Abstract
Many environmental toxins have been shown to suppress the immune system across taxa. The foreign red blood cell (RBC) challenge is an important part of a complement of tests used to assess immunocompetence in the laboratory because it can assess an individual’s humoral response without impacting its health. This challenge is used commonly across species and measures antibody titers in response to an intraperitoneal, intravenous, or subcutaneous injection of foreign RBCs. Determination of the best appropriate foreign RBC challenge is therefore important when designing tests for evaluation of humoral responses. The northern bobwhite (Colinus virginianus) is a commonly used species for avian toxicity tests, however little is known about the relative sensitivities of its humoral responses to foreign erythrocytes. In this pilot study, we exposed adult quail to intravenous injections of 5% solutions of sheep, rat, rabbit, bovine, or chicken erythrocytes and performed antibody titers [hemagglutination assay for total immunoglobulin (Ig), IgG, and IgM] for primary and secondary responses. Although the bobwhites appeared to respond strongly to rat RBCs, high variability in responses were observed among individuals. Chicken RBCs elicited the poorest responses for both primary and secondary challenges. Sheep and bovine RBCs were adequate antigens for this test in bobwhites. We found that rabbit erythrocytes elicited the strongest responses with the least amount of variability between individuals. Rabbit RBCs, therefore, appear to be the ideal antigen for this test of the humoral response in this species.
Introduction
Immunotoxicology is the study of adverse or inappropriate changes in the structure or function of the immune system caused by exposure of an organism to a foreign substance. It is a relatively recent sub-discipline of toxicology, with the first immunotoxicity study (Friend and Trainer, Citation1970) believed to have been conducted in the late 1960s (Grasman, Citation2002). Although many advances in immunotoxicology have occurred in the past few decades, not many well established and commonly used methods to assess immune function in birds have been developed and used consistently in laboratory and field studies. Birds occupy a wide variety of ecological niches and are good representatives of many different trophic levels, making them good indicators for environmental health assessments. The foreign red blood cell challenge is used commonly across species and measures antibody titers in response to an intraperitoneal or intravenous injection of foreign red blood cells typically in a saline solution.
The two most often used species in laboratory avian toxicity tests are the northern bobwhite (Colinus virginianus) and the Japanese quail (Coturnix japonica). Its relatively fast maturation cycle and ease of laboratory husbandry make the Japanese quail the most appropriate model for controlled reproductive and developmental studies and it has also been used in immunotoxicity tests (Touart, Citation2004). Its minimal sensitivities to sheep, human, chicken, turkey, and duck erythrocytes and a complete inability to produce immunoglobulins in response to human, bovine, and mouse albumins, however, are limiting in the context of designing a test of the humoral response without the use of a virulent antigen (Benton et al., Citation1977; Pardue, Citation1981). Japanese quail do mount a very strong antibody response to chukar (Alectoris chukar) erythrocytes; however, commercial red blood cells are not available for this species. The chukar red blood cells can easily be collected from donors at game farms, but are often only seasonally available.
The northern bobwhite may be more appropriate for humoral response tests, as they are able to mount adequate antibody responses to commercially available antigens. Sheep red blood cells are used most often in these tests as well as bovine serum albumin to a lesser extent (Fairbrother et al., Citation2004). The objective of this preliminary study was to determine relative sensitivities to challenges of commercially available foreign red blood cells in the northern bobwhite in order to optimize the ability to detect effects on the immune system by exposure to environmental chemicals.
Methods
Husbandry
Adult northern bobwhites were obtained from Trace Pheasantry, Inc. (Douglassville, PA). Upon arrival to the U.S. Army Center for Health Promotion and Preventive Medicine (CHPPM), birds were kept individually in 24-cage units, each cage (11 in wide × 12 in tall × 15 in deep) constructed of 0.5 in × 1 in polyvinylchloride (PVC)-coated wire. Quail arrived at CHPPM at 12 weeks of age and were used for this study at approximately 21 weeks-of-age. This was an opportunistic study that used surviving, non-treated sentinels from a prior study to gain useful information that would enhance future immunotoxicity studies prior to their euthanasia.
