Abstract
The objective of this study is to investigate the effects of two different yeast-derived β-glucans on macrophage functionality and blood cholesterol levels in mice. After establishing their ability to act as a strong stimulator of macrophages, we measured the capacities of the glucans to lower blood cholesterol. Our results showed that consumption of diets containing yeast-derived β-glucan indicated a dose-dependent decrease in plasma cholesterol levels, with Betamune having a significantly stronger effect than WGP (Whole Glucan Particles). In hypercholesterolemic subjects, the cholesterol- and triglycerides-lowering effects of Betamune were again significantly stronger. The results of this investigation demonstrated that highly purified yeast-derived β-glucans modify plasma cholesterol levels and other indicators associated with artherogenic progression in mice. The fact that these glucans also strongly stimulated both monocytes and macrophages supports the hypothesis that there could be a macrophage-cholesterol (metabolism) axis involvement in these outcomes.
Keywords::
Introduction
β1,3-Glucan’s role as a biologically-active immunomodulator has been well documented for over 40 years. Interest in the immunomodulatory properties of polysaccharides was initially raised after experiments showing that a crude yeast cell preparation stimulated macrophages via activation of the complement system (Benacerraf and Sebestyen, Citation1957). Further work identified the immunomodulatory active component as glucan (Rigi and Di Luzio, Citation1961). Numerous studies (currently more than 9,600 publications) have subsequently shown that glucans, either particulate or soluble, exhibit immunostimulating properties, including antibacterial and anti-tumor activities (Di Luzio et al., Citation1979; Mimura et al., Citation1985).
The National Center for Health Statistics reports that, in the United States alone, over 650,000 people die of heart disease each year. The link between elevated cholesterol levels and the risk of coronary diseases has been clearly defined (Schaefet et al., Citation1997). Since none of the current cholesterol-lowering drugs are without side-effects, the search for a natural modulator of cholesterol concentrations is an important task. The possible effects of dietary fiber were first suggested by Keys et al. (Citation1960). These effects were later found to be associated with glucans (Tietyen et al., Citation1990). Glucans belong to a group of physiologically active compounds, collectively termed biological response modifiers. In general, β-glucan is the chemical name of a polymer of β-glucose. Homopolymers of glucose having a linear molecule with (1-3)-β-d-glycosidic linkages or a branched one with side chains bound by (1-6)-β-d-glycosidic linkages, are usually commonly termed “β-glucans” in spite of being chemically heterogeneous.
There are various natural sources of β-glucans; however, they are most frequently prepared from fungal cell walls. In addition to the fungal cell walls, β-glucan is also isolated from seaweed laminaran from Laminaria hyperborea, linear β(1 →3)-d-glucan (Black et al., Citation1951) and commercially sold as Phycarine (Vetvicka and Yvin, Citation2004). It is also produced by bacteria (curdlan from Alcaligenes faecalis) (Harada et al., Citation1968) and is an ingredient of cereal. The composition of the cereal β-glucan is somewhat different (it contains, in addition, β(1 → 4) bound glucose).
The cholesterol-lowering effects of fibers are routinely associated with β-glucans. Due to the high consumption of oats or oat bran, attention has focused mainly on the relationship between oat-derived glucan and cholesterol levels. These glucans were shown to reduce serum cholesterol in both hypercholesterolemic animals (Fadel et al., Citation1987; Kahlon et al., Citation1993; Delaney et al., Citation2003) and humans (Queenan et al., Citation2007). However, some studies found no such effects (Keogh et al., Citation2003). These studies held the problem of routinely using poorly defined glucans thus making interpretation of the results extremely difficult. In addition, grain glucans are 1,4 glucan, whereas yeast glucans are 1,3 or 1,3-1,6 glucans.
With regard to yeast-derived glucans, less information is available and the results are often conflicting (Nicolosi et al., Citation1999; Babicek et al., Citation2007). To overcome the deficiencies of older studies, we decided to use two highly-characterized yeast-derived glucans (Hong et al., Citation2004; Vetvicka and Vetvickova, Citation2007; Vetvicka et al., Citation2008) on normal and hypercholesterolemic mice. Unlike the older investigation, this report is the first to use two different, highly-purified glucans rather than crude cereal extracts.
