Abstract
T-Cell leukemias and lymphomas represent a less common and heterogeneous group of lymphoid neoplasms. Overall, they respond less well to chemotherapy and have a poorer prognosis than their B-cell counterparts. T-Cell tumors express a number of potential targets for receptor-directed antibody therapy; however, there is no available therapeutic monoclonal antibody for these diseases with comparable activity to that of rituximab in B-cell disorders. Despite this, alemtuzumab, a humanized anti-CD52 monoclonal antibody has demonstrated meaningful anti-tumor activity in a variety of T-cell malignancies. A number of other antibodies, modified antibodies and immunotoxins directed against targets such as CD2, CD4, CD5, CD25, CD30 and CD122 expressed on malignant T-cells are under investigation. The current status of receptor-directed antibody therapy for T-cell leukemia and lymphoma is reviewed.
| Abbreviations | ||
| ACID, | = | Activation-induced cell death |
| ATL, | = | Adult T-cell leukemia/lymphoma |
| ALCL, | = | Anaplastic large cell lymphoma |
| ADCC, | = | Antibody-dependent cellular cytotoxicity |
| AIL, | = | Angioimmunoblastic T-cell lymphoma |
| CD, | = | Cluster designation |
| CHOP, | = | Cyclophosphamide, doxorubicin, vincristine and prednisone |
| CTCL, | = | Cutaneous T-cell lymphoma |
| CMV, | = | Cytomegalovirus |
| CLL, | = | Chronic lymphocytic leukemia |
| DT, | = | Diphtheria toxin |
| EPOCH, | = | Etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin |
| EBV-LPD, | = | Epstein-Barr virus-related B-cell lymphoproliferative disease, GPI, Glycophosphoinositol |
| GvHD, | = | Graft-versus-host disease |
| HAMA, | = | Human anti-mouse antibodies |
| HL, | = | Hodgkin's lymphoma |
| HTLV-1, | = | Human T-cell lymphotrophic virus type-1 |
| IL, | = | Interleukin |
| JAK, | = | Janus kinase |
| Kd, | = | Dissociation constant |
| LAK, | = | Lymphokine-activated killer |
| LFA, | = | Lymphocyte function antigen |
| MHC, | = | Major histocompatibility complex, NK, natural killer |
| NOD/SCID, | = | non-obese diabetic/severe combined immunodeficiency |
| PE, | = | Pseudomonas exotoxin A |
| PTCL, | = | Peripheral T-cell lymphoma |
| LGL, | = | large granular lymphocyte |
| T-PLL, | = | T-cell prolymphocytic leukemia |
| TCR, | = | T-cell receptor |
| TNFα, | = | Tumor necrosis factor-alpha |
| WHO, | = | World Health Organization |
INTRODUCTION
T-Cell leukemias and lymphomas represent a heterogeneous group of neoplasms that account for 7–15% of non-Hodgkin's lymphomas, contributing about 6,500 new cases annually in the United States (Groves et al., 1995; Non-Hodgkin's Lymphoma Classification Project, Citation1997; Armitage and Weisenberger, Citation1998). In contrast to the more common B-cell leukemias and lymphomas, T-cell malignancies often lack specific immunophenotypic profiles (Feldman et al., Citation2006). While the expression of certain antigens is associated with specific diseases, this association is usually not disease-specific (). The same antigen may be expressed across several different disorders.
TABLE 1 T-cell lymphoma immunophenotypes
For example, CD30, a tumor necrosis factor receptor family member, is over-expressed in anaplastic large cell lymphoma (ALCL), Hodgkin's lymphoma (HL), and some cutaneous T-cell lymphomas (CTCL) (Chittal et al., Citation1988; Stein et al., Citation2000; Marti et al., Citation2003; Barberio et al., Citation2007; Medeiros and Elenitoba-Johnson, Citation2007). T-Cell malignancies are categorized into two major groups in the current WHO Classification of Lymphoid Tumors: (1) immature or pre-thymic T-cell leukemias and lymphomas; and, (2) the more mature post-thymic or peripheral T-cell disorders () (Jaffe et al., Citation2001). Pre-thymic malignancies such as T-cell acute lymphoblastic lymphoma are typically pediatric diseases and will not be discussed in this review.
TABLE 2 World Health Organization (WHO) classification of T-cell and NK cell lymphoid tumorsFootnotea
In comparison to the B-cell disorders, T-cell malignancies exhibit a marked geographical variation in incidence. The incidence of adult T-cell leukemia/lymphoma (ATL) tracks the seroprevalence for infection with human T-cell lymphotrophic virus type-1 (HTLV-1) with higher frequencies in Japan, the Caribbean basin, and West Africa (Lennert et al., Citation1985). Enteropathy-associated T-cell lymphoma is increased in the British Isles associated with the incidence of celiac disease in this region (Holmes, Citation2002); and NK/T-cell sinonasal lymphoma occurs more often in Asia, and Central and Latin America (Abbondanzo and Wenig, Citation1995). In the United States, the most frequently diagnosed T-cell neoplasms are peripheral T-cell lymphoma (PTCL), ALCL, angioimmunoblastic T-cell lymphoma (AIL) and CTCL (mycosis fungoides/Sezary syndrome) (Criscione and Weinstock, Citation2007; Jermal et al., Citation2007).
Patients with malignant T-cell disorders often present with high-grade lesions, advanced stage and have systemic symptoms at diagnosis (Lopez-Guillermo, 1998). Historically, these diseases have been treated with the same combination chemotherapy regimens that were used to treat aggressive B-cell lymphomas. With few exceptions, the outcomes are poorer with lower response rates, shorter time to progression, shorter median survivals, and a lower chance of cure compared to patients with B-cell lymphomas (Gisselbrecht et al., Citation1998). A number of new agents have recently entered the clinical setting for the treatment of T-cell lymphoma. These include the histone deacetylase inhibitor voronistat (Zolinza; Merck & Co.) approved for CTCL (Mann et al., Citation2007). Also approved for the treatment of CTCL in the last decade are the retinoid beraxotene (Targretin; Ligand Pharmaceuticals, Inc.) and the receptor-targeted interleukin-2-diphtheria toxin fusion protein, denileukin diftitox (Ontak; Ligand Pharmaceuticals, Inc.) (Hurst, Citation2000; Wong et al., Citation2007).
The development of the chimerized anti-CD20 monoclonal antibody rituximab (Rituxan; Genetech, Inc.) was a major advance for the treatment of B-cell lymphoma. Studies have demonstrated the major impact of rituximab on the survival of patients with B-cell lymphoma especially when combined with chemotherapy (Coiffer et al., 2002; Sehn et al., Citation2005). To date; however, no antibody has received similar indication for treatment of T-cell leukemia or lymphoma. Presently a number of antibodies targeting cell-surface receptors on T-cells are being evaluated for the treatment of T-cell leukemia and lymphoma. The current status of these agents will be the focus of this review.
Targeting T-Cells
The ideal target for receptor-directed therapy should exhibit certain characteristics including: (1) restricted expression to the malignant T-cells. If the target is expressed on non-malignant cells, the loss of these cells should not result in serious complications such as life-threatening immunosuppression. This leads to an inherent difficulty targeting T-cell tumors due to the requirement for functional T-cells to prevent the reactivation of many serious viral, fungal and parasitic infections. Therapy directed against broadly expressed T-cell targets results in the depletion of both CD4+ and CD8+ T-cell populations and an increased risk of opportunistic infection (Nosari et al., Citation2004). On the other hand, antigens with limited expression restrict the spectrum of diseases that can be effectively targeted; (2) the receptor should be expressed at high numbers on the surface of the malignant T-cell to provide an adequate number of binding sites for the targeting agent. Escape mutants that lose expression will be unaffected by the antibody because there is no target for it to bind to. This problem may be overcome by using radiolabeled antibodies because of a radiobiological “bystander” killing effect; (3) the target receptor should be non-modulating. Some antigens when engaged with antibody undergo internalization, thus reducing the amount of target available on the cell surface. Modulation can be used to an advantage with immunotoxins and ligand-toxin fusion proteins that are activated when taken inside the cell, but in general, modulation reduces the effectiveness of monoclonal antibodies; and, (4) engagement of the receptor by the targeting agent should not lead to serious systemic side effects. Some antibodies can stimulate the massive systemic release of inflammatory cytokines with serious consequences.
Ideally, the targeting agent itself should exhibit several mechanisms of antitumor activity. The anti-CD25 antibody daclizumab blocks the binding of IL-2 to its receptor, depriving T-cells of a necessary growth factor (Tkaczuk et al., Citation2002). The anti-CD52 antibody alemtuzumab activates serum complement, leading to lysis of its target cells. (Hale et al., Citation1983) Some antibodies will engage the Fc receptors on effector cells such as macrophages and cytolytic T-cells, resulting in destruction of the target cell through antibody-dependent cellular cytotoxicity (ADCC). Daclizumab and alemtuzumab use several of these mechanisms (Zhang et al., Citation2004; Lowenstein et al., Citation2006). A number of anti-CD30 antibodies can activate apoptotic signals when they bind to their receptor causing cell death (Wahl et al., Citation2002).
Antibodies can also be chelated with or covalently bound to isotopes (radio-immunotherapy) that emit ionizing radiation, or the antibody may be linked to lethal toxins (immunotoxins) to enhance their ability to destroy tumor. In addition, the targeting agent should exhibit pharmacokinetics that facilitates patient dosing and it should also be non-immunogenic. The majority of antibodies in clinical use have been engineered so that most of the molecule except for the receptor-binding domains is identical to that of a human antibody and some are now fully human in their sequences (Lonberg, Citation2005; Mascelli et al., Citation2007). This reduces immunogenicity and the risk of induction of neutralizing responses against the antibody.
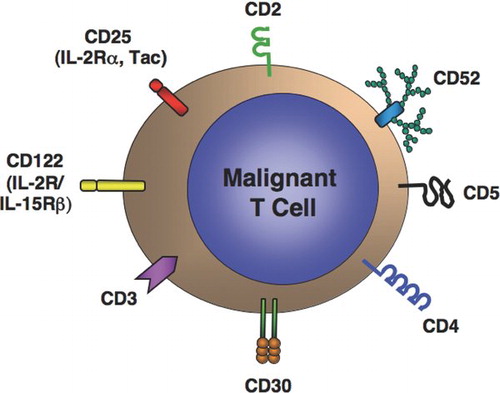
T-Cell leukemias and lymphomas represent a “target-rich environment” for receptor-directed therapy. T-Cells express a series of surface molecules that serve as receptors for a number of currently available and investigational monoclonal antibodies, immunotoxins or toxin-ligand fusion proteins (). These include the pan-T-cell antigen CD2, the T-cell receptor complex (CD3), the CD4 co-receptor, CD5, various cytokine receptors and their subunits including CD25, CD30, and CD122, and the surface glycoprotein CD52 ().
TABLE 3 Receptor-directed therapy for T-cell leukemia and lymphoma
CD2
CD2 (LFA-2, T11, sheep erythrocyte (E)-rosette receptor) is a 50 kDa glycoprotein whose extracellular region consists of two immunoglobulin-like domains (Moingeon et al., Citation1989). It has a proline-rich cytoplasmic tail involved in signal transduction. It is expressed at high density on thymocytes, most T-cells and on NK cells (Sanchez-Madrid et al., Citation1982). CD2 is one of the earliest markers expressed in T-cell development. Its ligand is CD58 (LFA-3), and possibly CD59 and CD48. CD2 is involved in T-cell activation, antigen co-stimulation, and cell adhesion. It enhances the physical interaction between T-cells and antigen-presenting cells, as well as between T-cells and NK cells, and their targets. In the cell membrane, CD2 associates with the T-cell receptor (TCR) and appears to enhance CD3 signaling during low affinity interactions with the major histocompatibility complex (MHC) molecules, enhancing Class I- and Class II-restricted antigen recognition (Moingeon et al., Citation1991).
A number of monoclonal antibodies and antibody-like agents targeting CD2 have been studied. Alefacept (Amevive; Astellas Pharma, Inc.), an LFA-3-immunoglobulin (Ig) Fc fusion protein that binds to CD2, depleted infiltrating T-cells in the skin and induced histological remissions in patients with psoriasis (Majeau et al., Citation1994; Chamian et al., Citation2005). BTI-322, a rat IgG2bκ anti-CD2 antibody suppressed renal allograft rejection as well as induced remissions in steroid-refractory graft-versus-host disease (GvHD) in patients undergoing allogeneic bone marrow or stem cell transplant (Przepiorka et al., Citation1998). Siplizumab (MEDI-507; MedImmune, Inc.) is a humanized BTI-322 monoclonal antibody that has been studied for the treatment of severe psoriasis and for prophylaxis of GvHD because of its T-cell depleting properties (Branco et al., Citation1999; Koenecke et al., Citation2003; Shaffer et al., Citation2007). Zhang and co-wokers examined the antitumor activity of siplizumab and found it resulted in long-term disease-free survival of mice that had been inoculated with MET-1 human ATL cells (Zhang et al., 2003). Animals receiving a 4-month course of siplizumab had a similar survival as tumor-free control mice. These results were superior to mice receiving a shorter course of siplizumab or mice treated with the humanized anti-CD25 antibody, daclizumab.
Based on this data, a Phase I/II trial of slipizumab in patients with malignant T-cell lymphoproliferative disorders was initiated (O'Mahony et al., Citation2007b). Twenty-nine patients with various T-cell malignancies including ATL, CTCL, PTCL, T-cell chronic lymphocytic leukemia, and T-cell large granular lymphocyte leukemia were treated. All patients except for one experienced a marked decline in circulating CD4+ and CD8+ T-cells and NK cells. Two complete and nine partial responses were observed. CMV reactivation was common, but was not associated with clinical disease. Four patients (13.7%), however, developed Epstein-Barr virus-related B-cell lymphoproliferative disease (EBV-LPD) (O'Mahony et al., 2007c). While it was anticipated that slipizumab would be depleting of T-cells and immunosuppressive, the increase in EBV-LPD was unexpected. It is hypothesized that the EBV-LPD was not solely due to T-cell depletion, as similar levels of T-cell depletion were observed in patients who did not develop EBV-LPD on this study.
This complication primarily occurred in patients treated on a weekly schedule with siplizumab that also produced a marked down-modulation in CD2 receptor expression on residual T-cells. CD2-deficient T-cells have been shown to have a similar cytolytic capacity when compared with those expressing CD2; however, they release less interferon-γ and proliferate more poorly in response to antigen stimulation (Teh et al., Citation1997; Sasada and Reinherz, Citation2001).
The depletion of T-cells that were directed against EBV-infected B-cells coupled with reduced CD2-signaling may allow for the unrestrained proliferation of EBV-infected B-cells and their malignant transformation. This event was not anticipated by the preclinical mouse model because of the highly selective targeting of human T-cells by siplizumab and little effect of the antibody on murine T-cells. Future clinical studies of slipizumab may incorporate rituximab (anti-CD20) in addition to suppress unrestrained B-cell proliferation.
CD3, CD4, and CD5
CD3 represents a series of intermediate molecular weight polypeptide chains (CD3γ, CD3δ, CD3ε and CDζ) closely associated with α and β -subunits of the TCR that recognizes antigen-peptide epitopes presented by MHC molecules in a class-restricted manner (Garcia and Adams, Citation2005). The intracellular regions of the CD3-subunits represent the signaling domains of the TCR complex that mediates T-cell activation. CD3 is expressed on most T-cells throughout development and thus represents a pan-T-cell antigen. The vast majority of T-cell neoplasms express CD3, although its expression may be reduced or lost in some lesions (Ohno et al., Citation1995).
Muromonab-CD3 (Orthoclone, OKT3; Janssen Pharmaceutica, Ltd.), a murine IgG2a monoclonal antibody directed against the 20 kDa CD3ζ -subunit is approved for the reversal of acute allograft rejection in patients undergoing cardiac, hepatic and renal transplants (Kirk, Citation2006). It has also been used for the depletion of T-cells from stem cell and bone marrow allografts to treat or reduce the risk of serious GvHD (Knop et al., Citation2007). Administration of muromonab-CD3 results in the rapid disappearance of CD3+ T-cells from the peripheral blood and lymphoid tissue through complement-mediated lysis, ADCC, apoptosis and the re-direction of T-lymphocytes to other compartments (Chantenoud, 2003). Binding of muromonab-CD3 to its target receptor also stimulates TCR signaling, activation and proliferation of T-cells with increased expression of HLA-DR and CD25.
In one report, a patient with refractory T-cell acute lymphoblastic leukemia who received muromonab-CD3 experienced a dramatic, albeit transient decline in circulating lymphoblasts and a reduction in splenomegaly (Gramatzki et al., Citation1995). Treatment with muromonab-CD3 improved the responsiveness of the patient's T-cells to IL-2 in vitro. A pilot Phase I trial in nine patients studied muromonab-CD3 treatment combined with systemic IL-2 (Borrione et al., Citation1996). Although some of the patients had lymphoproliferative disorders, no patient in this study was suffering from a T-cell malignancy and no responses were reported. The general use of muromonmab-CD3 therapy is made difficult because engagement of the antibody with CD3ζ increases TCR signaling and can result in the coordinated release of inflammatory cytokines that can cause life-threatening cytokine release syndrome that can reoccur. Muromonab-CD3 is also mitogenic for T-cells and its use may risk increasing the proliferation of malignant T-cells. Muromonmab-CD3 therapy is also associated with profound suppression of cell-mediated immunity and increased risk of opportunistic infections and secondary malignancies including EBV-LPD, lymphoma, skin cancers and Kaposi's sarcoma.
CD4 is a 55 kDa membrane glycoprotein with four immunoglobulin-like domains, a hydrophobic transmembrane domain and a long cytoplasmic tail (Leahy, Citation1995). CD4 acts as a co-receptor for the TCR complex. It is expressed on helper and regulatory T-cells, and it recognizes antigens presented by MHC Class II molecules in association with the TCR. CD4 represents an attractive target since the majority of the post-thymic T-cell malignancies manifest a CD4+ phenotype.
In an early study, Knox et al. (Citation1991) treated seven CTCL patients with a chimeric murine antibody composed of the IgG1κ human constant regions and the mouse variable regions directed against CD4 (anti-Leu3a; Becton-Dickinson). Patients were dosed in cohorts of 10, 20, 40 or 80 mg intravenously twice a week for three weeks. At the 80 mg dose, the antibody was detected in skin lesions and also coating circulating CD4+ T-cells in the peripheral blood; however, no significant depletion of CD4+ cells was observed. In a follow-up study, this group administered a single intravenous dose of another chimeric murine anti-CD4 monoclonal antibody (cM-T412; Centocor, Inc.) to eight previously treated CTCL patients (Knox et al., Citation1996). Following the antibody infusion there was a significant suppression of peripheral blood CD4+ cells in seven of the eight patients. Response in this trial was defined as freedom from progression. Seven of the eight patients were reported to have responded and the median response duration was 25 weeks. One patient developed a neutralizing anti-chimeric antibody response. Toxicity was grade 2 or less and usually manifested as infusional reactions, mayalgias, and rashes.