Cage assignments were randomized. Each unit contained two adjustable-height, automatic nipple drinkers and individual 18-gauge stainless steel feeding troughs. Each enclosure had a length of sisal rope hanging from the top for environmental enrichment. Birds were kept in constant photoperiod durations of 16-hr light: 8-hr dark with humidity maintained between 30 and 70% and the temperature between 18 and 26°C. Water and feed (Nutrena Premium Gamebird Feed; Cargill, Minneapolis, MN) were provided ad libitum.
This study was conducted through an approved protocol with the Institutional Animal Care and Use Committee. The investigators and technicians adhered to the following guidelines: the Public Health Service Policy on Humane Care and Use of Laboratory Animals, “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training,” and the Animal Welfare Act.
Antigen exposure
This study consisted of five treatments: four female birds in each of four treatments and three birds for one treatment (N = 19). Three birds each received a 0. 1 ml intravenous injection (jugular) of 5.0% sheep red blood cells in phosphate buffered saline (PBS), a standard concentration used to produce a strong antibody response in this species (Lochmiller et al., 1993). Four birds from each other treatment received an injection of either a 5.0% suspension (in PBS) of bovine, chicken, rabbit, or rat red blood cells. Red blood cells were purchased from Innovative Research (Novi, MI). At least 1 ml of blood was collected from the jugular in heparin-rinsed syringes 6 days following exposure for quantification of the strength of the primary antibody response. All quail were challenged again 3 weeks after the initial blood collection with identical treatments. A second blood collection occurred 6 days following the second challenge for assessment of the secondary antibody response. Grasman (Citation2002) and Fairbrother et al. (Citation2004) provide further descriptions of the standard methods for this and other commonly used immunotoxicity tests used in birds.
Hemagglutination assay
To measure plasma antibody activity, 50 μl of PBS was added to each well of a 96-well microtiter plate. Fifty microliters of plasma was then added to the first well of each row with serial two-fold dilutions being made of each sample. After another 50 μl of the appropriate foreign red blood cell suspension was added to each well, the plates were gently agitated and then incubated for 3 hours at 37°C. Titers were determined as the log 2 of the reciprocal of the highest dilution showing agglutination. The agglutination appeared as a diffuse red disc across the entire bottom of the well, and lack of agglutination appeared as a small red accumulation of red blood cells in the middle of the bottom of the well. This measures the activity of total hemagglutinating antibodies.
Additional plasma was incubated in 2-mercaptoethanol (2-ME) prior to serial dilutions being made. Incubation of plasma in 2-ME dissociates immunoglobulin (Ig) M and leaves IgG intact. The IgG titers were determined after incubation as described above; the IgM titers were then calculated by subtracting the IgG titers from the total hemagglutinating titers.
Statistical analysis
Data were analyzed using a one-way analysis of variance (ANOVA). If significant, a pairwise multiple comparison procedure was conducted using the Holm–Sidak method. Statistical significance was defined at the p < 0.05 level. Tests were conducted using SigmaStat Version 3.0 (SPSS, Chicago, IL).
Results
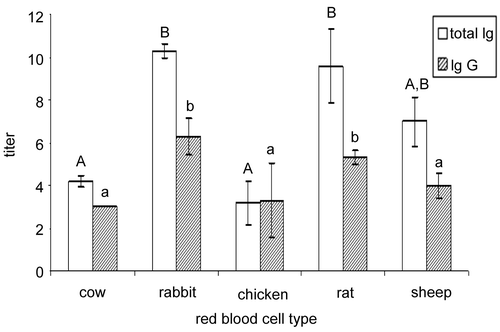
The bobwhite primary antibody responses were strongest for the rabbit and rat red blood cell challenges (). The primary responses to bovine, chicken, and sheep red blood cells did not differ from one another, although the response against the sheep erythrocytes was marginally greater than against those of the bovine and chicken.
Figure 1. Mean total immunoglobulin (Ig) and IgG titers produced in northern bobwhite (Colinus virginianus) adults in response to primary foreign red blood cell challenges (bovine, rabbit, chicken, rat, and sheep) with standard errors of the mean (SE). Differing uppercase letters indicate significant (p < 0.05) differences in total immunoglobulin production; differing lowercase letters indicate significant (p < 0.05) differences in immunoglobulin G (IgG) production.