The mechanisms of the cholesterol-lowering activity of glucans is still unclear. Some studies suggest the role of macrophages in cholesterol metabolism (Schmitz et al., Citation1986; Frey and De Maio, Citation2007). Since macrophages are strongly influenced by glucan (Vetvicka and Yvin, Citation2004), we first evaluated if the studied glucans are stimulating cells of the macrophage lineage.
Material and methods
Animals
Female 8-wk-old BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). Average weight of these animals was 22.3 g. All animal work was done according to the University of Louisville IACUC protocol. Animals were sacrificed by CO2 asphyxiation.
Diet
All diets were formulated and prepared by Purina (Richmond, IN). Laboratory Rodent Diet 5001 consists of 23.9% protein, 4.6% fat, 5.5% fiber, with 75.5% total digestible nutrients. This diet was supplemented with either glucan or cholesterol corresponding to the final daily doses of 100 or 400 mg of glucan or 16 mg of cholesterol, respectively. Cholesterol was obtained from Sigma Chemical Co. (St. Louis, MO). Diet ingredients for all groups were identical except for the proportion of glucan and/or cholesterol. The glucan-enhanced diet contained various doses of individual glucan, cholesterol-enhanced diet contained cholesterol. The β-glucans were quantified by using a Megazyme kit.
β-1,3 glucans
Yeast-derived insoluble whole glucan particle (WGP) glucan was purchased from Biothera (Eagan, MN), and insoluble yeast-derived Betamune glucan was purchased from Biorigin, Sao Paulo, Brazil.
Phagocytosis
The technique employing phagocytosis of synthetic polymeric microspheres was previously described (Vetvicka et al., Citation1988). In brief, peripheral blood or peritoneal cells obtained from mice fed with glucans for 72 hr, were incubated with 0.05 ml of 2-hydroxyethyl methacrylate particles (HEMA; 5 × 108/ml). The test tubes were incubated at 37°C for 60 min., with intermittent shaking. Smears were stained with Wright’s stain. The cells with three or more HEMA particles were considered positive.
Biochemical analysis
Mice were deprived of food for 12 hr and then sacrificed. Serum was collected via the retro-orbital sinus and stored at −80°C for less than a week. Biochemical analyses were performed by Antech Diagnostics (Indianapolis, IN) using Hitachi 747 chemistry analyzers.
Statistical analysis
The data from each experimental group was pooled from three separate trials for statistical analysis. Statistical differences between treatment groups were determined by ANOVA (p < 0.05).
Results
Glucans are generally considered to be potent stimulators of cellular immunity, with macrophages and neutrophils being the most important targets. Not surprisingly, we started our evaluation of glucan activities by phagocytosis. We used the synthetic polymeric microspheres, HEMA, since their use, dose and timing are already well established in glucan studies (Vetvicka and Vetvickova, Citation2007). Results summarized in show the significant effects of glucan samples on encapsulation of synthetic particles by both peripheral blood monocytes and peritoneal macrophages. In every case, Betamune glucan showed stronger and longer-lasting stimulation of the cells of the macrophage lineage.
Table 1. Effect of glucans on phagocytic activity.
After establishing that both glucans stimulate monocytes and macrophages, we studied the effect of long-term feeding with glucan-added diet. The mice adapted well to all diets and all survived the dietary treatment. Body weights and individual organ weights did not differ among the groups at each timepoint ().
Table 2. Effect of glucans on host body and organ weights (all values in grams).
In each subsequent experiment, we used three independent groups of mice totaling at least 10 animals per experimental point. To address the possibility that the tested glucans were damaged during the preparation of commercial diet, we also measured the effects of these glucans when added to drinking water or in forced feeding. All obtained results were identical with the use of glucan-enriched diets (data not shown). Our data showed the strong time-dependent effects of glucan on lowering cholesterol. The fastest and most pronounced effects were achieved with a higher dose of insoluble Betamune (). When total triglycerides were measured, Betamune lowered the triglyceride levels in all tested intervals (400 mg/mouse), whereas WGP glucan showed no significant activity ().
Figure 1. Effect of long-term feeding with glucan (see Materials and Methods) on blood cholesterol levels. Each value represents the mean of three independent experiments ± SD. *Represents significant differences between control (PBS) and glucan samples. **Represents differences between individual doses of glucan. Mice obtained either 100 or 400 μg of glucan.
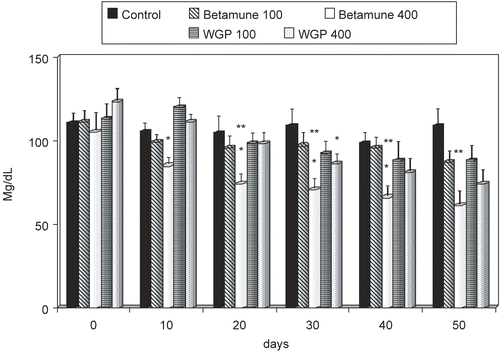
Figure 2. Effect of long-term feeding with glucan (see Materials and Methods) on blood triglyceride levels. Each value represents the mean of three independent experiments ± SD. *Represents significant differences between control (PBS) and glucan samples. **Represents differences between individual doses of glucan. Mice obtained either 100 or 400 μg of glucan.
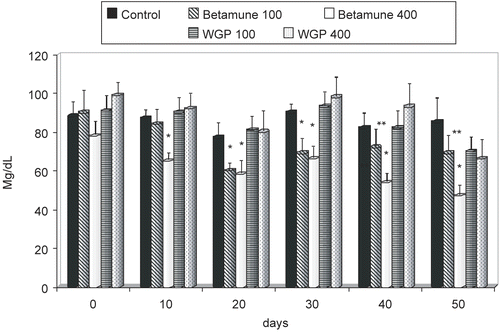
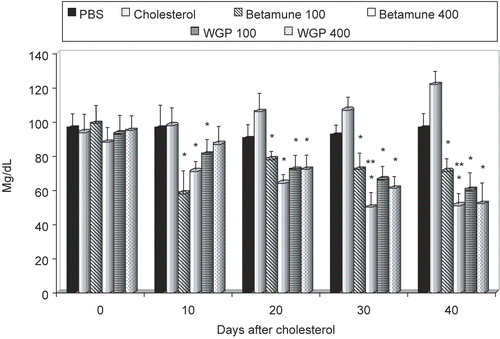
The mice were then given a diet with added cholesterol (0.15 g/100 g) for a period of 2 weeks (Delaney et al., Citation2003). The blood cholesterol and triglyceride levels obtained on Day 14 (last day of the cholesterol diet) were used as a positive control. The cholesterol-rich diet was followed by 40 days of feeding with a glucan-rich diet. Individual groups of mice were sacrificed at 10-day intervals and cholesterol and triglyceride levels were evaluated. The results summarized in showed that higher doses of glucan significantly lowered the cholesterol levels from Day 10. In the case of Betamune, the lower levels of cholesterol were observed in all tested time intervals (10–40 days), but the effects of WGP were significant only at Day 20. A different result was observed in the case of tryglycerides. The effects of Betamune were once again faster; however but 20 days after glucan-feeding both glucans (and both doses) were able to significantly lower the levels of tryglycerides in blood ().
Figure 3. Effect of long-term feeding with glucan on blood cholesterol levels in experimentally-induced hypercholesterolemia. The feeding with glucan started after two weeks of cholesterol-high diet (see Materials and Methods). Each value represents the mean of three independent experiments ± SD. *Represents significant differences between control (PBS) and glucan samples. **Represents differences between individual doses of glucan. Mice obtained either 100 or 400 μg of glucan.
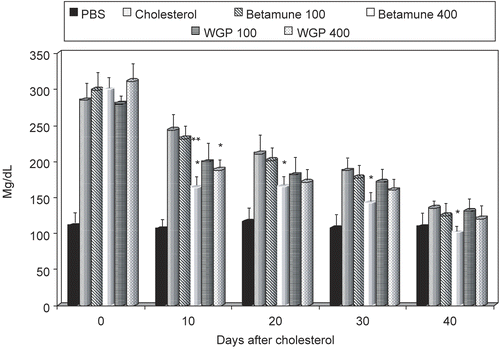
Figure 4. Effect of long-term feeding with glucan on blood triglyceride levels in experimentally-induced hypercholesterolemia. The feeding with glucan started after 2 weeks of cholesterol-high diet (see Materials and Methods). Each value represents the mean of three independent experiments ± SD. *Represents significant differences between control (PBS) and glucan samples. **Represents differences between individual doses of glucan. Mice obtained either 100 or 400 μg of glucan.
