1/5 LD50 value) or 84 days (at 1/12 LD50) induced several markers of immunosuppression (Lukowicz and Krenchniak, Citation1992). Humoral immune responses in these animals—determined by agglutinin and hemagglutinin titer against sheep red blood cell antigen as well as by the number of plaque-forming cells producing IgM antibodies—were significantly decreased. Cell-mediated responses—assessed by a-naphthyl acetate esterase activity, formation of EAC (erythrocyte-antibody-complement) rosettes, and footpad reaction tests, were also hindered. An earlier study by Kowalczyk-Bronisz et al. (Citation1990) using mice yielded conflicting results—an outcome that the authors themselves attributed to their particular exposure regimen.
Several methods have been developed to determine the potential immunosuppressive action of xenobiotics using dose and time dependence of direct and indirect effects on the immune system as well as development of in vitro systems to reflect biological outcomes that might occur in vivo (Kacmar et al., Citation1999). With respect to the latter, long-term animal studies (such as those of Lukowicz and Krenchniak [Citation1992] and Kowalczyk-Bronisz et al. [1990]) and determining the toxicological risk of immunotoxicants (including pesticides) are time-consuming and expensive to perform; ethical factors also play an increasingly important role. Rather than follow the tendency to reduce the number of test animals in the experiments for the quick screening of the immunotoxic potency of pesticides (as well as other classes of chemicals), there have been increasing calls for simple, reproducible, and reliable in vitro test systems to perform this function (see Pistl et al., Citation2003). To address this need, the study reported here sought to determine the potential utility of primary avian lymphocytes as tools to assess the eventual immunotoxic effects of environmentally- and/or occupationally-encountered chemicals, like pesticides.
The field of avian immunology has progressed to where it is technically robust (extensively reviewed in Fairbrother et al., Citation2004). Given this technical capacity, it now is possible to screen chemicals for immunotoxicologic properties following the same tiered approach that has been established for mammals. In fact, the potential for chemicals to affect the immune system of birds has been studied and documented for various pesticides, organochlorine agents, mycotoxins, petroleum hydrocarbons, heavy metals, organometallics, and radiation (e.g., Bishop et al., Citation1998a and Citation1998b; Bunn et al., Citation2000; Quist et al., Citation2000; Fair and Ricklefs, Citation2002). Nearly all studies with avian hosts have measured hematological parameters and the relative percentages of each major immune cell type after exposure. The most commonly conducted in vivo assays have been the wing web mitogen stimulation test and the antibody response to SRBC. Ex vivo studies have routinely included assessments of lymphocyte blastogenesis to B- or T-lymphocyte-specific antigens. Studies of the ability of blood/peritoneal macrophages to ingest foreign objects or the splenic plaque-forming cell assay have been attempted with less frequency. Though the ultimate test of hosts resistance against pathogenic challenge has been used infrequently, it appears that immune suppression in birds resulting from contaminants and low-level stressors (e.g., crowding, food loss, climate change) caused chronic morbidity and mortality associated with multiple pathogens. It is important to note that the same types and magnitudes of altered immune function that have been demonstrated in birds have been associated with increased susceptibilities to challenge infections in laboratory animals.
In building upon the increasing utility of avian immunology as an alternative to the use of mammalian studies for assessing immunotoxic potentials of environmentally and/or occupationally encountered chemicals, the in vitro studies reported here were designed to determine if easily obtainable, freshly harvested cells from White Leghorn chickens could be used first to detect an effect on a critical immune cell type by any given (here, deltamethrin) toxicant. In addition, these studies sought to determine if these cells could also potentially provide a vehicle for elucidating potential mechanisms underlying effects previously described for the given test agent in in vivo investigations.
MATERIALS AND METHODS
Chemical Reagents
All chemicals and culture reagents were obtained from Sigma Diagnostics, St. Louis, MO (USA), unless otherwise indicated in the text. The same commercial preparation of deltamethrin (Decis 2.8% EC; 〈http://www.bayercropscienceus.com/products/view/decis/labels.html〉) used by regional farmers was obtained from Bayer Crop Science India Ltd, Mumbai, India. This is composed of (by weight) 31% deltamethrin, with the remainder being inert ingredients; this formulation is designed to yield the equivalent to 2.8 lb. active ingredient per gallon. Pure deltamethrin ([S]-α-cyano-3-pehoxybenzyl(1R)-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopro-ane carboxylate) was not tested as this study sought to determine the toxic impact from exposures to the formulation of this agent that is routinely encountered by farmers and their livestock. Use of pure deltamethrin here would only have resulted in the eventual dosages employed being adjusted to accommodate the relative content of the pesticide (i.e., 31% vs. 98%).
Animals
White Leghorn chickens (5–6-weeks-old; Gallus domesticus) were maintained from birth at a poultry research station (PRC) located at the Govindh Ballabh Pant University of Agriculture and Technology, Pantnagar, Nainital, Uttaranchal, India. The birds were provided with standard hygienic conditions and were given feed and clean water ad libitum. At all times, the birds were monitored under veterinary supervision to assure optimal health was maintained prior to and on the day of peripheral blood harvesting.
Isolation of Peripheral Blood Lymphocytes (PBLs) and Treatment with Deltamethrin
Peripheral blood lymphocytes (PBLs) were isolated by the method of Haddad and Marshaly (Citation1992). Cells in the whole blood were harvested aseptically using Histopaque-1077 (and centrifugation at 400 × g, 30 min, 4°C) and suspended in RPMI-1640 growth medium supplemented with 10% fetal calf serum (FCS). The percentage cell viability was determined on a hemocytometer using a 0.4% trypan blue dye exclusion test (Boyse et al., Citation1964) and the final count was adjusted to 107 live cells/ml.
A commercial formulation of deltamethrin (Decis 2.8% EC) was dissolved in ethanol to yield a stock final concentration of 1%. A fresh solution of deltamethrin was prepared before each experiment at a stock concentration 10−3 M so that when added to the lymphocyte cultures at a level of 1% (by volume), the actual concentration would be 10−5 M. The rationale for selection of this level as the optimal dilution was based on a report by Enan et al. (Citation1996) that showed that 50 μM deltamethrin induced cytotoxicity (≈45% after 24-hr exposure) in suspensions of murine thymocytes. Though it was possible that extrapolation across species might not yield the same results, it was expected that use of a level one-fifth of that used by Enan's group (and for a far shorter timeframe) would not evince very high levels of cell death outright yet still be able to induce changes in the treated avian cells. In the assays reported here, all treatments with the pesticide were at 37°C for 60 or 120 min only.
Lymphocyte Proliferation
Lymphocyte proliferation assays were carried out using the method of Rai-el-Balhaa et al. (Citation1987) with slight modifications. In these assays, lipopolysachharide (LPS, derived from Escherichia coli 0111:B4) and concanavalin-A (Con-A, Type V from jack bean Canavalia ensiformis) were used as B- and T-cell mitogens, respectively. The reduction of the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) dye to formazan was used as an indicator of cell proliferation (Altman, Citation1976; Mosmann, Citation1983). Briefly, following the treatment for 60 and 120 min, 100 μl of diluted cell suspension was cultured in triplicate in the wells of a 96-well plate. LPS or Con-A were added (each at 5 μg/ml final concentration) to dedicated triplicate wells and the volume in each was adjusted to 0.2 ml. After the cells had been incubated for 72 hr, at 37°C in a humidified atmosphere bearing 5% CO2, the extent of the cell proliferation in the period was determined. An aliquot of a 10% MTT (5 mg/ml) solution was added to each well and the plates were incubated at 37°C for an additional 4 hr. The blue formazan precipitate was then dissolved in acidic isopropanol and the optical density (OD) in each well was measured at 570 nm using microscan ELISA reader (ECIL, India). All results were reported as mean change (Δ) in optical density (mean Δ OD = mean OD of mitogen-stimulated wells − mean OD of unstimulated wells).
Annexin-V Binding
Annexin-V binding assays were performed using an immunoperoxidase technique. Triplicate smears of treated and control cells were made on glass slides and fixed in methanol. The slides were then washed in phosphate buffered saline [(PBS), 3 changes] for 5 min each and any residual peroxidase activity in the cells present then quenched by immersion in 10% H2O2 for 10 min. The slides were then incubated overnight at 4°C in a solution of annexin-V-biotin conjugate (at a dilution of 1:1000) and then in a solution of avidin-peroxidase conjugate (at a dilution of 1:500) for 4 hr at 37°C. The slides were then washed in PBS (3 changes) for 5 min each before being allowed to react with diaminobenzidine (DAB) substrate. The stained slides were then washed with distilled water (2 changes), dehydrated through an alcohol series leading up to xylene (2 changes), mounted with DPX mountant, and 200 cells/slide examined at 400X using a light microscope. Results for these studies were then expressed as the mean percentage of positively-staining cells in the population analyzed on each slide.