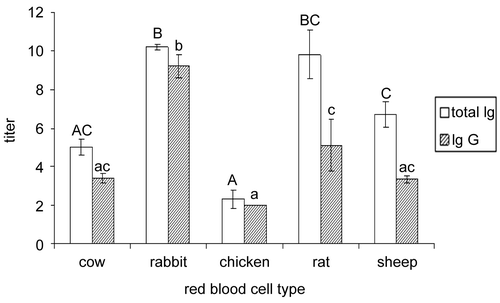
The total antibodies produced to the secondary challenge did not differ from those produced in response to the first (). Only IgG levels from the rabbit red blood cell challenges increased appreciably from the secondary challenge compared to the primary; IgG levels produced from the secondary challenge to rabbit erythrocytes were approximately 45% greater than those from the first inoculation. Similar to what was observed from the primary response, antibody titers from the rabbit and rat challenges were highest among the secondary challenges. The titer from the chicken red blood cell challenge was weakest among the secondary responses.
Figure 2. Mean total immunoglobulin (Ig) and IgG titers produced in northern bobwhite (Colinus virginianus) adults in response to secondary foreign red blood cell challenges (bovine, rabbit, chicken, rat, and sheep) 3 weeks post primary challenge with standard errors of the mean (SE). Differing uppercase letters indicate significant (p < 0.05) differences in total immunoglobulin production; differing lowercase letters indicate significant (p < 0.05) differences in immunoglobulin G (IgG) production.
Discussion
Since mean antibodies produced to rabbit and rat red blood cell inoculations were highest among the challenges, it would first appear that these would be the optimal red blood cell types to use in humoral test responses, followed by rat red blood cells. The mean responses to rabbit erythrocytes were approximately two times greater than what was observed with the standard foreign red blood cell challenge, sheep erythrocytes. The bobwhites responded poorest to the chicken red blood cells in both the primary and secondary challenges. In addition to overall antibody production, variability, secondary response vs. primary response, and quality of the appearance of the agglutination in the microtiter plates must also be considered.
In general, greater variability in responses could be expected to be observed across treatments just due to the low sample sizes used in this experiment. The titers observed from the bovine and rabbit red blood cell challenges, however, showed little variability in the primary and secondary responses and were the least variable among the five. As mentioned above, the only titer that showed an indication of isotype switch from the secondary challenge was that from the rabbit red blood cells. Perhaps more time was needed between the first and second red blood cell challenges for class switching, evidence of affinity maturation, to be observed in the other treatments. The almost absence of IgM production in response to the chicken red blood cell challenges was particularly peculiar; whatever the reason for this may be, this observation lends further support to the conclusion that chicken red blood cells would be the least likely candidate for this type of humoral response test.
Overall, chicken red blood cells elicited the poorest responses for both primary and secondary challenges and showed no evidence of immunoglobulin class switch. Sheep, which are most commonly used in immunotoxicity tests to assess humoral responses in many avian species, and bovine red blood cells are adequate antigens for this test in bobwhites. The responses and variability among samples were moderate. Rat red blood cells elicited a strong response, but had the greatest amount of variability between samples. Rabbit erythrocytes both elicited the strongest responses and displayed the least amount of variability between individuals. With the additional ability to elicit immunoglobulin class switching in a secondary challenge 3 weeks following the primary, rabbit red blood cells appear to be the ideal antigen for this test of the humoral response in this species, and may be better able to detect effects on the humoral arm of the immune system by exposure to environmental chemicals than the standard sheep erythrocyte challenge.
Acknowledgment
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Benton, E. H., Morgan, G. W., and Thaxton, P. 1977. Antibody responses to xenogenic red blood cell challenge in the Japanese quail. Immunol. Commun. 6:259–265.
- Fairbrother, A., Smits, J., and Grasman, K. 2004. Avian immunotoxicology. J. Toxicol. Environ. Health 7:105–137.
- Friend, M., and Trainer, D. O. 1970. Polychlorinated biphenyl: Interaction with duck hepatitis virus. Science 170:1314–1316.
- Grasman, K. A. 2002. Assessing immunological function in toxicological studies on avian wildlife. Integ. Comp. Biol. 42:34–42.
- Pardue, S.L., Thaxton, J. P., and Morgan, G. W. 1981. Humoral immunity in Japanese quail following surgical bursectomy at various ages. Poult. Sci. 60:2713–2719.
- Touart, L. W. 2004. Factors considered in using birds for evaluating endocrine-disrupting chemicals. ILAR J. 45:462–468.