Discussion
High numbers of individual glucans have been described in the literature. Due to the huge differences in activities among various glucans isolated from numerous sources, it is imperative to evaluate its biological properties before any suggestions for use of a particular glucan in clinical practice. This investigation focused on the biological activities of two different types of glucans isolated from the same source – yeasts.
Most experimental studies dealing with cholesterol-lowering effects of glucans used oats- or barley-derived glucans (Fadel et al., Citation1987; Kahlon et al., Citation1993), without finding significant differences between them (Delaney et al., Citation2003). The composition of these cereal (1,4) β-glucans is, however, significantly different from other commercial and scientific glucans that are used more frequently. High numbers of individual glucans have been described in the literature. Due to the significant differences in activities among various glucans isolated from numerous sources, it is necessary to evaluate their biological properties before any suggestion is made for the use of a particular glucan in clinical practice. This investigation focused on the cholesterol-lowering activities of two different types of glucan. We evaluated two yeast-derived glucans - insoluble WGP and insoluble Betamune. Both these glucans and their activities are well documented (Yan et al., Citation2003, Citation2005), including full toxicological assessment (Babicek et al., Citation2007), and are considered to be highly active glucans (Vetvicka et al., Citation2008). As the effectiveness of orally-administered glucans was fully established (Hong et al. Citation2004; Li et al., Citation2006), glucans are currently nearing use in clinical practice. In addition, orally effective lentinan is already being used in Japan (Hamuro, Citation2005). Therefore, it was important to learn if these glucans also possess a cholesterol-lowering activity.
The cholesterol-lowering effects of glucans are well-established in numerous models, including humans (Fadel et al., Citation1987; Queenan et al., Citation2007; Reyna-Villasmil et al., Citation2007). These effects are usually described as the result of fiber intake and subsequent decreased absorption of bile acids (Malkki, Citation2001), albeit without any substantiating proof. Most of these studies suffer from the fact that they did not evaluate the effects of isolated glucans, and the fact that only crude extracts were used (Wang et al., Citation1997). This is compounded by the lack of any knowledge as to whether these glucans are even digested. Various glucan modifications and substances with different concentrations of poorly defined β-glucans are routinely tested (Davidson et al., Citation1991; Kahlon et al., Citation1993). This is addition to the fact that some of these glucans (Keogh et al., Citation2003) had previously been found to be inactive (Vetvicka and Vetvickova Citation2007).
Despite significant research on the role of glucan in lowering cholesterol levels, the exact mechanisms are still illusive. Among the possibilities, the macrophage-cholesterol metabolism was suggested (Schmitz et al., Citation1986). In addition, substances activating macrophages were also found to lower cholesterol levels (Pandey et al., Citation2005). Therefore, we first established that glucans used in this paper affect cells of the monocyte-macrophage lineage.
Several papers dealing with yeast-derived glucans showed the superiority of this type of glucan. However, information regarding the molecular structure and source of these glucans is, once again, lacking (Nicolosi et al., Citation1999). The current study is not only the first to directly compare the cholesterol-lowering activity of two different yeast-derived β-glucans but also the first to compare normal animals and mice with experimentally-induced cholesterolemia.
Consumption of diets containing yeast-derived β-glucan indicated a dose-dependent decrease in plasma cholesterol levels with insoluble Betamune having a much stronger effect. In the case of triglycerides, insoluble WGP showed only slight and sometimes non-significant effects. The effects were not only dose-dependent, but also showed a major difference when based on the type of glucan. Despite the fact that Betamune has a stronger effect on immune reactions (Vetvicka et al., Citation2008), the fact that both glucans are insoluble suggests that the answer to the question as to whether the cholesterol-lowering effects are due to the differences in solubility is not relevant. The question of whether these effects are affected by solubility of glucans is pertinent however, since both soluble and insoluble glucans are processed by macrophages to an approximately 25-kDa active moiety that subsequently primes cells for biological activity (Hong et al., Citation2004; Li et al., Citation2006). The studies showing the results of in vivo glucan processing also invalidate the hypothesis of the importance of molecular weight of glucan (Bengtsson et al., Citation1989; Newman et al., Citation1992).