More recently, zanolimumab (HuMax-CD4®; Genmab, Inc.), a fully human IgG1κ anti-CD4 monoclonal antibody was shown to deplete CD4+ T-cells from the skin, reduce dermal inflammatory infiltrates, and induce remissions in psoriasis patients (Skov et al., Citation2003). Zanolimumab was evaluated in two separate Phase II trials in a total of 47 CTCL/Sezary syndrome patients (Kim et al., Citation2007). The dose of the antibody was stratified based on the stage and severity of disease. Patients received between 280–980 mg weekly for up to 17 weeks. Response was assessed using a Composite Assessment Grading Scale and reported as objective complete response, clinical complete response, partial response, stable or progressive disease. Zanolimumab caused a dose-dependent and profound CD4+ lymphocytopenia; however, the recovery of CD4+ cells in the peripheral blood was not dose-dependent and occurred at an average rate of 0.137 x 109 CD4+ cells/L per year.
Overall, 13 of 38 (34.2%) CTCL patients and 2 of 9 (22.2%) patients with Sezary cell leukemia responded to the antibody. Adverse events included nine infections attributed to therapy. One patient died of liver cancer three months after completion of therapy and one patient died of progressive CTCL complicated by pneumonia and CMV infection nearly a year after completion of therapy. Based on these results, a pivotal study of zanolimumab has been initiated in CTCL.
CD5 (Leu-1) is a 67 kDa cysteine-rich scavenger receptor family glycoprotein expressed on T-cells and the B1a subset of B-cells (Ledbetter et al., Citation1980; Lozano et al., Citation2000). CD5 acts as a co-receptor and appears to regulate the signaling strength of the TCR. It may also play a similar role in modulating B-cell receptor signaling (Raman, Citation2002). Current evidence indicates that CD5 is a key regulator of immune tolerance. Two small clinical trials have examined the use of anti-CD5 antibodies in patients with T-cell lymphoma. In one trial, 7 patients with refractory Leu-1+ (CD5+) T-cell lymphoma, six with CTCL and one with PTCL were treated with murine anti-Leu-1 monoclonal antibody at doses of 0.25 to 100 mg administered 2-3 times per week (Miller et al., Citation1983).
A rapid decrease in circulating T-cells was observed. The decline in T-cells was short-lived with a return to baseline levels occurring within 24-48 hours. The target antigen demonstrated down-modulation suggesting that CD5 might be a less suitable target for an antibody strategy. These workers reported five short-lived responses and the majority of patients treated developed neutralizing antibody limiting the usefulness of the murine antibody. In another trial, T101, a murine IgG2a anti-CD5 monoclonal antibody, was administered to eight patients with CD5+ T-cell malignancies, four of which had CTCL (Dillman et al., Citation1984). Short-lived clinical improvements were noted in two CTCL patients. Again, the induction of neutralizing antibodies was limiting. More recent trials of CD5-targeted therapy have focused on treatment of B-cell-induced autoimmune diseases and purging of T-cells from bone marrow to prevent GvHD and have used immunotoxin conjugates of anti-CD5 (Ravel et al., Citation1992; Strand et al., Citation1993; Przepiorka et al., Citation1994; Olsen, et al., Citation1996).
CD25 (IL-2Rα)
Interleukin-2 (IL-2) is a potent immunomodulatory cytokine whose function is the activation of T- and B-lymphocytes, NK cells, and macrophages (Ma et al., Citation2006). IL-2 mediates its biological effects though its receptor (IL-2R) (Nelson and Willerford, Citation1998). Three different IL-2 receptors are recognized. These include a (1) high affinity (Kd≈ 10− 11 M) heterotrimer composed of a 55 kDa α -subunit (IL-2Rα, CD25), a 75 kDa β -subunit (CD122) shared with the IL-15 receptor, and a 64 kDa common γ -chain (CD132) shared with the IL-4, IL-7, IL-9, IL-15 and IL-21 receptors; (2) an intermediate affinity (Kd≈ 10− 9 M) heterodimer receptor composed of the 75 kDa β -subunit (CD122) and the 64 kDa common γ -chain (CD132); and, (3) a non-signaling low affinity receptor (Kd≈ 10− 8 M) composed solely of the 55 kDa IL-2Rα (CD25). The α -subunit confers the specific binding of IL-2 and up-regulates receptor sensitivity, the β -subunit also plays a role in binding of IL-2, and the β - and the common γ -subunits mediate signal transduction through the JAK1 and JAK3 pathways.
Less than 5% of unstimulated peripheral blood T-cells express the IL-2Rα; however, it is highly expressed on activated T-cells and on many B- and T-cell neoplasms (Robb et al., Citation1982). IL-2Rα over-expression is seen in ATL, ALCL, CTCL, hairy cell leukemia, and on the Reed-Sternberg cells of HL (Waldmann, Citation1986). IL-2Rα is enzymatically cleaved at the cell surface and can be measured in the serum as a 45 kDa soluble fragment (Junghans et al., Citation1996). The soluble-IL-2Rα level is proportional to IL-2Rα expression and can be used as a measure of tumor burden and response to therapy.
In 1981, Uchiyama and coworkers isolated the first monoclonal antibody, murine anti-Tac, that defined the human IL-2Rα (CD25) (Uchiyama et al., Citation1981a). Since then a number of antibodies have been developed that are directed against the IL-2Rα and have been used for diagnostic purposes as well as studied as potential therapy. Anti-Tac binds to IL-2Rα and inhibits IL-2 binding (Uchiyama et al., Citation1981b). The murine 7G7/B6 antibody targets a separate epitope on IL-2Rα and does not block IL-2 binding (Rubin et al., Citation1985). Anti-Tac inhibits IL-2-stimulated proliferation of T-cells.
Murine monoclonal antibodies are clinically limited due to their immunogenicity and the rapid induction of neutralizing human anti-mouse antibodies (HAMA). In addition, they lack effector function due to reduced binding to human Fc receptors. Major efforts have gone into engineering non-human antibodies to reduce their immunogenicity and to improve their effector function. In daclizumab (Zenapax; Hoffmann-La Roche, Inc.), ≈ 90% of the murine IgG2a has been replaced with a human IgG1κ sequence (Queen et al., Citation1989). It has the advantages of a low frequency neutralizing antibodies, a significantly prolonged serum half-life compared to murine anti-Tac, and the ability to mediate antibody dependent cellular cytotoxicity (ADCC) through its humanized Fc-domain (Zhang et al., Citation2004). Daclizumab inhibits IL-2-induced activation of T-cells and it is approved for the prophylaxis of renal allograft rejection in combination with other immunosuppressive drugs (Nashan et al., Citation1999).
Since IL-2Rα is over-expressed in many T-cell lymphoproliferative disorders, it warranted investigation as a potential target for receptor-directed anti-tumor therapy. The Metabolism Branch at the NCI has a lengthy experience with anti-CD25-directed therapy for the treatment of T-cell leukemias and lymphomas using murine anti-Tac, and more recently the humanized molecule, daclizumab, as well as these molecules chelated with radioisotopes. ATL has served as a clinical model due to the high level of IL-2Rα expression in this disease. ATL cells express 10,000–40,000 target molecules per cell, whereas resting T-cells express fewer than 500.
ATL is an aggressive lymphoproliferative disorder resulting from infection with the retrovirus, human T-cell lymphotrophic virus type-1 (HTLV-1) (Poiesz et al., Citation1980). After infection, the viral tax protein acts as a promiscuous transcriptional activator increasing the expression of numerous cellular genes, notably IL-2, IL-2Rα, IL-15, IL-15Rα, OX-40 and NFκ B that result in autostimulatory growth loops and abnormal T-cell proliferation (Johnson et al Citation2001). Over time, 2-4% of infected individuals will develop an aggressive T-cell leukemia or lymphoma, ATL (Poiesz et al., Citation2001). On flow cytometry, typical ATL cells are characterized as CD3dimCD4+CD25bright. Clinically these patients exhibit high leukemic cell counts, rapidly growing lymphadenopathy, hypercalcemia, lytic bone lesions, and skin and solid organ infiltration by the malignant T-cells. Median survivals for the aggressive forms of ATL are 4–10 months and current treatments have had little impact on survival (Matutes, Citation2007).
Murine anti-Tac and daclizumab inhibit the ex vivo proliferation of primary ATL cells as measured by [3H]-thymidine uptake (Tendler et al., Citation1990). Preclinical studies where human primary MET-1 ATL cells were injected into non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice showed that the untreated mice progress to death from leukemia in 4–6 weeks (Phillips et al., Citation2000). Treatment with murine anti-Tac, daclizumab, or the 7G7/B6 antibody all increased the survival of the tumor-bearing mice to varying degrees, indicating that CD25-targeted therapy of ATL is active.
A series of patient trials studying the activities antibodies directed against IL-2Rα as a therapeutic approach to HTLV-1-associated ATL have been reported. In an early study, 19 patients with ATL were treated with murine anti-Tac (Waldmann et al., Citation1993). Ten of the patients had been unresponsive to prior chemotherapy. Two patients achieved complete remissions and four patients had partial responses. The duration of response ranged from 9 weeks to more than 12 years in one patient. Toxicities included fever and pancytopenia. The short serum half-life of murine anti-Tac and the development of HAMA limited the usefulness of this treatment. To enhance the killing ability of the antibody, the murine anti-Tac was chelated to the β -emitting radioisotope yttrium-90 (Y90; Waldmann et al., 1995). Eighteen ATL patients were treated with up to 15 mCi of radiolabeled antibody. Two complete and seven partial responses were observed. Toxicity was primarily granulocytopenia and thrombocytopenia; however, a significant fraction of patients also developed HAMA limiting the ability to administer repeated treatments.
To overcome the immunogenicity of the murine antibody, recombinant technology was used to “humanize” the anti-Tac molecule in which the murine antigen-binding regions were joined to a human immunoglobulin framework resulting in daclizumab (Queen et al., Citation1989). With the availability of daclizumab, a series of clinical trials targeting a variety of T-cell malignancies were under taken. In one Phase I/II trial, up to 8 mg/kg of daclizumab was administered to ATL patients (Morris et al., Citation2003). Endpoints of the study were to determine the maximum tolerated dose of daclizumab, the dose of antibody required to achieve ≥ 95% saturation of IL-2Rα on circulating ATL cells and ATL cells in lymph nodes, and to determine the response rate.
Cohorts of patients were treated with daclizumab 2 mg/kg on Days 1 and 2, or 4, 6, or 8 mg/kg as a single intravenous dose on Day 1 of each cycle. Treatment was repeated every 2 or 3 weeks to complete six doses. Flow cytometry analysis of the peripheral blood 72 hours after the first dose and at Weeks 2, 5, and 14 showed that ≥ 95% saturation of IL-2Rα on circulating ATL cells could be achieved and maintained. In six patients that underwent lymph node fine needle aspiration, receptor saturation was documented in only half and it was not maintained suggesting that the impeded access of large antibody molecules into tumor is a potential blockade to receptor-directed therapy.
Radioimmunotherapy targeting IL-2Rα has shown promise in patients with relapsed HL. While it has been reported that 50-70% of Reed-Sternberg cells (Hsu et al., 1990; Tesch et al., Citation1993) express CD25, using paraffin-fixed tissue for immunostaining we demonstrated less than 10% of cases with significant staining of the malignant cells. However, virtually all Hodgkin's lesions demonstrate infiltration with “non-malignant” T-cells that express CD25 that surround the HL Reed-Sternberg cells. Treatment with Y-90 radiolabeled daclizumab is able to induce complete responses in more than half of these patients (O'Mahony et al., 2006a). Local irradiation from the labeled antibodies bound to the infiltrating CD25+ T-cells caused the regression of HL even if the tumor cells did not express the target receptor by a bystander killing effect.
Other approaches to CD25 receptor-targeted therapy use immunotoxins and ligand-toxin fusion molecules. Immunotoxins are monoclonal antibodies or antibody fragments linked to a toxin that target a specific cell-surface receptor. After binding to the receptor, the immunotoxin-receptor complex is internalized (modulated) and the toxin is released inside the cell to kill the cell. Because the antibody targets a specific receptor, immunotoxins exhibit highly specific cytotoxicity. Characteristics of an ideal toxin for this approach include a molecule with distinct receptor-binding and toxin domains that can be separated. When cleaved into individual subunits, the toxin should not bind to cells expressing its natural receptor and hence the isolated toxin fragment exhibits little toxicity. However, when introduced into the cell by other means, the toxin fragment should remain highly potent.
A variety of toxins meet these requirements. Most are plant or bacterial products that disrupt ribosomal function and, hence protein synthesis. The toxin ricin, Diphtheria toxin-A (DT), and Pseudomonas exotoxin A (PE) are the most extensively studied. A PE-single chain anti-CD25 (sc)Fv-fragment fusion protein (LMB-2) showed promising results in IL-2Rα -expressing lymphoid malignancies including responses in patients with ATL and CTCL (Kreitman et al., Citation2000).
Denileukin diftitox (DAB389IL-2, Ontak) is a recombinant fusion protein composed of the toxin and translocation domains of DT and a human IL-2 fragment (Foss et al., Citation1998). It is able to direct its cytotoxic action to cells expressing a functional IL-2 receptor and when internalized, active DT is released to kill tumor cells. In a pivotal trial in 71 patients with advanced refractory CTCL, denileukin diftitox produced a 30% response rate with 10% complete responses (Olsen et al., Citation2001). Common toxicities included fever and chills, hypotension, nausea and vomiting, edema, and liver enzyme abnormalities. CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy has been studied in combination with denileukin difitox in 41 patients with PTCL (Foss et al., Citation2007). An overall response rate of more than 90% was reported. Denileukin diftitox is being evaluated in a clinical trial as a potential treatment in ATL due to over expression of the IL-2R in this disease.
CD30
CD30 (Ki-1) is a 105-120 kDa transmembrane glycoprotein, a member of the tumor necrosis factor (TNF) receptor superfamily (Falini et al., Citation1995). Although its function has not been clearly defined, it appears to be involved in the negative selection of autoreactive lymphocytes. CD30 is expressed on activated T- and B-cells, the Reed-Sternberg cells of HL, ALCL, some CTCL, and mediastinal B-cell lymphomas (Vergier et al., Citation2000). Over-expression of CD30 by HL is thought to result in ligand-independent nuclear factor-κ B (NF-κ B) signaling that enhances tumor cell survival (Schneider and Hübinger, Citation2002).
A number of antibodies targeting CD30 have been developed, including Ber-H2, HeFi-1, MDX-060 and SGN30 (Hecht et al., Citation1985; Norton and Isaacson, Citation1987; Bowen et al., Citation1993; Wahl et al., Citation2002; Borchmann et al., Citation2003, Hu and Ping, Citation2005). Many anti-CD30 antibodies induce apoptosis of ALCL and HL cell lines in vitro and a number of these are currently being evaluated in clinical trials. In a Phase I/II trial, the anti-CD30 antibody, MDX-060 (Medarex, Inc.) was administered to 21 patients: 16 withHL, three with ALCL, and two with other CD30+ T-cell lymphomas (Ansell et al., Citation2007). The antibody was well tolerated and a maximum dose was not established. In Phase II, an additional 51 patients were treated, 47 withHL and four with ALCL. Six responses were reported, primarily confined to patients with ALCL.
In another Phase I trial, SGN-30 (Seattle Genetics, Inc.), an antibody that binds to a different epitope of CD30, was administered to 24 CD30+ lymphoma patients, 21 of which had HL (Bartlett et al., Citation2007). One response was seen in a patient with ALCL and six patients had stable disease. Infusional reactions accounted from most of the adverse events observed in this study.
Several CD30-directed immunotoxin molecules have been evaluated in preclinical studies. Most have used the murine Ber-H2 antibody conjugated to a variety of toxins, most commonly ricin. Ki-4(scFv)-ETA', an anti-CD30 scFv fragment-Pseudomonas exotoxin fusion molecule demonstrated inhibition of protein synthesis in CD30+ HL cell lines and a single intravenous dose cured more than 90% of CD30+ tumor-bearing mice in one study (Barth et al., Citation2000). Using the Ber-H2 antibody linked to the plant toxin saporin, an early clinical trial treated 4 patients with advanced refractory HL (Falini et al., Citation1992). Binding of the immunotoxin to tumor was demonstrated and three patients experienced short-lived responses.
In another Phase I trial, 17 relapsed HL and CD30+ non-Hodgkin's lymphoma patients received escalating doses of an anti-CD30 antibody linked to deglycoslyated ricin (Schnell et al., Citation2002). A single partial response was observed. Vascular leak syndrome was dose-limiting and anti-ricin antibodies were developed in 40% of the patients. SGN-35 (Seattle Genetics, Inc.), a CD30-targeted immunotoxin composed of the chimeric murine anti-CD30 monoclonal antibody (cAC10) linked to the cytotoxic agent monomethyl auristatin entered a Phase I trial in patients with relapsed HL and other CD30+ lymphomas (Hamblett et al., Citation2005). The results of this study are pending at this time.
CD52
CD52 is a 21-28 kDa glycoprotein with a 12 amino acid core peptide, an N-glycosylation site, and a glycophosphoinositol (GPI) anchor attached to the outer cell membrane (Treumann et al., Citation1995). It is expressed on T- and B-lymphocytes, NK cells, monocytes, macrophages, eosinophils, and the epithelial cells of the male genital tract. It is expressed at high density; it is non-modulating and is expressed on many types of malignant T-cells making it a target for antibody therapy.
The development of therapeutic anti-CD52 antibodies is reviewed elsewhere (Dyer, Citation1999). Alemtuzumab (Campath-1H, Campath; Bayer Healthcare Pharmaceuticals, Inc.) is a humanized rat IgG2b monoclonal antibody that defines the CD52 antigen. It was initially approved for the treatment of alkylating agent and fludarabine-resistant B-cell CLL; however, its indication was recently extended to first-line use (Ravandi and O'Brien, Citation2006). Alemtuzumab shows activity against a large number of T-cell neoplasms. A 76% response rate was reported in 39 patients with T-cell prolymphocytic leukemia (T-PLL), most of who had progressed on prior treatment.
Sixty percent of the patients achieved a complete remission with a median response duration of 7 months (range, 4–45 months) (Dearden et al., Citation2001). Another Phase II study evaluated the safety and efficacy of alemtuzumab in 22 patients with advanced mycosis fungoides/Sezary syndrome, and found an overall response rate of 55% with 32% complete remissions with acceptable toxicity (Lundin et al., Citation2003).
Alemtuzumab has been combined with chemotherapy in an attempt to improve on the outcome for patients with PTCL. In a small Phase II trial, 20 newly-diagnosed PTCL patients were treated with a combination of CHOP chemotherapy and intravenous alemtuzumab 10 mg/m2 on Day 1 and 20 mg/m2 on Day 2 of cycle 1, followed by 30 mg/m2 on Day 1 of each subsequent chemotherapy cycle (Kim et al., Citation2007). An overall 80% response rate with 65% complete responses and an estimated 1-year event-free survival of 43.3% was reported. Grade 4 neutropenia occurred in 90% of patients and febrile neutropenia was seen in 55% of patients receiving this treatment. Of note, 25% of patients had CMV reactivation and 15% experienced clinical pneumonitis or retinitis. Similarly, Gallamini and colleagues reported a 71% complete response rate with CHOP chemotherapy combined with alemtuzumab in 24 patients with PTCL (Gallamini et al., Citation2007). Granulocytopenia and CMV reactivation were the most commonly observed side effects. A Phase III randomized trial is currently underway in Europe comparing CHOP chemotherapy to CHOP chemotherapy plus alemtuzumab in patients with T-cell lymphoma. EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) infusional chemotherapy combined with 30, 60 or 90 mg of alemtuzumab was studied in patients with a variety of aggressive T-cell lymphomas (Janik et al., Citation2005). It this trial, doses of alemtuzumab above 30 mg resulted in dose-limiting bone marrow aplasia in three of six patients. Infectious complications were common and the majority of patients required hospitalization at some time during treatment.
