ABSTRACT
This review describes different postharvest fungal diseases like blue mold, Anthracnose and fruit rot of Indian gooseberry along with its management strategies that covers physical, chemical and biological methods. The physical methods of management include osmo-drying, hot water treatment, UV-C treatment, application of high electric field current, efficient packaging in low and high density polythene pouches.Chemical methods involve treatment with different synthetic fungicides like Bavistin, Mancozeb, Gibberellic acid, Carbendazim, Calcium nitrate and Calcium sulphate. Lastly, the biological control methods have been mentioned, which includes application of microbial antagonists and naturally occurring antifungal compounds.
Introduction
Emblica officinalis Gaertn belongs to the family Euphorbiaceae, and it is known to be an important minor crop of India (Kumar and Sagar, Citation2009). It is also referred to as an underutilized crop (Scartezzini et al., Citation2006). However, underutilized crops often have the potential to contribute to the food security of the nation (Mayes et al., Citation2012). Emblica officinalis grows abundantly in the tropical and subtropical areas of India, China, Indonesia, Myanmar, Sri Lanka and the Malay Peninsula (Benthal, Citation1946; Liu et al., Citation2008; Macmillan, Citation1943; Perianayagam et al., Citation2005; Thakur et al., Citation1989). Indian gooseberry has also been reported to grow naturally in other parts of the world like Cuba, Puerto Rico, Iran, Iraq (Hooper and Field, Citation1937), Hawaii, Florida (Barrett, Citation1956; Sturrock, Citation1959), Java, West Indies and Trinidad (Webster, Citation1956). It is found to grow profusely in many northeastern states of India like Mizoram, Tripura, Assam particularly in the forests of Khasi and Garo hills of Meghalaya (Pandey et al., Citation1993). However, intensive cultivation is done in Uttar Pradesh, mainly in the districts of Pratapgarh, Azamgarh, Varanasi, and Jaunpur (Bajpai and Shukla, Citation2002). Cultivation of Indian gooseberry is also done in other states of India like Maharashtra, Gujarat, Rajasthan, Madhya Pradesh, Jharkhand Chhattisgarh, Bihar, West Bengal, Orissa, Andhra Pradesh, Karnataka, Haryana, and Himachal Pradesh (Chandra and Singh, Citation2015; Rawat and Uniyal, Citation2003).
Indian gooseberry can be grown in variable soil conditions-from sandy loam to clay. It grows well in arid and semi-arid regions. Farmers can particularly benefit by its cultivation because Indian gooseberry can thrive well in marginal lands as well. Indian gooseberry is known to possess a number of health benefits. It has been used frequently in the Ayurveda and Unani system of medicine (Pathak, Citation2003). It has very high Ascorbic acid, i.e., Vitamin C content, about 20 times higher than that of orange (Tarwadi and Agte, Citation2007). Juice of Indian gooseberry being highly acidic protects the vitamin C from degradation during heating or drying (Hassan et al., Citation2014). Ascorbic acid can act as an antioxidant by means of its free radical scavenging properties (Cort, Citation1982). However, in addition to ascorbic acid Indian gooseberry also contains polyphenols like ellagic acid, gallic acid, and hydrolysable tannins (Emblicanin A, Emblicanin B, punigluconin, pedunculagin) which have been reported to contribute to the antioxidant properties of the fruit (Bhattacharya et al., Citation1999; Ghosal et al., Citation1996). Ihantola-Vormisto et al. (Citation1997) have demonstrated that Indian gooseberry has anti-inflammatory and antipyretic properties. Different types of extracts of fruits have been reported to show Hepatoprotective activity, like 50% hydroalcoholic extract (Tasduq et al., Citation2005), or even 5% aqueous extracts (Reddy et al., Citation2010). The anti-tumor properties of the extracts of Indian gooseberry can be attributed to the polyphenolic compounds principally tannins and flavonoids. Pyrogallol obtained from Indian gooseberry extracts, have an anti-proliferative effect on human lung cancer cell-lines (Yang et al., Citation2009); while gallic acid extracted from leaves of Indian gooseberry result in apoptosis of human hepatocellular carcinoma cells (Huang and Zhong, Citation2011). Extracts of E. officinalis fruits inhibit transcription factor AP1 as well as interfere with the expression of viral oncogenes that could lead to cervical cancer development and progression. Therefore, the fruit extracts could be a potential source for drug development against Human Papilloma Virus (HPV) induced cervical cancer (Mahata et al., Citation2013). Apart from these, fruits of Indian gooseberry have a wide range of other properties like hypolipidemic (Thakur and Mandal, Citation1984; Yokozawa et al., Citation2007), hypoglycaemic (Jamwal et al., