Abstract
Increased expectations from a number of regulatory agencies, e.g., Environmental Protection Agency (EPA), Food and Drug Administration (FDA), European Medicines Agency (EMEA), and the Ministry of Health, Labour and Welfare (MHLW) of Japan, call for the evaluation of potential adverse effects on the immune system. As recently summarized in the ICH S8 guideline, the T-cell-dependent antibody response (TDAR) has been identified in a regulatory context as a main functional test of immunotoxicity. While the characterization of immunotoxic potential is pertinent to both the chemical and pharmaceutical industries, the use of immunotoxicology data for hazard identification and/or risk assessment in each case is different. Therefore, multiple approaches to immunotoxicity testing have evolved. The assays that evaluate TDAR function include both well-established tests, e.g., anti-sheep red blood cell plaque-forming cell (PFC) assay, and newer models, e.g., anti-keyhole limpet hemocyanin (KLH) antibody ELISA. These tests vary in the study design, antigen application and analytical methods. However, they all evaluate the same endpoint—a competent immune (e.g., antibody) response to an antigen. Numerous issues have been identified in the application of TDAR tests, including high animal to animal variability; differences in antigen source and potency; a lack of established “normal range” of the immune response and uncertainty about the degree of inhibition of the TDAR to be considered toxicologically important. As such, the need for a forum to discuss these issues was recognized by the immunotoxicology community, and was addressed at the 2006 Society of Toxicology (SOT) Workshop. A series of papers will summarize that forum with the ultimate objectives being to build a consensus among immunotoxicologists on the implications of these factors on using TDAR results in hazard identification and/or risk assessment, and to establish a criteria to classify compounds as immunotoxicants.
INTRODUCTORY REMARKS
Studies in animals and humans have indicated that the immune system is a potential target organ, and that damage to this system can be associated with morbidity and even mortality. Immunotoxicology is a scientific discipline focused on the evaluation of potential adverse effects of xenobiotics (chemicals, drugs and biotechnology derived products) on the host immune mechanisms that can lead to harmful changes in host responses manifested as an increased susceptibility to infectious diseases and tumorigenesis, the induction of hypersensitivity reactions, or an increased incidence of autoimmune disease. Recognition by regulatory agencies that the immune system is an important as well as sensitive target organ for chemical- and drug-induced toxicity is an indication of the growth of this subdiscipline of toxicology.
With the availability of sensitive, reproducible, and predictive tests, immunotoxicology testing has emerged in recent years as a significant adjunct to routine safety evaluation of environmental chemicals and pharmaceuticals in development, and has been addressed by guidelines issued by several regulatory authorities, including the United States Environmental Protection Agency (EPA, Citation1998) and Food and Drug Administration (FDA, Citation2002), the European Medicines Agency (EMEA) (CPMP, Citation2000), and the International Conference On Harmonisation (ICH, Citation2006).
Regulatory approaches to immunotoxicity have evolved with the science, and all immunotoxicity testing strategies to date have recognized the complexity of the immune system as a target organ, and that no single immune parameter can be used with sufficient confidence to test for the hazard of immunotoxicity. Historically, immunotoxicity has been assessed by a battery of assays—usually structured in a multi-tiered approach—and an appreciation of this historical perspective is essential to understand why the T-cell-dependent antibody response (TDAR) has emerged as a main functional test of immunotoxicity.
In an effort conducted as part of the National Toxicology Program (NTP) over 50 chemicals were studied, including a variety of chemical classes, such as catalysts, solvents, dyes, lubricants, pesticides, disinfectants, drugs, food additives and natural products (Luster et al., Citation1988). The NTP studies utilized a multi-tiered approach with Tier I providing an assessment of general toxicity (immunopathology, hematology, body and organ weights) as well as end-line functional assays (proliferative responses, the TDAR assay—measured as PFC assay, and the NK assay). Tier I was designed to detect potential immunotoxic compounds at concentrations that do not produce overt toxicity. Tier II was designed to further define an immunotoxic effect, and included tests for cell-mediated immunity (e.g., cytotoxic T-lymphocyte [CTL] assay and delayed-hypersensitivity response [DHR]), secondary antibody responses, enumeration of lymphocyte populations, and host resistance models.