Preclinical studies showed alemtuzumab to be active in the MET-1 mouse model of human ATL (Zhang et al., 2003). As a result, a Phase II clinical trial of alemtuzumab in ATL was initiated (Sharma et al., Citation2006). While the trial is still ongoing, more than 20 patients have been treated and some preliminary observations can be made: (1) the CD52 target is highly expressed on ATL cells. Only one of more than 30 patients whose peripheral blood, lymph nodes or tumor that was examined lacked CD52 expression on their ATL cells; (2) responses were seen in almost half of the patients with the acute (leukemic) subtype of ATL, but only rarely in patients suffering from the lymphomatous form of the disease. These results are consistent with that reported in patients with CLL treated with alemtuzumab where responses were significantly lower in patients with lymphadenopathy (Keating et al., Citation2002); (3) the median duration of response was 5 months; and, (4) CMV reactivation was a near universal event in these patients indicating a need for antiviral prophylaxis and CMV monitoring while they are receiving alemtuzumab.
CD122
CD122 (IL-2R/IL-15Rβ) represents the β -subunit shared by the heteromeric IL-2 and IL-15 receptors (Anderson et al., Citation1995). It is a 75 kDa glycoprotein with a 286 amino acid intracellular domain (Zamai et al., Citation2001). CD122 functions during receptor signaling by recruiting the Janus kinase, JAK1 that activates the signal transducer and activator of transcription-3 (STAT3). The intermediate and high affinity IL-2 receptors and heteromeric IL-15 receptors also share the common γ -chain (CD132). CD132 activates JAK3 that in turn phosphorylates STAT5. Phosphorlyated-STAT transcription factors translocate to the nucleus where they activate specific gene expression.
Interleukin-15 induces maturation of T-cells and NK cells, enhances the survival of CD8+ memory T-cells, and stimulates the expression of TNFα, IL-1β, and other pro-inflammatory cytokines (Shanmugham et al., Citation2006). Unlike IL-2, IL-15 is not expressed by lymphocytes, but rather is produced by macrophages, dendritic cells, and other non-lymphoid cells. IL-2 promotes tolerance through the activation-induced cell death (AICD) of T-lymphocytes (Waldmann, Citation2006). In contrast to IL-2, IL-15 inhibits self-tolerance. Receptor specificity for IL-2 or IL-15 is conferred by each cytokine's receptor's private α -chain. Abnormalities of IL-15 and its receptor have been reported to play a role in the progression of ALL, ATL, CTCL and T-cell large granular lymphocyte (T-LGL) leukemia, as well as a number of autoimmune and inflammatory conditions (Bamford et al., Citation1996; Döbbeling et al., Citation1998; McInnes and Gracie, Citation2004, Cario et al., Citation2007).
T-LGL leukemia is a rare lymphoproliferative disorder characterized by elevated numbers of CD3+CD8+CD57+ large granular lymphocytes (LGL) in the peripheral blood, spleen and bone marrow (O'Malley, Citation2007). T-LGL leukemia is often associated with granulocytopenia, or red cell aplasia and less so thrombocytopenia. Rheumatoid arthritis and autoimmune hemolytic anemia may also occur. IL-2 and IL-15 have been shown to induce lymphokine activated killer (LAK) cell activity in LGL. Resting NK cells and LGL lack the IL-2Rα subunit (CD25); however, its expression can be induced by the addition IL-2 to cells that express the IL-2R/IL-15β (CD122) and common γ -subunits (CD132) of the IL-2R demonstrating that the intermediate affinity receptor complex can signal for the induction of NK activity in LGL (Alileche et al., Citation2001). In addition, LGL can also be stimulated by IL-15 through the same receptor complex (Giri et al., 2005). It is hypothesized that the clinical hemocytopenias seen in T-LGL leukemia are a result of the enhanced NK activity of the leukemic cells. The Mik-β 1 antibody (anti-CD122), directed against the IL-2R/IL-15Rβ, can inhibit the trans-presentation of IL-15 to NK cells and T-cells, and subsequent IL-15-mediated effects (Tsudo et al., Citation1989; Kanegane and Tostato, Citation1996; Guex-Crosier et al., Citation1997; Kobayashi, Citation2005).
In a Phase I trial, 12 patients with T-LGL leukemia and hemocytopenias received the murine Mik-β 1 antibody at doses of 0.5, 1.0 or 1.5 mg/kg on Days 1, 4, 7, and 10 (Morris et al., Citation2006). Surprisingly, no patient developed a neutralizing antibody response to the murine antibody. Greater than 95% saturation of CD122 was achieved in all patients, and down-modulation of CD122 was observed in seven patients. Clinical side effects were minor; however, no responses in terms of decreases in T-LGL cell counts or improvement in hemocytopenias were seen. The lack of response may be the result of the short half-life of the murine antibody in man. In addition, down-modulation of CD122 after binding of the antibody reduced the amount of the receptor on the surface of the LGL cells and may have impacted the efficacy of the Mik-β 1 antibody. A Phase I safety and pharmacokinetic study of the humanized form of the antibody, Hu-Mik-β 1, in T-LGL leukemia is currently in progress. Hu-Mik-β 1 is expected to have a more efficacious pharmacokinetic profile (Hakimi et al., Citation1993; Tinubu et al., Citation1994).
CONCLUSIONS
T-Cell leukemias and lymphomas represent a heterogeneous group of lymphoid neoplasms. Tumor responses and the remission duration achieved by these patients with currently available chemotherapy regimens are suboptimal. These patients have an overall poorer outcome when compared to their B-cell lymphoma counterparts. Malignant T-cells express a number of potential targets for receptor-directed therapy. Monoclonal antibodies such as alemtuzumab (anti-CD52) have shown meaningful clinical activity in a variety of T-cell malignancies; however, no monoclonal antibody with therapeutic activity comparable to that of rituximab in B-cell lymphoma has been introduced for the treatment of T-cell lymphoma. A number of investigational antibodies and immunotoxins directed against targets such as CD2, CD4, CD25, CD30, and CD122 hold promise for the future. Receptor-directed therapy of T-cell malignancies will no doubt continue to be an area of active interest and expanding clinical trials.
REFERENCES
- Abbondanzo S. L., Wenig B. M. Non-Hodgkin's lymphoma of the sinonasal tract. A clinicopathologic and immunophenotypic study of 120 cases. Cancer 1995; 75: 1281–1291
- Alileche A., Goldman C. K., Waldmann T. A. Differential effects of IL-2 and IL-15 on expression of IL-2 receptor-α. Biochem. Biophys. Res. Commun. 2001; 285: 1302–1308
- Anderson D. M., Kumaki S., Ahdieh M., Bertles J., Tometsko M., Loomis A., Giri J., Copeland N. G., Gilbert D. J., Jenkins N. A., Valentine V., Shapiro D. N., Morris S. W., Park L. S., Cosman D. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15Rα and IL2Rα genes. J. Biol. Chem. 1995; 270: 29862–29869
- Ansell S. M., Horwitz S. M., Engert A., Khan K. D., Lin T., Strair R., Keler T., Graziano R., Blanset D., Yellin M., Fischkoff S., Assad A., Borchmann P. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J. Clin. Oncol. 2007; 25: 2764–2769
- Armitage J. O., Weisenburger D. D. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J. Clin. Oncol. 1998; 16: 2780–2795
- Bamford R. N., Battiata A. P., Burton J. D., Sharma H., Waldmann T. A. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA 1996; 93: 2897–2902
- Barberio E., Thomas L., Skowron F., Balme B., Dalle S. Transformed mycosis fungoides: Clinicopathological features and outcome. Br. J. Dermatol. 2007; 157: 284–289
- Barth S., Huhn M., Matthey B., Tawadros S., Schnell R., Schinköthe T., Diehl V., Engert A. Ki-4(scFv)-ETA', a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood 2000; 95: 3909–3914
- Bartlett N. L., Younes A., Carabasi M. H., Forero A., Rosenblatt J. D., Leonard J. P., Bernstein S. H., Bociek R. G., Lorenz J. M., Hart B. W., Barton J. A Phase 1 multi-dose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 2007, [Epub ahead of print]
- Borchmann P., Treml J. F., Hansen H., Gottstein C., Schnell R., Staak O., Zhang H. F., Davis T., Keler T., Diehl V., Graziano R. F., Engert A. The human anti-CD30 antibody 5F11 shows in vitro in vivo activity against malignant lymphoma. Blood 2003; 102: 3737–3742
- Borrione P., Montacchini L., Beggiato E., Pileri A., Bianchi A., Massaia M. Clinical and immunological studies in advanced cancer patients sequentially treated with anti-CD3 monoclonal antibody (OKT3) and interleukin-2. Leuk. Lymphoma 1996; 21: 325–330
- Bowen M. A., Olsen K. J., Cheng L., Avila D., Podack E. R. Functional effects of CD30 on a large granular lymphoma cell line, YT. Inhibition of cytotoxicity, regulation of CD28 and IL-2R, and induction of homotypic aggregation. J. Immunol. 1993; 151: 5896–5906