DNA Fragmentation
Treated and control cells were each washed with PBS, suspended in lysis buffer (25 mM Tris-HCl [pH 8.0], 10 mM EDTA, 10% SDS) with Proteinase K (200 μg/ml; Sigma), and then incubated overnight at 37°C. DNA samples were then extracted from each preparation with an equal volume of phenol:chloroform (1:1) and the total DNA contained in the aqueous phase was precipitated with a 1/10 vol of ethanol. DNA pellets were prepared by centrifugation and then washed with 70% ethanol, air dried, and resuspended in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) containing RNAse (50 μg/ml). After incubation for 1 hr at 37°C, assessment of fragmentation was performed via DNA laddering analyses; here, 20 μg of the recovered DNA was loaded into a lane and electrophoresed over a 1% agarose gel containing 0.1 mg/ml ethidium bromide, and visualized using an ultraviolet light box. The sizes of the fragments within the laddered materials were estimated by comparing migration distances to those in the GeneRuler™ 100 bp DNA Ladder Plus (MBI Fermantas, Hanover, MD) that yields a 14 fragment ladder with pieces spanning in size from 100–3000 bp in a standard agarose gel.
Scanning Electron Microscopy (SEM)
Electron microscopy was performed by the method of Malorni et al. (Citation1998). Cells were washed in PBS and then fixed in 2.5% glutaraldehyde. After a wash with 0.1 M cacodylate buffer, the cells were then dehydrated in a series of ethanol solutions of decreasing dilution (with water). Post-treatment by critical point dryer and ion sputter was then performed and observed on an SEM (Leo 435 Variable Pressure, Leo Electron Microscopy Ltd., Cambridge, England). A total of 50 cells per section were analyzed for each sample.
Transmission Electron Microscopy (TEM)
Cells were fixed in PBS containing 2.5% glutaraldehyde. Samples were post-fixed in 0.1 M cacodylate buffer containing 1% osmium tetroxide, and embedded. Ultrathin sections (500 Å) were prepared, then stained with uranyl acetate and lead citrate, and finally examined by TEM in an FEI Morgagni 268D instrument (Eindhoven, the Netherlands). The apoptotic cells were identified by their nuclear morphology resulting from chromatin condensation. A total of 50 cells per section were analyzed for each sample.
Statistical Analyses
The Student's t-test was used for determining if there were statistically significant differences between the treated and control cell populations for the various endpoint measured herein. In the case of the lymphocyte proliferation assay results, all values were expressed as mean Δ OD ± SE.
RESULTS
Lymphocyte Proliferation
The lymphoproliferative activities (mitogen-induced and spontaneous) of White leghorn lymphocytes were assessed after treatment with deltamethrin or vehicle for 60 and 120 min. At the end of respective incubation periods, the cells were washed and either incubated with vehicle or with one of two mitogens (Con-A or LPS) for 72 hr. At the end of this incubation, the cells were washed and the degree of proliferation in the 72-hr period assessed by monitoring the amount of reduction of MTT dye. All results were then recorded as mean Δ OD for both the LPS- and Con-A-stimulated cultures ().
TABLE 1 In vitro effect of deltamethrin on B- and T-lymphocyte proliferation in the presence LPS or Con-A mitogens
The mean Δ OD for deltamethrin-treated cells in the presence of LPS was 0.194 ± 0.029 and 0.154 ± 0.027 and in the presence of Con-A was 0.185 ± 0.050 and 0.147 ± 0.057, respectively, as a result of the 60- and 120-min treatments three days earlier. With the control cells, these values were 0.379 ± 0.036 (LPS) and 0.299 ± 0.045 (Con-A), respectively. This indicated significant (p < 0.05) treatment length-dependent reductions in proliferative capacity of the deltamethrin-treated cells when compared to the controls. Specifically, for LPS, the treatment resulted in a 48.82% (60-minutes exposure) reduction that increased to 59.37% after an additional 60 min of exposure to the pesticide. For the Con-A regimen, these values were 38.13% rising to 50.84%, respectively. These reductions in proliferative capacities with both LPS and Con-A indicated that there was ultimately a significant potential risk for depression of both the wings (humoral [HI] and cell-mediated [CMI]) of the leghorn's immune system due to these effects of the pesticide on this normal function of the host's lymphocytes.
Annexin-V Binding
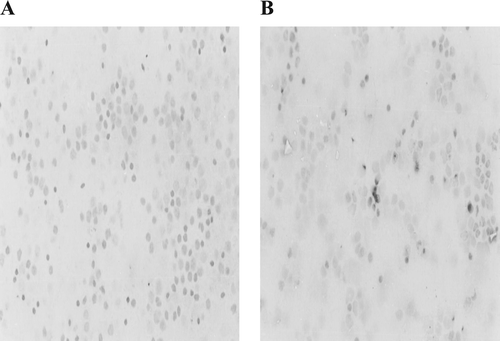
Loss of plasma membrane asymmetry is a universal phenomenon of apoptosis. Evaluation of select annexins, a family of structurally related proteins with Ca2+-dependent phospholipid binding capacity, is a valuable protocol for determining the extent of this asymmetry, and thus apoptosis, in toxicant-exposed cells. In this study, biotinylated annexin-V was used for the identification of translocated phosphatidyl serine (PS) following exposures to deltamethrin. Qualitative analyses indicated that treated cells displayed significant amounts of brown coloration on their surface (indicated the specific binding of annexin-V) as compared to what was seen with the control lymphocytes (). Quantitatively, the mean percentage of positively-staining cells after exposure to deltamethrin for 60 min was 6.20% and 10.75% after a 120-min regimen; both of these values were significantly greater (p < 0.05) in comparison to their respective control values of 2.20 and 4.25%. Because exposure of PS is commonly observed on the surface of cells undergoing apoptosis (Fadok et al., Citation1992), this appearance of an increase in annexin-V positive cells indicated that there was likely a time-dependent induction of apoptotic damage in the avian lymphocytes induced by deltamethrin. The slight increase among the control cell staining percentages is most likely due to background normal cell death.
FIG. 1 Induction of apoptosis in White Leghorn lymphocytes treated with 10−5 M deltamethin in vitro. Photomicrographs (at X400) are of avian lymphocytes after immunoperoxidase staining to detect presence of Annexin-V (A) Control lymphocytes. (B) Lymphocytes treated with deltemethrin with translocated phosphatidylserine on their surface.
Induction of Internucleosomal Cleavage
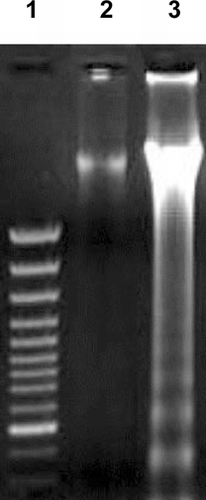
Because DNA fragmentation is a well-defined biochemical marker of apoptosis (Schwartzman and Cidlowski, Citation1993), the ability of deltamethrin to induce DNA fragmentation in lymphocytes was examined. Intense fragmentation of DNA, a pattern highly specific to apoptosis was observed after 120 min of treatment with 10−5 M deltamethrin (). In contrast, the lane containing genetic material from the vehicle-treated control cells showed no DNA fragmentation. Since the DNA fragmentation is also a hallmark of cells undergoing apoptosis (Nagata, Citation2000), the appearance of the DNA ladder by genetic material from the treated cells confirmed that there was some measure of apoptotic damage induced in the leghorn lymphocytes by the deltamethrin.
FIG. 2 DNA fagmentation in avian lymphocytes exposed to 10−5 M of deltamethrin. After treatments, DNA was harvested and 20 μg per sample electrophoresed over a 1% agarose gel. Lane 1: GeneRuler 100 bp DNA Ladder Plus Marker; Lane 2: DNA from control cells; Lane 3: DNA from cells after exposure to deltamethrin.
Electron Microscopy Observations
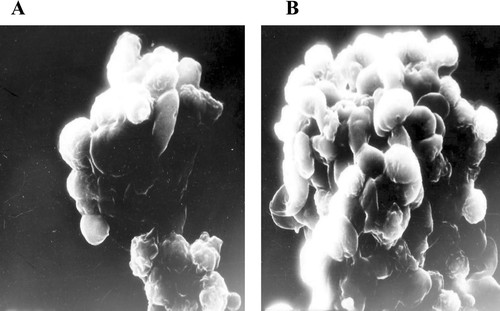
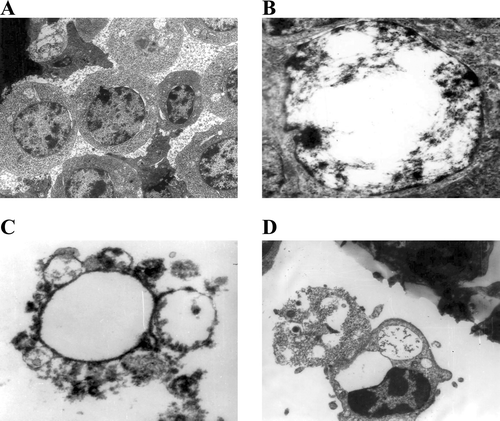
Apoptosis induces characteristic morphological changes in cells, such as condensation of chromatin and membrane blebbing (Chiou et al., Citation2000; Wyllie et al., Citation1980). Deltamethrin-treated cells (120 min,10−5 M) displayed margination of chromatin and membrane blebbing, confirming apoptosis. The SEM analyses of the treated cells—when compared to the controls—showed the smoothing of the cell surface and the formation of membrane blebs (). The TEM analyses of treated cells revealed condensation of chromatin and margination toward the nuclear envelope, as well as the formation of apoptotic bodies and their engulfment by neighboring cells (). All of these items are typical structural features of apoptotic cells. Based on this, it can be concluded again that, deltamethrin induced apoptotic damage in avian lymphocytes.
DISCUSSION
The present investigation was carried out to evaluate the toxic effects of deltamethrin on avian lymphocytes. Though there are a few reports of deltamethrin-induced immunotoxicity, the mechanism of induction of the immunotoxic effect is not known. Moreover, these earlier observations were obtained via in vivo studies in standard laboratory animals (Stelzer and Gordon, Citation1984; Desi et al., Citation1986; Bhaumik and Gupta, Citation1988; Tammang et al., Citation1988; Tandan, Citation1990) This present investigation reports the potential for deltamethrin-induced immunotoxicities based on in vitro studies of the toxicity of this pesticide against avian lymphocytes. Specifically, the extent of toxicity (which would likely give rise to immunotoxicity in situ) was determined by analyses of several endpoints in the lymphocyte targets. These included assessments of proliferative activity, annexin-V binding, and DNA fragmentation, as well as electron microscopy to determine if ultrastructural alterations were induced, in the exposed cells.
The MTT-based lymphocyte proliferation assays (Rai-el-Bahlaa, Citation1985; Dennizot and Lang, Citation1986; Carmichael et al., Citation1987; Vohr, Citation1995) revealed reductions in both LPS- and Con-A-stimulated lymphoproliferation. Furthermore, the data indicated that the effect on both B- and T-lymphocytes was treatment length-dependent. From this it can be concluded that deltamethrin has the potential to eventually suppress both the arms (HI and CMI) of the immune system. Our results are consistent with earlier in vivo findings of deltamethrin-induced immunotoxicity in mice. In that study, mice exposed to deltamethrin in twice-daily doses of 6 mg/kg for 84 days and 15 mg/kg for 14 days, showed reductions in both HI and CMI responses, when they were immunized with sheep red blood cells (Lukowicz and Krenchniak, Citation1992).
The annexin V binding assays here indicated that the deltamethrin-treated cells displayed significantly more brown color on their surface (indicating specific binding of biotin-conjugated annexin-V to the translocated PS) as compared to that on control lymphocytes. In normal cells, phospholipids are asymmetrically distributed between inner and outer leaflets of the plasma membrane. Phosphatidylcholine (PC) and sphingomyelins are found predominantly in the outer leaflet whereas PS is confined to the inner leaflet. Hence, PS is not normally available for binding by annexin-V. During the early stages of apoptosis, due to structural alterations in the plasma membrane, PS moves out onto the outer surface (Fadok et al., Citation1992). As such, translocation of PS in cells undergoing apoptosis is a readily identifiable and quantifiable hallmark of this process. It is interesting to note that other reports using flurochromes coupled to annexin-V and then fluorescent-activated cell sorting (FACs) (Knoopman et al., Citation1994; Vermes et al., Citation1995) have shown that not only is PS increased on the cell surface during apoptosis, but expression of phosphatidylethanolamine (PE) is elevated as well (Emoto et al., Citation1997). Recently, the exposure of PS on the cell surface has been shown to be of importance in the phagocytic uptake of apoptotic cells and apoptotic cell remnants (Krahling et al., Citation1999; Callahan et al., Citation2000) by leukocytes. In the studies here, the exposure length-related increases in detectable surface PS on treated avian lymphocytes indicated strongly that there was deltamethrin-induced apoptotic damage. The findings of induced apoptosis due to deltamethrin were further supported by the results of the DNA ladder assays, since an increase in DNA fragmentation is a distinct feature of apoptosis (Wyllie, Citation1980; Arends et al., Citation1990; Compton Citation1992; Schwartzman and Cidlowski, Citation1993; Nagata, Citation2000).
Both TEM and SEM showed prominent ultrastructural changes in the cells exposed to deltamethrin. The TEM analyses demonstrated the condensation of chromatin, its margination towards nuclear periphery, and the formation of apoptotic bodies; SEM revealed cells with a loss of surface microvilli and formation of membrane blebs. The presence of all of these phenomena are distinct features of cells undergoing apoptosis. This then again indicates that exposure of avian lymphocytes to deltamethrin results in their apoptotic demise. These various structural alterations in the cell are intimately related to a cascade of physiological, biochemical, and molecular changes that occur during apoptosis. Furthermore, their relative presence has also been shown to be useful in helping to determine/analyze the various stages of apoptosis (Kerr et al., Citation1972; Sun et al., Citation2000). For example, the detection of condensed chromatin in certain cells indicated that they were in an early stage of apoptosis, while the appearance of apoptotic bodies revealed if cells were in their final stage of the process.
The above results confirming the apoptotic damage induced by deltamethrin are consistent with earlier reports. Enan et al. (Citation1996) reported thymic atrophy (due to apoptosis) in male Balb/c mice following deltamethrin treatment. El-Gohary et al. (Citation1999) demonstrated a deltamethrin-elicited testicular apoptosis in male albino rats. A more recent report also demonstrated that deltamethrin induced apoptosis in cultured cerebral cortical neurons (Wu et al., Citation2003). Therefore, the present in vitro findings confirm that an induction of apoptotic damage of lymphocytes could be one of the ways by which this pesticide causes immunotoxicity in exposed hosts. The exact mechanism of deltamethrin-induced apoptosis in these cells has not yet been elucidated. It has, however, been demonstrated that deltamethrin induces apoptosis by up-regulation of p53 and Bax and down-regulation of the Bcl-2 family of genes—leading to activation of nitric oxide synthase (NOS) and the synthesis of nitric oxide (NO)—which induce neuronal apoptosis in vivo and in vitro (Le et al., Citation1995; Nicotera et al., Citation1995; Palluy and Rigaud, Citation1996; Wu et al., Citation2003).
In summary, our results demonstrated that deltamethrin at a very low concentration is toxic to White Leghorn lymphocytes and that this effect is due, in part, to an induction of apoptotic damage. This study also advocates that deltamethrin possesses the potential to cause immunotoxicity in vivo following host chronic exposure to very low doses of the agent, as can occur with consumption of contaminated foodstuffs. The present study also establishes that this particular in vitro system may be a promising alternative to using laboratory animals for experiments to analyze the potential for induction of immunotoxicity by chemical agents, including pesticides.
ACKNOWLEDGMENTS
Authors are thankful to ICAR for financial support in the form of National Fellow Project, to the Dean, College of Veterinary and Animal Sciences and to the Director of the Experiment Station, G.B. Pant University of Agriculture and Technology, Pantnagar for providing facilities.
REFERENCES
- Altman F. P. Tetrazolium salts and formazans. Prog. Histochem. Cytochem. 1976; 9: 1–56, [CSA]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis: The role of the endonuclease. Am. J. Pathol. 1990; 136: 593–608, [CSA]
- Aziz M. H., Agrawal A. K., Adhami V. M., Shukla Y., Seth P. K. Neurodevelopmental consequences of gestational exposure (GD14-GD20) to low dose deltamethrin in rats. Neurosci. Lett. 2001; 300: 161–165, [CSA]
- Bhaumik A., Gupta P. K. Sub-acute toxicity of deltamethrin in rats. Advances in Toxicology and Environmental Health, P. K. Gupta, V. Ravi Prakash. Jagmander Book Agency, New Delhi, India 1988; 148–158
- Bishop C. A., Boermans H. J., Ng P., Campbell G. D., Struger J. Health of tree swallows (Tachycineta bicolor) nesting in pesticide-sprayed apple orchards in Ontario, Canada. I. Immunological parameters. J. Toxicol. Environ. Health. A 1998a; 55: 531–559, [CSA]
- Bishop C. A., van Der Kraak G., Ng P., Smits J. E. G., Hontela A. Health of tree swallows (Tachycineta bicolor) nesting in pesticide-sprayed apple orchards in Ontario, Canada. II. Sex and thyroid hormone concentrations and testes development. J. Toxicol. Environ. Health 1998b; 55: 561–581, [CSA]
- Black R. D., Scott D., Oehme F. W. Immunotoxicity in the bovine animal: A review. Vet. Human Toxicol. 1992; 34: 438–442, [CSA]
- Boyse E. A., Old L. J., Chouroulinkov I. Cytotoxic test for demonstration of mouse antibody. Meth. Med. Res. 1964; 10: 39–47, [CSA]
- Bunn T. L., Marsh J. A., Dietert R. R. Gender differences in developmental immunotoxicity to lead in the chicken: Analysis following a single early, low-level exposure in ovo. J. Toxicol. Environ. Health 2000; 61: 677–693, [CSA]
- Callahan M. K., Williamson P., Schlegel R. A. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000; 7: 645–653, [CSA]
- Carmichael J., Degraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987; 47: 936–942, [CSA]
- Casida J. E., Gammon D. W., Glickman A., Lawrence L. J. Mechanism of selective action of pyrethroid insecticides. Ann. Rev. Pharmacol. Toxicol. 1983; 23: 413–438, [CSA]
- Chiou P. P., Kim C. H., Ormonde P., Leong J. A. infectous hematopoietic necrosis virus matrix protein inhibits host-directed gene expression and induces morphological changes of apotosis in cell cultures. J. Virol. 2000; 74: 7619–7627, [CSA]
- Compton M. M. A biochemical hallmark of apoptosis: Internucleosomal degradation of the genome. Cancer Metast. Rev. 1992; 11: 105–109, [CSA]
- Dennizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Meth. 1986; 89: 271–277, [CSA]
- Immunotoxicity of pesticides. Immunotoxicology of Drugs and Chemicals, J. Descotes. Elsevier, Amsterdam 1988; 347–363
- Desi I., Dobronyl I., Varga. Immuno, neuro and general toxicologic animal studies and synthetic pyrethroid: Cypermethrin. Ecotoxicol. Environ. Safety 1986; 12: 220–232, [CSA]
- Poultry Immunology, R. R. Dietert, K. A. Golemboski, H. Kwak, R. Ha, T. L. Miller, T. F. Davison, T. R. Morris, L. N. Payne. Carfax Publishing, Abingdon, UK, 343–356
- El-Gohary M., Awara W. M., Nassar S., Hawas S. Deltamethrin induced testicular apoptosis in rats: The protective effect of nitric oxide synthase inhibitor. Toxicology 1999; 132: 1–8, [CSA]
- Emoto K., Toyama-Sorimachi N., Karasuyama H., Inoue K., Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp. Cell Res. 1997; 232: 430–434, [CSA]
- Enan E., Pinkerton K. E., Peake J., Matsumuro F. Deltamethrin induced thymus atrophy in male Balb/c mice. Biochem. Pharmacol. 1996; 51: 447–454, [CSA]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992; 148: 2207–2216, [CSA]
- Fair J. M., Ricklefs R. E. Physiological, growth, and immune responses of Japanese quail chicks to the multiple stressors of immunological challenge and lead shot. Arch. Environ. Contam. Toxicol. 2002; 42: 77–87, [CSA]
- Fairbrother A., Smits J., Grasman K. A. Avian immunotoxicology. J. Toxicol. Environ. Health 2004; 7: 105–137, [CSA]
- Faith R. E., Luster M. L., Vos J. G. Effects on immunocompetence by chemicals of environmental concern. Reviews in Biochemical Toxicology 2, E. Hodgson, J. R. Bend, R. M. Philpot. Elsevier, Amsterdam 1980; 173–211
- Haddad E. E., Marshaly M. M. Augmentation of natural cell mediated cytotoxicity by supernatants from in vitro mitogen activated, in vivo hormone-treated lymphocytes in mature made (K strain) chickens. Immunol. Invest. 1992; 21: 365–375, [CSA]
- Chemistry of Plant Protection 4: Synthetic Pyrethroid Insecticides: Structures and Properties, G. Haug, H. Hoffman. Springer-Verlag, Berlin 1990
- Kacmar P., Pistl J., Mikula I. Immunotoxicology and veterinary medicine. Acta Vet. 1999; 68: 57–79, [CSA]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: Basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972; 26: 239–257, [CSA]
- Knoopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., Vanoers M. H. Annexin-V for flow cytometric detection of phosphatidylserine expression on B-cells undergoing apoptosis. Blood 1994; 84: 1415–1420, [CSA]
- Kowalczyk-Bronisz S. H., Gieldanowski J., Bubak B. Immunological profile of animals exposed to pesticide—deltamethrin. Arch. Immunol. Ther. Exp. 1990; 38: 229–238, [CSA]
- Krahling S., Callahan M. K., Williamson P., Schlegel R. A. Exposure of phosphatidylserine is a general feature in phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999; 6: 183–189, [CSA]
- Le W. D., Colom L. V., Xie W. J., Smith G., Allexianu M., Appel S. H. Cell death induced by beta amyloid I-40 in MES 23.5 hybrid clone: The role of nitric oxide and NMDA-gated channels activation leading to apoptosis. Brain Res. 1995; 686: 49–60, [CSA]
- Loose L. D., Pittman K. A., Benitz K. F., Silkworth J. B., Mueller W., Coulston F. Environmental chemical-induced immune dysfunction. Ecotoxicol. Environ. Safety 1978; 2: 173–198, [CSA]
- Lukowicz R. J., Krenchniak J. Effects of deltamethrin on the immune system in mice. Environ. Res. 1992; 59: 467–475, [CSA]
- Malorni W., Fais S., Fiorentini. Morphological aspects of apoptosis. Apoptosis: Laboratory Manual of Experimental Methods, A. Cossarizza, D. Boraschi. Purdue University, West Lafayette, INUSA 1998
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983; 65: 55–63, [CSA]
- Nagata S. Apoptotic DNA fragmentation. Exp. Cell Res. 2000; 256: 12–18, [CSA]
- Nicotera P., Bonfoco E., Brune B. Mechanisms for NO-induced cell death: Involvement of apoptosis. Adv. Neuroimmunol. 1995; 5: 411–420, [CSA]
- Palluy O., Rigaud M. Nitric oxide induces cultured cortical neuron apoptosis. Neurosci. Lett. 1996; 208: 1–4, [CSA]
- Patro N., Patro I. K. Effects of deltamethrin on granule cell migration during postnatal development of rat cerebellum. Indian J. Exp. Biol. 2005; 43: 158–162, [CSA]
- Pistl J., Kovalkovicova N., Holovska V., Legath J., Mikula I. Determination of the immunotoxic potential of pesticides on functional activity of sheep leukocytes in vitro. Toxicology 2003; 188: 73–81, [CSA]
- Quist C. F., Bounous D. I., Kilburn J. V., Nettles V. F., Wyatt R. D. The effect of dietary aflatoxin on wild turkey poults. J. Wild. Dis. 2000; 36: 436–444, [CSA]
- Rai-el-Balhaa G., Pellerin J. L., Bodin G., Abdulla A. Lymphoblastic transformation assay of sheep peripheral blood lymphocytes, a new rapid and easy to read technique. Comp. Immunol. Microbiol. Inf. Dis. 1985; 8: 311–318, [CSA]
- Rai-el-Balhaa G., Pellerin J. L., Bodin G., Abdullah A. Importance of the hour sampling in the lymphoblastic transformation assay of sheep peripheral blood lymphocytes. Vet. Immunol. Immunopathol. 1987; 16: 67–76, [CSA]
- Raszyk J., Toman M., Gajduskova V., Nezveda K., Ulrich R., Jarosova A., Docekalova H., Salava J., Palac J. Effect of environmental pollutants on the porcine and bovine immune systems. Vet. Med. Czech. 1997; 42: 313–317, [CSA]
- Schwartzman R. A., Cidlowski J. A. Apoptosis: The biochemistry and molecular biology of programmed cell death. Endocrine Rev. 1993; 14: 133–151, [CSA]
- Stelzer K. J., Gordon M. Effect of pyrethroids on lymphocyte mitogenic responsiveness. Res. Commun. Chem. Pathol. Pharmacol. 1984; 66: 137–150, [CSA]
- Immunotoxicology of Drugs and Chemicals, J. Descotes. Elsevier, Amsterdam, 315–332
- Sun Y., Slinkenbeard K. D., Own C. L., Cudd L., Clarke C. R., Highlander S. K. Ultrastructural characterization of apoptosis in bovine lymphocytes exposed to Pasteurella haemolytica leucotoxin. Am. J. Vet. Res. 2000; 61: 51–56, [CSA]
- Tammang R. K., Jha G. J., Gupta M. K., Chauhan H. V., Tiwary B. K. In vivo immunosuppression by synthetic pyrethroid (cypermethrin) pesticide in mice and goats. Vet. Immunol. Immunopathol 1988; 19: 299–305, [CSA]
- Tandan. Studies on pharmacological and toxicological aspects of fenvelerate—A synthetic pyrethroid. Ph.D. Thesis, Deemed University, IVRI, Izatnagar, India 1990
- Vermes I., Haanen C., Steffensnakker H., Reutelingsperger C. A novel assay for apoptosis: Flow cytometric detection of phosphatidylserine expression in early apoptotic cells using fluorescence labeled annexin-V. J. Immunol. Meth. 1995; 184: 39–51, [CSA]
- Vohr H. W. Experiences with an advanced screening procedure or the identification of chemicals with an immunotoxic potential in routine toxicology. Toxicology 1995; 104: 149–158, [CSA]
- Vos J. G., Van Genderen H. Toxicological aspects of immunosuppression. Pesticides and the Environment: A Continuing Controversy, W. B. Deichmann. Intercontinental Medical Book Corporation, New York 1973; 527–545
- The Pesticide Manual: A World Compendium. Eighth edition, C. R. Worthing. The British Crop Protection Council. 1987
- Wu A., Li L., Liu Y. Deltamethrin induces apoptotic cell death in cultured cerebral cortical neurons. Toxicol. Appl. Pharmacol. 2003; 187: 50–57, [CSA]
- Wu A., Liu Y. Prolonged expression of c-Fos and c-Jun in the cerebral cortex of rats after deltamethrin treatment. Brain Res. Mol Brain Res. 2003; 110: 147–151, [CSA]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980; 284: 555–556, [CSA]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980; 68: 251–306, [CSA]