In conclusion, the results of our investigation demonstrated that highly-purified, yeast-derived β-glucans modify plasma cholesterol levels as well as other indicators associated with artherogenic progression in mice. The fact that these glucans also strongly stimulate monocytes and macrophages supports the hypothesis of the macrophage-cholesterol axis.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Babicek, K., Cechova, I., Simon, R. R., Harwood, M., and Cox, D. J. 2007. Toxicological assessment of a particulate yeast (1,3/1,6)-β-D-glucan in rats. Food. Chem. Toxicol. 45:1719–1730.
- Benacerraf, B., and Sebestyen, M. M. 1957. Effect of bacterial endotoxins on the reticuloendothelial system. Fed. Proc. 16:860–867.
- Bengtsson, S., Aman, P., Graham, H., Newman, C. W., and Newman, P. K. 1989. Chemical studies on mixed linked β-glucans in naked barley cultivars giving different hypocholesterolemic responses in chicken. J. Agric. Food Sci. 52:435–445.
- Black, W. A., Cornhill, W. J., Dewar, E. T., and Woodward, F. N. 1951. Manufacture of algal chemicals. III. Laboratory scale isolation of laminarin from brown marine algae. J. Appl. Chem. 1:505–507.
- Davidson, M. H., Dugan, L. D., Burns, J. H., Bova, J., Story, K., and Drennan, K. B. 1991. The hypocholesterolemic effects of β-glucan in oatmeal and oat bran. A dose-controlled study. JAMA 265:1833–1839.
- Delaney, B., Nicolosi, R. J., Wilson, T. A., Carlson, T., Frazer, S., Zheng, G. H., Hess, R., Ostergren, K., Haworth, J., and Knutson, N. 2003. β-Glucan fractions from barley and oats are similarly anti-atherogenic in hypercholesterolemic Syrian golden hamsters. J. Nutr. 133:468–475.
- Di Luzio, N. R., Williams, D. L., McNamee, R. B., Edwards, B. F., and Kitahama, A. 1979. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int. J. Cancer 24:773–779.
- Fadel, J. G., Newman, R. K., Newman, C. W., and Barnes, A. E. 1987. Hypocholesterolemic effects of β-glucans in different barley diets fed to broiler chicks. Nutr. Rep. Int. 35:1049–1058.
- Frey, T., and De Maio, A. 2007. Increased expression of CD14 in macrophages after inhibition of the cholesterol biosynthetic pathway by lovastatin. Mol. Med. 13:592–604.
- Hamuro, J. 2005. Anti-cancer immunotherapy with perorally effective lentinan. Gan. To Kagaku Ryoho 32:1209–1215.
- Harada, T., Misaki, A., and Saito, H. 1968. Curdlan: A bacterial gel-forming β-1,3-glucan. Arch. Biochem. Biophys. 124:292–298.
- Hong, F., Yan, J., Baran, J. T., Allendorf, D. J., Hansen, R. D., Ostroff, G. R., Xing, P. X., Cheung, N. K., and Ross, G.D. 2004. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of anti-tumor monoclonal antibodies in murine tumor models. J. Immunol. 173:97–806.
- Kahlon, T. S., Chow, F. I., Knuckles, B. E., and Chiu, M. M. 1993. Cholesterol-lowering effects in hamsters of β-glucan-enriched barley fraction, dehulled whole barley, rice bran, and oat bran and their combinations. Cereal Chem. 70:435–440.
- Keogh, G. F., Cooper, G. J., Mulvey, T. B., McArdle, B. H., Coles, G. D., Monro, J. A., Poppitt, S. D. 2003. Randomized controlled crossover study of the effect of a highly β-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 78:711–718.
- Keys, A., Anderson, J. T., and Grande, F. 1960. Diet-type (fast constant) and blood lipid in man. J. Nutr. 70:257–266.
- Li, B., Allendorf, D. J., Hansen, R., Marroquin, J., Ding, C., Cramer, D. E., and Yan, J. 2006. Yeast β-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-Phosphatidylinositol 3-kinase pathway. J. Immunol. 177:1661–1669.
- Malkki, Y. 2001. Oat fiber; In: Handbook of Dietary Fiber(Eds. Cho, S. S., and Dreher, M. L.), New York: Marcel Dekker, pp. 497–512.
- Mimura, H., Ohno, N., Suzuki, I., and Yadomae, T. 1985. Purification, anti-tumor activity, and structural characterization of β-1,3-glucan from Peziza vesiculosa. Chem. Pharm. Bull. 33:5096–5099.
- Newman, R. K., Klopfenstein, C. F., Newman, C. W., Guritno, N., and Hofer, P. J. 1992. Comparison of the cholesterol-lowering properties of whole barley, oat bran, and wheat red dog in chicks and rats. Cereal Chem. 69:240–244.
- Nicolosi, R., Bell, S. J., Bistrian, B. R., Greenberg, I., Forse, R. A., and Blackburn, G. L. 1999. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am. J. Clin. Nutr. 70:208–212.
- Pandey, R. S., Birenda, K. T., and Tripathi, Y. B. 2005. Exact of gum resins of Boswellia serrata L. inhibits lipopolysaccharide induced nitric oxide production in rat macrophages along with hypolipidemic property. Indian J. Exp. Biol. 43:509–516.
- Queenan, K. M., Stewart, M. L., Smith, K. N., Thomas, W., Fulcher, R. G., and Slavin, J. L. 2007. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutrition J. 6:1–8.
- Reyna-Villasmil, N., Bermudez-Pirela, V., Mengual-Moreno, E., Arias, N., Cano-Ponce, C., Leal-Gonzales, E., Suiki, A., Inglett, G. E., Israili, Z. H., Hernandez-Hernandez, R., and Velasco, M. 2007. Oat-derived β-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am. J. Therapeut. 14:203–212.
- Rigi, S. J., and Di Luzio, N. R. 1961. Identification of a reticuloendothelial stimulating agent in zymosan. Am. J. Physiol. 200:297–300.
- Schaefer, E. J., Lamon-Fava, S., Ausman, L. M., Ordovas, J. M., Clevidence, B. A., Judd, J. T., Goldin, B. R., Woods, M., Gorbach, S., and Lichtenstein, A. H. 1997. Individual variability in lipoprotein cholesterol response to National Cholesterol Education Program Step 2 diets. Am. J. Clin. Nutr. 65:823–830.
- Schnmitz, G., Robenek, H., and Assmann, G. 1986. Role of the high density lipoprotein-receptor cycle in macrophage-cholesterol metabolism. Klin. Wochenschrift 64:979–985.
- Tietyen, J. L., Nevins, D. J., and Schneeman, B. O. 1990. Characterization of the hypocholesterolemic potential of oat brand. FASEB J. 4:A527.
- Vetvicka, V., Holub, M., Kovaru, H., Siman, P., and Kovaru, F. 1988. α-Fetoprotein and phagocytosis in athymic nude mice. Immunol. Lett. 19:95–98.
- Vetvicka, V., Vashishta, A., Saraswat-Ohri, S., and Vetvickova, J. 2008. Immunological effects of yeast- and mushroom-derived β-glucans. Biofactors (in press).
- Vetvicka, V., and Vetvickova, J. 2007. An evaluation of the immunological activities of commercially available β-1,3-glucans. JANA 10:25–31.
- Vetvicka, V., and Yvin, J. C. 2004. Effects of marine β-1,3 glucan on immune reactions. Int. Immunopharmacol. 4:721–730.
- Wang, L., Behr, S. R., Newman, R. K., and Newman, C. W. 1997. Comparative cholesterol-lowering effects of barley β-glucan and barley oil in golden Syrian hamsters. Nutr. Res. 17:77–88.
- Yan, J., Zong, H., Shen, A., Chen, S., Yin, X., Liu, W., Gu, X., and Gu, J. 2003. The β-(1-6)-branched β-(1-3) glucohexaose and its analogues containing an α-(1-3)-linked bond have similar stimulatory effects on the mouse spleen as Lentinan. Int. Immunopharmacol. 3:1861–1871.
- Yan, J., Allendorf, D. J., and Brandley, B. 2005. Yeast whole glucan particle (WGP) β-glucan in conjunction with anti-tumor monoclonal antibodies to treat cancer. Expert Opin. Biol. Ther. 5:691–702.