- Branco L., Barren P., Mao S. Y., Pfarr D., Kaplan R., Postema C., Langermann S., Koenig S., Johnson S. Selective deletion of antigen-specific, activated T-cells by a humanized MAB to CD2 (MEDI-507) is mediated by NK cells. Transplantation 1999; 68: 1588–1596
- Cario G., Izraeli S., Teichert A., Rhein P., Skokowa J., Möricke A., Zimmermann M., Schrauder A., Karawajew L., Ludwig W. D., Welte K., Schünemann H. J., Schlegelberger B., Schrappe M., Stanulla M. High interleukin-15 expression characterizes childhood acute lymphoblastic leukemia with involvement of the CNS. J. Clin. Oncol. 2007; 25: 4813–4820
- Chamian F., Lowes M. A., Lin S. L., Lee E., Kikuchi T., Gilleaudeau P., Sullivan-Whalen M., Cardinale I., Khatcherian A., Novitskaya I., Wittkowski K. M., Krueger J. G. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc. Natl. Acad. Sci. USA 2005; 102: 2075–2080
- Chatenoud L. CD3-specific antibody-induced active tolerance: From bench to bedside. Nat. Rev. Immunol. 2003; 3: 123–132
- Chittal S. M., Caverivière P., Schwarting R., Gerdes J., Al Saati T., Rigal-Huguet F., Stein H., Delsol G. Monoclonal antibodies in the diagnosis of Hodgkin's disease. The search for a rational panel. Am. J. Surg. Pathol. 1988; 12: 9–21
- Coiffier B., Lepage E., Briere J., Herbrecht R., Tilly H., Bouabdallah R., Morel P., Van Den Neste E., Salles G., Gaulard P., Reyes F., Lederlin P., Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. New Engl. J. Med. 2002; 346: 235–242
- Criscione V. D., Weinstock M. A. Incidence of cutanenous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007; 143: 854–859
- Dearden C. E., Matutes E., Cazin B., Tjønnfjord G. E., Parreira A., Nomdedeu B., Leoni P., Clark F. J., Radia D., Rassam S. M., Roques T., Ketterer N., Brito-Babapulle V., Dyer M. J., Catovsky D. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood 2001; 98: 1721–1726
- Dillman R. O., Shawler D. L., Dillman J. B., Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J. Clin. Oncol. 1984; 2: 881–891
- Döbbeling U., Dummer R., Laine E., Potoczna N., Qin J. Z., Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood 1998; 92: 252–258
- Dyer M. J. The role of CAMPATH-1 antibodies in the treatment of lymphoid malignancies. Semin. Oncol. 1999; 26(5S14)52–57
- Falini B., Bolognesi A., Flenghi L., Tazzari P. L., Broe M. K., Stein H., Dürkop H., Aversa F., Corneli P., Pizzolo G., Barbabietola G., Sabattini E., Pileri S., Martelli M. F., Stirpe F. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet 1992; 339: 1195–1196
- Falini B., Pileri S., Pizzolo G., Dürkop H., Flenghi L., Stirpe F., Martelli M. F., Stein H. CD30 (Ki-1) molecule: A new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995; 85: 1–14
- Feldman A. L., Pittaluga S., Jaffe E. S. Classification and histopathology of the lymphomas. The Lymphomas, 2nd Edition, G. P. Canellos, T. A. Lister, B. Young. Saunders-Elsevier, Philadelphia 2006; 2–38
- Foss F. M., Saleh M. N., Krueger J. G., Nichols J. C., Murphy J. R. Diphtheria toxin fusion proteins. Curr. Top. Microbiol. Immunol. 1998; 234: 63–81
- Foss F., Sjak-Shie N., Goy A., Jacobsen E., Advani R., Smith M., Komrokji R., Pendergrass K., Bolejack V., Watts K., Acosta M. Denileukin diftitox (ONTAK) plus CHOP chemotherapy in patients with peripheral T-cell lymphomas (PTCL), the CONCEPT trial. Blood 2007; 110: 3449, (ASH Annual Meeting Abstracts)
- Gallamini A., Zaja F., Patti C., Billio A., Specchia M. R., Tucci A., Levis A., Manna A., Secondo V., Rigacci L., Pinto A., Iannitto E., Zoli V., Torchio P., Pileri S., Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007; 110: 2316–2323
- Garcia K. C., Adams E. J. How the T-cell receptor sees antigen—A structural view. Cell 2005; 122: 333–336
- Giri J. G., Anderson D. M., Kumaki S., Park L. S., Grabstein K. H., Cosman D. IL-15, a novel T-cell growth factor that shares activities and receptor components with IL-2. J. Leukocyte Biol. 1995; 57: 763–766
- Gisselbrecht C., Gaulard P., Lepage E., Coiffier B., Brière J., Haioun C., Cazals-Hatem D., Bosly A., Xerri L., Tilly H., Berger F., Bouhabdallah R., Diebold J. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Blood 1998; 92: 76–82
- Gramatzki M., Burger R., Strobel G., Trautmann U., Bartram C. R., Helm G., Horneff G., Alsalameh S., Jonker M., Gebhart E., Kalden J. R. Therapy with OKT3 monoclonal antibody in refractory T-cell acute lymphoblastic leukemia induces interleukin-2 responsiveness. Leukemia 1995; 9: 382–390
- Groves F. D., Linet M. S., Travis L. B., Devesa S. S. Cancer surveillance series: Non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J. Natl. Cancer Inst. 2000; 92: 1240–1251
- Guex-Crosier Y., Raber J., Chan C. C., Kriete M. S., Benichou J., Pilson R. S., Kerwin J. A., Waldmann T. A., Hakimi J., Roberge F. G. Humanized antibodies against the alpha-chain of the IL-2 receptor and against the β -chain shared by the IL-2 and IL-15 receptors in a monkey uveitis model of autoimmune diseases. J. Immunol. 1997; 158: 452–458
- Hakimi J., Ha V. C., Lin P., Campbell E., Gately M. K., Tsudo M., Payne P. W., Waldmann T. A., Grant A. J., Tsien W. H., Schneider W. P. Humanized Mik β 1, a humanized antibody to the IL-2 receptor β -chain that acts synergistically with humanized anti-TAC. J. Immunol. 1993; 151: 1075–1085
- Hale G., Bright S., Chumbley G., Hoang T., Metcalf D., Munro A. J., Waldmann H. Removal of T-cells from bone marrow for transplantation: A monoclonal anti-lymphocyte antibody that fixes human complement. Blood 1983; 62: 873–882
- Hamblett K. J., Barton J., Cerveny C. G., Andreyka J. B., Kissler K. M., Okeley N. M., Stone I., Sutherland M. K., Sun M. M., Senter P. D., Wahl A. F., Ihle N. C. SGN-35, an anti-CD30 antibody-drug conjugate, exhibits potent anti-tumor activity for the treatment of CD30+ malignancies. Blood 2005; 106: 610, (ASH Annual Meeting Abstracts)
- Hecht T. T., Longo D. L., Cossman J., Bolen J. B., Hsu S. M., Israel M., Fisher R. I. Production and characterization of a monoclonal antibody that binds Reed-Sternberg cells. J. Immunol. 1985; 134: 4231–4236
- Holmes G. K. Coeliac disease and malignancy. Dig. Liver Dis. 2002; 34: 229–237
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am. J. Pathol. 1985; 118: 209–217
- Hu X. F., Xing P. X. MDX-060. Medarex. Curr. Opin. Invest. Drugs 2005; 6: 1266–1271
- Hurst R. E. Bexarotene ligand pharmaceuticals. Curr. Opin. Invest. Drugs 2000; 1: 514–523
- Jaffe E. S., Harris N. L., Stein H., Vardiman J. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press, LyonFrance 2001
- Janik J. E., Dunleavy K., Pittaluga S., Jaffe E. S., Grant N., Shovlin M., Stetler-Stevenson M., Wilson W. H. A pilot trial of Campath-1H and dose-Adjusted EPOCH in CD52-expressing aggressive T-cell malignancies. Blood 2005; 106: 3348, (ASH Annual Meeting Abstracts)
- Jermal A., Siegel R., Ward E., Murray T., Xu J., Thun M. J. Cancer statistics, 2007. CA Cancer J. Clin. 2007; 57: 43–66
- Johnson J. M., Harrod R., Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus Type-1 (HTLV-1). Int. J. Exp. Pathol. 2001; 82: 135–147
- Junghans R. P., Stone A. L., Lewis M. S. Biophysical characterization of a recombinant soluble interleukin-2 receptor (Tac). J. Biol. Chem. 1996; 271: 10453–10460
- Kanegane H., Tosato G. Activation of naive and memory T-cells by interleukin-15. Blood 1996; 88: 230–235
- Keating M. J., Flinn I., Jain V., Binet J. L., Hillmen P., Byrd J., Albitar M., Brettman L., Santabarbara P., Wacker B., Rai K. R. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood 2002; 99: 3554–3561
- Kim J. G., Sohn S. K., Chae Y. S., Cho Y. Y., Yang D. H., Lee J. J., Kim H. J., Shin H. J., Chung J. S., Cho G. J., Lee W. S., Joo Y. D., Sohn C. H., Oh S. J. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: A Phase II study. Cancer Chemother. Pharmacol. 2007; 60: 129–134
- Kim Y. H., Duvic M., Obitz E., Gniadecki R., Iversen L., Osterborg A., Whittaker S., Illidge T. M., Schwarz T., Kaufmann R., Cooper K., Knudsen K. M., Lisby S., Baadsgaard O., Knox S. J. Clinical efficacy of zanolimumab (HuMax-CD4): Two Phase 2 studies in refractory cutaneous T-cell lymphoma. Blood 2007; 109: 4655–4662
- Kirk A. D. Induction immunosuppression. Transplantation 2006; 82: 593–602
- Knop S., Hebart H., Gratwohl A., Kliem C., Faul C., Holler E., Apperley J., Kolb H. J., Schaefer A., Niederwieser D., Einsele H. Treatment of steroid-resistant acute GVHD with OKT3 and high-dose steroids results in better disease control and lower incidence of infectious complications when compared to high-dose steroids alone: A randomized multi-center trial by the EBMT Chronic Leukemia Working Party. Leukemia 2007; 21: 1830–1833
- Knox S., Hoppe R. T., Maloney D., Gibbs I., Fowler S., Marquez C., Cornbleet P. J., Levy R. Treatment of cutaneous T-cell lymphoma with chimeric anti-CD4 monoclonal antibody. Blood 1996; 87: 893–899
- Knox S. J., Levy R., Hodgkinson S., Bell R., Brown S., Wood G. S., Hoppe R., Abel E. A., Steinman L., Berger R. G., Gaiser C., Young G., Bindl J., Hanham A., Reichert T. Observations on the effect of chimeric anti-CD4 monoclonal antibody in patients with mycosis fungoides. Blood 1991; 77: 20–30
- Kobayashi H., Dubois S., Sato N., Sabzevari H., Sakai Y., Waldmann T. A., Tagaya Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 2005; 105: 721–727
- Koenecke C., Shaffer J., Alexander S. I., Preffer F., Dombkowski D., Saidman S. L., Dey B., McAfee S., Spitzer T. R., Sykes M. NK cell recovery, chimerism, function, and recognition in recipients of haploidentical hematopoietic cell transplantation following nonmyeloablative conditioning using a humanized anti-CD2 mAb, Medi-507. Exp. Hematol. 2003; 31: 911–923
- Kreitman R. J., Wilson W. H., White J. D., Stetler-Stevenson M., Jaffe E. S., Giardina S., Waldmann T. A., Pastan I. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J. Clin. Oncol. 2000; 18: 1622–1636
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T-cell surface antigens. Science 1979; 206: 347–349
- Leahy D. J. A structural view of CD4 and CD8. FASEB J. 1995; 9: 17–25
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T-Cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J. Exp. Med. 1980; 152: 280–295
- Lennert K., Kikuchi M., Sato E., Suchi T., Stansfeld A. G., Feller A. C., Hansmann M. L., Müller-Hermelink H. K., Gödde-Salz E. HTLV-positive and -negative T-cell lymphomas. Morphological and immunohistochemical differences between European and HTLV-positive Japanese T-cell lymphomas. Int. J. Cancer 1985; 35: 65–72
- Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005; 23: 1117–1125
- López-Guillermo A., Cid J., Salar A., López A., Montalbán C., Castrillo J. M., González M., Ribera J. M., Brunet S., García-Conde J., Fernández de Sevilla A., Bosch F., Montserrat E. Peripheral T-cell lymphomas: Initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann. Oncol. 1998; 9: 849–855
- Lowenstein H., Shah A., Chant A., Khan A. Different mechanisms of Campath-1H-mediated depletion for CD4 and CD8 T cells in peripheral blood. Transpl. Int. 2006; 19: 927–936
- Lozano F., Simarro M., Calvo J., Vilà J. M., Padilla O., Bowen M. A., Campbell K. S. CD5 signal transduction: Positive or negative modulation of antigen receptor signaling. Crit. Rev. Immunol. 2000; 20: 347–358
- Lundin J., Hagberg H., Repp R., Cavallin-Ståhl E., Fredén S., Juliusson G., Rosenblad E., Tjønnfjord G., Wiklund T., Osterborg A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood 2003; 101: 4267–4272
- Ma A., Koka R., Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Ann. Rev. Immunol. 2006; 24: 657–679
- Majeau G. R., Meier W., Jimmo B., Kioussis D., Hochman P. S. Mechanism of lymphocyte function-associated molecule 3-Ig fusion proteins inhibition of T-cell responses. Structure/function analysis in vitro and in human CD2 transgenic mice. J. Immunol. 1994; 152: 2753–2767
- Mann B. S., Johnson J. R., Cohen M. H., Justice R., Pazdur R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007; 12: 1247–1252
- Marti R. M., Pujol R. M., Servitje O., Palou J., Romagosa V., Bordes R., González-Castro J., Miralles J., Gallardo F., Curcó N., Gómez X., Domingo A., Estrach T. Sézary syndrome and related variants of classic cutaneous T-cell lymphoma. A descriptive and prognostic clinicopathologic study of 29 cases. Leuk Lymphoma 2003; 44: 59–69
- Mascelli M. A., Zhou H., Sweet R., Getsy J., Davis H. M., Graham M., Abernethy D. Molecular, biologic, and pharmacokinetic properties of monoclonal antibodies: Impact of these parameters on early clinical development. J. Clin. Pharmacol. 2007; 47: 553–565
- Matutes E. Adult T-cell leukaemia/lymphoma. J. Clin. Pathol. 2007; 60: 1373–1377
- McInnes I. B., Gracie J. A. Interleukin-15: A new cytokine target for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 2004; 4: 392–397
- Medeiros L. J., Elenitoba-Johnson K. S. Anaplastic large cell lymphoma. Am. J. Clin. Pathol. 2007; 127: 707–722
- Miller R. A., Oseroff A. R., Stratte P. T., Levy R. Monoclonal antibody therapeutic trials in seven patients with T-cell lymphoma. Blood 1983; 62: 988–995
- Moingeon P. E., Lucich J. L., Stebbins C. C., Recny M. A., Wallner B. P., Koyasu S., Reinherz E. L. Complementary roles for CD2 and LFA-1 adhesion pathways during T-cell activation. Eur. J. Immunol. 1991; 21: 605–610
- Moingeon P., Chang H. C., Sayre P. H., Clayton L. K., Alcover A., Gardner P., Reinherz E. L. The structural biology of CD2. Immunol. Rev. 1989; 111: 111–144
- Morris J. C., Janik J. E., Turner M., Lee C., Quinn C., Pittaluga S., Stetler-Stevenson M., Fleisher T. A., Albert P., Waldmann T. A. A Phase I trial of humanized anti-Tac (daclizumab) for the treatment of human T-cell lymphotrophic virus Type-1-associated leukemia/lymphoma. Proc. Am. Soc. Clin. Oncol 2003; 22: 695
- Morris J. C., Janik J. E., White J. D., Fleisher T. A., Brown M., Tsudo M., Goldman C. K., Bryant B., Petrus M., Top L., Lee C. C., Gao W., Waldmann T. A. Preclinical and Phase I clinical trial of blockade of IL-15 using Mik-β -1 monoclonal antibody in T-cell large granular lymphocyte leukemia. Proc. Natl. Acad. Sci. USA 2006; 103: 401–406
- Nashan B., Light S., Hardie I. R., Lin A., Johnson J. R. Reduction of acute renal allograft rejection by daclizumab. Daclizumab Double Therapy Study Group. Transplantation 1999; 67: 110–115
- Nelson B. H., Willerford D. M. Biology of the interleukin-2 receptor. Adv. Immunol. 1998; 70: 1–81
- Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997; 89: 3909–3918
- Norton A. J., Isaacson P. G. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. Am. J. Pathol. 1987; 128: 225–240
- Nosari A., Montillo M., Morra E. Infectious toxicity using alemtuzumab. Haematologica 2004; 89: 1414–1419
- O'Mahony D., Morris J. C., Carrasquillo J. A., Le N., Paik C., Whatley M., Pittaluga S., Fleischer T. A., Lee C., Gao W., O'Hagan D., Waldmann T. A., Janik J. E. A Phase I/II study of yttrium-90-labeled humanized monoclonal anti-Tac antibody and calcium-DTPA in patients with CD25-expressing lymphoid malignancies. J. Nucl. Med. 2006; 47(S1)279
- O'Mahony D., Morris J. C., Moses L., O'Hagan D., Matthews H., Stetler-Stevenson M., Urquhart N., Kaucic K., Hammershaimb L., Warfe G., Waldmann T. A., Janik J. E. A Phase I trial of siplizumab in CD2-positive lymphoproliferative disease. AIDS Res. Hum. Retrovir 2007a; 23: 598
- O'Mahony D., Morris J. C., Stetler-Stevenson M., Matthews H., Pittaluga S., Albert P., Kaucic K., Hammershaimb L., Waldmann T. A., Janik J. E. EBV-related lymphoproliferative disease complicating therapy with siplizumab, a novel anti-CD2 mediated T- and NK cell depleting agent, in patients with T-cell malignancies. Blood 2007b; 110: 1043, (ASH Annual Meeting Abstracts)
- Ohno T., Yamaguchi M., Oka K., Miwa H., Kita K., Shirakawa S. Frequent expression of CD3 epsilon in CD3 (Leu 4)-negative nasal T-cell lymphomas. Leukemia 1995; 9: 44–52
- Olsen E., Duvic M., Frankel A., Kim Y., Martin A., Vonderheid E., Jegasothy B., Wood G., Gordon M., Heald P., Oseroff A., Pinter-Brown L., Bowen G., Kuzel T., Fivenson D., Foss F., Glode M., Molina A., Knobler E., Stewart S., Cooper K., Stevens S., Craig F., Reuben J., Bacha P., Nichols J. Pivotal Phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J. Clin. Oncol. 2001; 19: 376–388
- Olsen N. J., Brooks R. H., Cush J. J., Lipsky P. E., St. Clair E. W., Matteson E. L., Gold K. N., Cannon G. W., Jackson C. G., McCune W. J., Fox D. A., Nelson B., Lorenz T., Strand V. A double-blind, placebo-controlled study of anti-CD5 immunoconjugate in patients with rheumatoid arthritis. The Xoma RA Investigator Group. Arthritis Rheum. 1996; 39: 1102–1108
- O'Malley D. P. T-Cell large granular leukemia and related proliferations. Am. J. Clin. Pathol. 2007; 127: 850–859
- Phillips K. E., Herring B., Wilson L. A., Rickford M. S., Zhang M., Goldman C. K., Tso J. Y., Waldmann T. A. IL-2Rα -directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Rα interaction. Cancer Res. 2000; 60: 6977–6984
- Poiesz B. J., Papsidero L. D., Ehrlich G., Sherman M., Dube S., Poiesz M., Dillon K., Ruscetti F. W., Slamon D., Fang C., Williams A., Duggan D., Glaser J., Gottlieb A., Goldberg J., Ratner L., Phillips P., Han T., Friedman-Kien A., Siegal F., Rai K., Sawitsky A., Sheremata L. W., Dosik H., Cunningham C., Montagna R. Prevalence of HTLV-I-associated T-cell lymphoma. Am. J. Hematol. 2001; 66: 32–38
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980; 77: 7415–7419
- Przepiorka D., LeMaistre C. F., Huh Y. O., Luna M., Saria E. A., Brown C. T., Champlin R. E. Evaluation of anti-CD5 ricin A chain immunoconjugate for prevention of acute graft-vs.-host disease after HLA-identical marrow transplantation. Ther. Immunol. 1994; 1: 77–82
- Przepiorka D., Phillips G. L., Ratanatharathorn V., Cottler-Fox M., Sehn L. H., Antin J. H., LeBherz D., Awwad M., Hope J., McClain J. B. A Phase II study of BTI-322, a monoclonal anti-CD2 antibody, for treatment of steroid-resistant acute graft-versus-host disease. Blood 1998; 92: 4066–4071
- Queen C., Schneider W. P., Selick H. E., Payne P. W., Landolfi N. F., Duncan J. F., Avdalovic N. M., Levitt M., Junghans R. P., Waldmann T. A. A humanized antibody that binds to the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA 1989; 86: 10029–10033
- Raman C. CD5, an important regulator of lymphocyte selection and immune tolerance. Immunol. Res. 2002; 26: 255–263
- Ravandi F., O'Brien S. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Invest. 2006; 24: 718–725
- Ravel S., Colombatti M., Casellas P. Internalization and intracellular fate of anti-CD5 monoclonal antibody and anti-CD5 ricin A-chain immunotoxin in human leukemic T-cells. Blood 1992; 79: 1511–1517
- Robb R. J., Munck A., Smith K. A. T-Cell growth factor receptors. J. Exp. Med. 1982; 154: 1455–1474
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma 1985; 4: 91–102
- Sanchez-Madrid F., Davignon D., Martz E., Springer T. A. Antigens involved in mouse cytolytic T-lymphocyte (CTL)-mediated killing: Functional screening and topographic relationship. Cell. Immunol. 1982; 73: 1–11
- Sasada T., Reinherz E. L. A critical role for CD2 in both thymic selection events and mature T cell function. J. Immunol. 2001; 166: 2394–2403
- Schneider C., Hübinger G. Pleiotropic signal transduction mediated by human CD30: A member of the tumor necrosis factor receptor (TNFR) family. Leuk Lymphoma 2002; 43: 1355–1366
- Schnell R., Staak O., Borchmann P., Schwartz C., Matthey B., Hansen H., Schindler J., Ghetie V., Vitetta E. S., Diehl V., Engert A. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin's and non-Hodgkin's lymphoma. Clin. Cancer Res. 2002; 8: 1779–1786
- Sehn L. H., Donaldson J., Chanabhai M., Fitzgerald C., Gill K., Klasa R., MacPherson N., O'Reilly S., Spinelli J. J., Sutherland J., Wilson K. S., Gascoyne R. D., Connors J. M. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J. Clin. Oncol. 2005; 23: 502–5033
- Shaffer J., Villard J., Means T. K., Alexander S., Dombkowski D., Dey B. R., McAfee S., Ballen K. K., Saidman S., Preffer F. I., Sachs D. H., Spitzer T. R., Sykes M. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp. Hematol. 2007; 35: 1140–1152
- Shanmugham L. N., Petrarca C., Frydas S., Donelan J., Castellani M. L., Boucher W., Madhappan B., Tete S., Falasca K., Conti P., Vecchiet J. IL-15 an immunoregulatory and anti-cancer cytokine. Recent advances. J. Exp. Clin. Cancer Res. 2006; 25: 529–536
- Sharma K., Janik J. E., O'Mahoney D., Lee C. C., O'Hagan D., Gao W., Wharfe G., Cranston B., Waldmann T. A., Morris J. C. A Phase II study of the efficacy and toxicity of alemtuzumab (CAMPATH-1H) for the therapy of human T-cell lymphotrophic virus-1 (HTLV-1)-associated adult T cell leukemia/lymphoma (ATL). Proc. Am. Soc. Clin. Oncol 2006; 24: 2535
- Skov L., Kragballe K., Zachariae C., Obitz E. R., Holm E. A., Jemec G. B., Sølvsten H., Ibsen H. H., Knudsen L., Jensen P., Petersen J. H., Menné T., Baadsgaard O. HuMax-CD4: A fully human monoclonal anti-CD4 antibody for the treatment of psoriasis vulgaris. Arch. Dermatol. 2003; 139: 1433–1439
- Stein H., Foss H. D., Dürkop H., Marafioti T., Delsol G., Pulford K., Pileri S., Falini B. CD30(+) anaplastic large cell lymphoma: A review of its histopathologic, genetic, and clinical features. Blood 2000; 96: 3681–3695
- Strand V., Lipsky P. E., Cannon G. W., Calabrese L. H., Wiesenhutter C., Cohen S. B., Olsen N. J., Lee M. L., Lorenz T. J., Nelson B. Effects of administration of an anti-CD5 plus immunoconjugate in rheumatoid arthritis. Results of two Phase II studies. The CD5 Plus Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 1993; 36: 620–630
- Teh S. J., Killeen N., Tarakhovsky A., Littman D. R., Teh H. S. CD2 regulates the positive selection and function of antigen-specific CD4− CD8+ T-cells. Blood 1997; 89: 1308–1318
- Tendler C. L., Greenberg S. J., Blattner W. A., Manns A., Murphy E., Fleisher T., Hanchard B., Morgan O., Burton J. D., Nelson D. L., Waldmann T. A. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: Pathogenic implications and a rationale for immunotherapy. Proc. Natl. Acad. Sci. USA 1990; 87: 5218–5222
- Tesch H., Günther A., Abts H., Jücker M., Klein S., Krueger G. R., Diehl V. Expression of interleukin-2Rα and interleukin-2Rβ in Hodgkin's disease. Am. J. Pathol. 1993; 142: 1714–1720
- Tinubu S. A., Hakimi J., Kondas J. A., Bailon P., Familletti P. C., Spence C., Crittenden M. D., Parenteau G. L., Dirbas F. M., Tsudo M., Bather J. D., Kasten-Sportes C., Martinucci J. L., Carolyn K, Goldman C. K., Clark R. E., Waldmann T. A. Humanized antibody directed to the IL-2 receptor β -chain prolongs primate cardiac allograft survival. J. Immunol. 1994; 153: 4330–4338
- Tkaczuk J., Yu C. L., Baksh S., Milford E. L., Carpenter C. B., Burakoff S. J., McKay D. B. Effect of anti-IL-2Rα antibody on IL-2-induced Jak/STAT signaling. Am. J. Transplant. 2002; 2: 31–40
- Treumann A., Lifely M. R., Schneider P., Ferguson M. A. Primary structure of CD52. J. Biol. Chem. 1995; 270: 6088–6099
- Tsudo M., Kitamura F., Miyasaka M. Characterization of the IL-2 receptor β chain using three distinct monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1989; 86: 1982–1986
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T-cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J. Immunol. 1981a; 126: 1393–1397
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T-cells. II. Expression of Tac antigen on activated cytotoxic killer T-cells, suppressor cells, and on one of two types of helper T-cells. J. Immunol. 1981b; 126: 1398–1403
- Vergier B., de Muret A., Beylot-Barry M., Vaillant L., Ekouevi D., Chene G., Carlotti A., Franck N., Dechelotte P., Souteyrand P., Courville P., Joly P., Delaunay M., Bagot M., Grange F., Fraitag S., Bosq J., Petrella T., Durlach A., De Mascarel A., Merlio J. P., Wechsler J. Transformation of mycosis fungoides: Clinicopathological and prognostic features of 45 cases. French Study Group of Cutaneous Lymphomas. Blood 2000; 95: 2212–2218
- Wahl A. F., Klussman K., Thompson J. D., Chen J. H., Francisco L. V., Risdon G., Chace D. F., Siegall C. B., Francisco J. A. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects anti-tumor activity in models of Hodgkin's disease. Cancer Res. 2002; 62: 3736–3742
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant T-cells. Science 1986; 232: 727–732
- Waldmann T. A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006; 6: 595–601
- Waldmann T. A., White J. D., Carrasquillo J. A., Reynolds J. C., Paik C. H., Gansow O. A., Brechbiel M. W., Jaffe E. S., Fleisher T. A., Goldman C. K., Top L. E., Bamford R., Zaknoen E., Roessler E., Kasten-Sportes C., England R., Litou H., Johnson J. A., Jackson-White T., Manns A., Hanchard B., Junghans R. P., Nelson D. L. Radioimmunotherapy of interleukin-2Rα -expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood 1995; 86: 4063–4075, 1995
- Waldmann T. A., White J. D., Goldman C. K., Top L., Grant A., Bamford R., Roessler E., Horak I. D., Zaknoen S., Kasten-Sportes C., England R., Horak E., Mishra B., Dipre M., Hale P., Fleisher T. A., Junghans R. P., Jaffe E. S., Nelson D. L. The interleukin-2 receptor: A target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood 1993; 82: 1701–1712
- Wong B. Y., Gregory S. A., Dang N. H. Denileukin diftitox as novel targeted therapy for lymphoid malignancies. Cancer Invest. 2007; 25: 495–501
- Zamai L., Del Zotto G., Papa S. CD122 (interleukin-2 receptor beta subunit). J. Biol. Regul. Homeost. Agents 2001; 15: 95–97, 2001
- Zhang M., Zhang Z., Garmestani K., Goldman C. K., Ravetch J. V., Brechbiel M. W., Carrasquillo J. A., Waldmann T. A. Activating Fc receptors are required for anti-tumor efficacy of the antibodies directed toward CD25 in a murine model of adult T-cell leukemia. Cancer Res. 2004; 64: 5825–5829
- Zhang Z., Zhang M., Goldman C. K., Ravetch J. V., Waldmann T. A. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD52 monoclonal antibody, Campath-1H. Cancer Res. 2003a; 63: 6453–6457
- Zhang Z., Zhang M., Ravetch J. V., Goldman C., Waldmann T. A. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507. Blood 2003b; 102: 284–288