The results of the NTP studies indicated that while none of the assays measured were 100% predictive alone, the TDAR, an assessment of lymphocyte subpopulations by cytofluorometric analysis, and an assessment of NK activity were the most valuable in terms of predicting immunotoxicity (Luster et al., Citation1992). Subsequent analysis indicated that an application of the full extent of the tiered approach was not necessary. The NTP studies indicated that several combinations of only two tests could give > 90% concordance, and that a number of combinations of just three tests gave 100% concordance. Subsequent results also indicated a good correlation between the results from functional tests and host resistance models (Luster et al., Citation1993), which indicated that the latter were not necessary to adequately identify immunosuppressive potential. The impacts of the NTP database are clear as indicated by the recently established immunotoxicity guidelines. Specifically, after several years of international debate, the importance of including functional immunotoxicity assessments in regulatory studies has been emphasized, with the TDAR emerging as a main functional test of immunotoxicity.
The testing approach in the EPA OPPTS 870.7800 immunotoxicity guideline (USEPA, Citation1998) reflected the continued evolution of the science of immunotoxicology and a more limited, case-by-case approach than previously described by earlier, more comprehensive, guidelines considered earlier by this agency. The cornerstone of OPPTS 870-7800 was the primary TDAR. If the chemical produces significant suppression of this response, surface marker assessment by flow cytometry may be performed. If the chemical produces no suppression of the TDAR, an assessment of innate immunity (NK assay) may be performed. Specific criteria for the conduct of these “optional” tests have never been identified. The tests do not represent a comprehensive assessment of immune function but are intended to complement assessment made in routine toxicity testing (hematological assessments, lymphoid organ weights, and histopathology).
The FDA Center for Drug Evaluation and Research (CDER) document, “Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs” (FDA, Citation2002) is arguably the most comprehensive description of approaches to immunotoxicology. Not only does the FDA CDER “Guidance for Industry” describe the spectrum of adverse events associated with the immunotoxicology continuum, including immune suppression, immunogenicity, hypersensitivity, autoimmunity and adverse immune stimulation, this document provides approaches at the level of specific methodology for evaluating each event. As with earlier guidance documents from the FDA, the new “Guidance for Industry” advocating the use of information derived from standard repeat-dose toxicity studies to provide the earliest indicators of immunotoxicity.
While immune function evaluation is an integral component of immunotoxicity assessments of chemicals and new drugs, immune function testing of pharmaceuticals takes into account drug characteristics, including mechanism of pharmacological action, therapeutic application and biological context of other toxicity observed in multiple studies. Furthermore, in pharmaceutical immunotoxicology testing, a distinction between a temporal immunomodulation and irreversible immunotoxicity may be more difficult to interpret than signals of any undesired effects caused by chemicals not intended to be directly administered to humans.
Internationally, one of the most significant advances in our approach to the assessment of immunotoxicity with human pharmaceuticals has been a guidance entitled, “S8 Immunotoxicity Studies for Human Pharmaceuticals” (ICH, Citation2006), which was prepared under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceutical for Human Use (ICH) and was made available in 2006. The objectives of this guideline are twofold: to provide recommendations on non-clinical testing approaches to identify compounds which have the potential for immunotoxicity; and to provide guidance on a weight-of-evidence decision making approach for immunotoxicity testing. The guidance applies to unintended immunosuppression and immunoenhancement, excluding allergenicity or drug-specific autoimmunity. According to the ICH S8 guideline evaluation of potential adverse effects on the immune system should be incorporated into standard drug development.
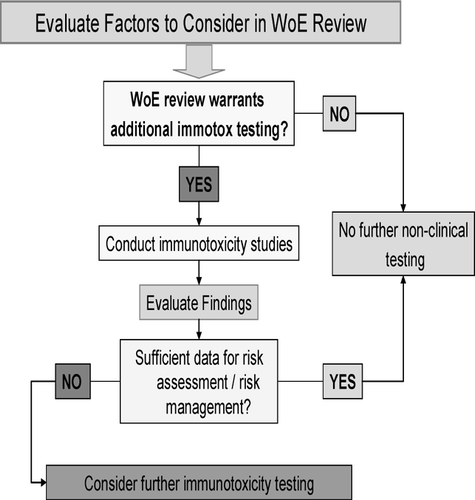
However, the functional immunotoxicology studies (anticipated to be performed for all environmental chemicals) should be conducted based on a weight-of-evidence review of multiple factors. The ICH S8 guideline is based on a cause-for-concern approach using a review of factors such as the following: (1) findings from standard toxicology studies; (2) the pharmacological properties of the drug; (3) the intended patient population; (4) structural similarities to known immunomodulators; (5) the disposition of the drug; and, (6) clinical information. The document provides a decision tree as illustrated here.
Recent immunotoxicology guidance documents recommend TDAR tests primarily because this assay represents a comprehensive evaluation of immune function based on assessment of the various components of the immune system (e.g., antigen-presenting cells, T-helper cells, and B-lymphocytes) involved in the antigen-specific antibody response. Any compound-induced alteration in antigen processing, presentation, cell proliferation, differentiation and/or secretion of interleukins, antibody production and cytokine-dependent isotype class switch (i.e., IgM- to IgG-specific antibody response), is thought likely to modify the response.
Originally this test was configured as an IgM/complement-dependent ex vivo plaque-forming cell (PFC) assay in mice, with sheep red blood cells (SRBC) as both the immunogen and the target for complement mediated lysis. Subsequently, an ELISA-based format was developed using SRBC as both immunogen and antigen in the antibody detection system (Temple et al., Citation1993). SRBC-based TDAR assays (PFC and ELISA) have also been used in rats, and have ultimately gained acceptance as validated tests for detecting immunosuppressant activity of drugs and chemicals (Ladics et al., Citation1998).
More recently, for testing immune function of drugs under development, a new rat model has been established using keyhole limpet hemocyanin (KLH) as the T-cell-dependent antigen (Gore et al., Citation2004). Injection of KLH results in induction of IgM- and IgG-specific antibodies. Usually, the peak of the primary (e.g., anti-KLH) IgM antibody response occurs 5 ± 2 days post-immunization and the peak of the primary (e.g., anti-KLH) IgG antibody response occurs 2 weeks post-immunization. Inclusion of IgG evaluation in the primary antibody response has not been emphasized in current immuntoxicology guidelines; however, published data (Herzyk and Gore, Citation2004) indicate that this additional endpoint may offer greater sensitivity and enhanced predictive value in the context of immunotoxicity testing.
A number of immunotoxicology laboratories have adopted KLH as their immunogen and/or antigen of choice in evaluating TDAR. However, to date there have been no formal cross validations of the assay, either in comparison to SRBC or across laboratories. Thus, there is a wealth of data, generated in different laboratories, but under somewhat differing protocols and different outcome measures. Such data do not lend themselves to direct comparison of effects of different compounds and evoke questions about a need for a rigorous cross-validation between SRBC tests and KLH tests in immunotoxicology applications.
Furthermore, there are other challenges in current immune function testing and data interpretation related to the following limitations: (1) a small pool of historical data in different toxicology species; (2) a lack of an established threshold for biologically meaningful immunotoxicity since all methods are being validated with known strong immunotoxicants; and, (3) a lack of translational knowledge and experience with signals from immune function tests in the preclinical studies in animals and human safety concerns.
OVERVIEW OF THE WORKSHOP
The purpose of the Workshop at the 2006 SOT meeting was to collect and discuss existing data on TDAR tests from a number of laboratories to better understand the comparability of the various test protocols and the current status of their applications in immune function testing and the assessment of immunotoxicity potential. As briefly mentioned, multiple test systems have been applied in TDAR evaluations, and there has been uncertainty whether the various study designs and assays are “validated” and/or acceptable for use in regulatory studies. A comparative review of existing data was needed to improve communication between immunotoxicologists, and to increase our understanding of the impact of immunotoxicity evaluation on safety of novel compounds. A number of experts from the immunotoxicology community were brought together to discuss their experiences in using TDAR tests in evaluation of immunotoxicity potential. Their insights are captured in the subsequent series of papers and are briefly summarized here.
In the paper by Dr. Gregory Ladics (DuPont Co.), the current applicability of the primary immune response to Sheep Red Blood Cells (SRBC), which he describes as the conventional TDAR test, is summarized. He emphasizes that the T-cell-dependent antigen of choice has historically been SRBC, and that the SRBC plaque forming cell (PFC) assay is considered the “gold standard” for TDAR based on extensive intra- and inter-laboratory validation in mice and the fact that it has been utilized for over 35 years. As noted above, results from the NTP indicated that the quantification of the primary PFC response (i.e., the specific IgM antibody-forming cell response) was found to provide one of the best predictors of immunotoxicity in mice. Dr. Ladics summarizes data from the application of using both the SRBC specific PFC and ELISA for the evaluation of potential immunotoxicity of chemicals in rodents, and the pros and cons and associated issues of each method.
In the subsequent paper, he discusses: (1) current state of regulatory guidelines pertaining to SRBC TDAR tests; (2) data from an inter-laboratory study comparing the PFC and ELISA in outbred rodents using both cyclophosphamide and dexamethasone; (3) characterization of an approach to developmental immunotoxicology assessment in the rat using SRBC as the antigen; and, (4) studies investigating the incorporation of the SRBC-specific IgM ELISA in rats on standard toxicology study.
In his paper, Dr. Kimber White, Jr. (Virginia Commonwealth University) emphasizes that the TDAR has emerged as a main functional test of immunotoxicity, and highlights the similarities and differences between measured endpoints using the primary immune responses to SRBC and KLH in rodents. He summarizes recent data indicating that the sensitizing dose of KLH used in rodents to elicit anti-KLH antibodies can differentially affect the responses, and that within the same species, different strains of mice and rats can produce different magnitudes of responses to the same sensitizing dose. Finally, he discusses the sensitivity of the SRBC PFC assay and the KLH and SRBC ELISA-based assays, when compared using several known immunosuppressive agents, including cyclophosphamide, dexamethasone, and azathioprine. He also addresses the comparability of results measuring the TDAR in B6C3F1 mice and F344 rats, the primary rodents used in the NTP immunotoxicological studies, with results measuring the TDAR in CD1 mice and Sprague–Dawley rats, the primary test models used by industry.
The paper by Dr. Peter Bugelski (Centocor) summarizes a meta-analysis of results generated across multiple laboratories as part of a consortia conducted under the aegis of the Immunotoxicology Technical Committee (ITC) of the ILSI Health and Environmental Sciences Institute (HESI). He emphasizes that TDAR assays were originally configured as an IgM/complement-dependent ex vivo PFC assay in mice with SRBC and that more recently KLH has been explored as an alternate antigen for TDAR. Since protocols for anti-KLH antibody responses had not been harmonized among laboratories using the tests, the comparative analysis of TDAR data was undertaken.
The purpose of this study was to apply meta-analysis to compare data from several laboratories, using SRBC or KLH, in two strains of rats, by multiple routes of immunization and with different primary TDAR assay formats. He notes that, although limitations imposed by the scale of the data set and the need to convert some data to an ordinal scale to allow comparisons restricted the scope and strength of the conclusions that could be drawn, the results of this study did show that the 2 antigens and the 3 TDAR assay formats (SRBC-PFC, SRBC-ELISA, or KLH-ELISA) gave comparable results. Notably, both antigens and assay formats showed the same pattern of response to strong immunotoxicants. As described in the subsequent paper, these results indicate that standardizing the choice of antigen or assay format may not be critical in the evaluation of immunotoxicity potential.
In her paper, Dr. Helen Haggerty (Bristol-Myers Squibb) summarizes immunotoxicity testing in non-rodent species. She notes that increased expectations from a number of regulatory agencies, as summarized in the ICHS8 Guidance document, have resulted in increased attention to the determination of potential adverse effects on the immune system, and that evaluation of the immunotoxicity potential of some pharmaceuticals, including immunomodulatory chemicals and biologics, cannot be limited to testing in rodents.
As such, immune function tests have also been applied in studies with non-human primates, and more recently, dogs, that assess various components of the immune system. These assays include TDAR with various immunogens, lymphocyte phenotyping, NK cell activity, delayed-type hypersensitivity, and macrophage function assays. Approaches for incorporating immune function testing in non-rodent species, results from these tests, and their interpretation with respect to drug safety assessment are discussed in the subsequent paper.
CONCLUDING REMARKS
Taken together, the discussion during the SOT workshop and the subsequent series of papers indicate clearly that assays measuring TDAR have been extensively characterized since the early days of the NTP studies, and that the applicability of these endpoints have been tremendously expanded. A critically important observation is that even though multiple test systems (i.e., different protocols using different antigens and different species) have been developed and applied, the evaluation of the TDAR appears to be a sufficiently robust response so that highly standardized protocols are not required for use in regulatory studies for the purposes of hazard identification. However, it can still be debated how many of the various study designs and assays are considered to be “validated.”
There is little question that based on the NTP studies (i.e., a comparison of > 50 chemicals in multiple laboratories using multiple immune endpoints), the SRBC PFC assay in mice comes as close to being “validated” as any immunotoxicological parameter. There is also no doubt that growing studies using the SRBC PFC assay in rats, and SRBC and KLH ELISA-based assays in multiple species contribute to the confidence in the TDAR assays, especially in the context of hazard identification in non-clinical studies for regulatory purposes.
One of the obvious and most important goals of experimental (e.g., non-clinical) immunotoxicity testing strategies is to enable the best extrapolations between the results generated in the animal models and the potential risk of immunotoxicity in humans. The significant homology of the rodent and human immune systems has facilitated our ability to make decisions on the immunotoxic hazard potential of test materials based on the results of these experimental models. However, the characterization of the risk associated with xenobiotic-induced immunotoxicity represents one of the key challenges for this discipline in the immediate future. In spite of the impressive growth in the approaches to immunotoxicity testing, there are still relatively few examples of where risk assessment, as it is traditionally defined, has been applied to immunotoxicology to the fullest extent.
In 1983, the National Research Council (NRC) of the National Academies of Science (NAS) published a “Redbook,” entitled “Risk Assessment in the Federal Government: Managing the Process,” which was intended to facilitate the development of uniform technical guidelines. The NRC Redbook defined “risk assessment” as “the characterization of the potential adverse health effects of human exposures to environmental hazards.” Importantly, the NRC identified the following basic steps of a human health risk assessment: hazard identification; dose-response assessment; exposure assessment; and risk characterization.
As emphasized here, the immune system has unquestionably been identified as a potential target organ for drugs and chemicals, and therefore, the potential for immunotoxic “hazard” certainly exists. Studies like those conducted by the NTP have had a big impact on the integration of the principles of risk assessment, especially in the context of hazard identification and dose-response assessment. “Exposure” is an equally important part of risk assessment, and is a function of the amount of chemical involved and the time of its interaction with people and/or the environment.
As such, the assessment of “risk” is often an assessment of the probability for exposure, because if there is no exposure, there is no risk—even to the most hazardous materials. In this context, differences in the integration of immunotoxicity results into the risk assessment of environmental chemicals and pharmaceuticals are greatest. For environmental chemicals, models are generally needed to estimate the potential for occupational and residential exposure, which is primarily unintended. For pharmaceuticals, exposure in man is intended, and the goal is to adequately characterize the immunotoxic risk during drug development for clinical use.
In conclusion, it is hoped that this workshop and the subsequent papers will enable the immunotoxicology community to move beyond questions about the validation status and the regulatory acceptance of the TDAR and can focus resources on the following future challenges:
Interpretation of the significance of minor or moderate immunotoxic effects in animal models in relation to human risk assessment.
Integrated consideration of exposure into risk assessment and immunotoxicology.
Design of human studies to assess the impact on the immune system in the species of greatest interest in the context of risk assessment.
As the regulatory pressures for immunotoxicology determinations continue to emerge in the years ahead, there is no question that opportunities to address these challenges will be forthcoming, and that the resolution of these challenges will facilitate the continued integration of the principles of risk assessment into immunotoxicology.
REFERENCES
- CPMP. Committee on Proprietary Medicinal Products. 2000, http://www.emea.eu.int CPMP/SWP/1042/99, Note for Guidance on Repeated-Dose Toxicity
- EPA. United States Environmental Protection Agency. Health Effects Test Guidelines: Immunotoxicity. 1998, http://www.epa.gov/opptsfrs/publications OPPTS 870.7800
- FDA. United States Food and Drug Administration. Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs. 2002, http://www.fda.gov/cder/guidance
- Gore E. R., Gower J., Kurali E., Sui J. L., Bynum J., Ennulat D., Herzyk D. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology 2004; 197: 23–35
- Herzyk D. J., Gore E. R. Adequate immunotoxicity testing in drug development. Toxicol. Lett. 2004; 149: 115–122
- ICH. International Conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. 2006, ICH Harmonised Tripartite Guideline Immunotoxicity Studies For Human Pharmaceuticals S8.
- Ladics G. S., Smith C., Elliott G. S., Slone T. W., Loveless S. E. Further evaluation of the incorporation of an immunotoxicological functional assay for assessing humoral immunity for hazard identification purposes in rats in a standard toxicology study. Toxicology 1998; 126: 137–152
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J. D., White K. L., Jr., Lauer L. D., Germolec D. R., Rosenthal G. J., Dean J. H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 1988; 10: 2–19
- Luster M. I., Portier C., Pait D. G., Rosenthal G. J., Germolec D. R., Corsini E., Blaylock B. L., Pollock P., Kouchi Y., Craig W., White K. L., Jr., Munson A. E., Comment C. E. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 1993; 21: 71–82
- Luster M. I., Portier C., Pait D. G., White K. L., Jr., Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992; 18: 200–210
- Temple L., Kawabata T. T., Munson A. E., White K. L., Jr. Comparison of ELISA and plaque-forming cell assay for measuring the humoral immune response to SRBC in animals treated with benzo(a)pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993; 21: 412–419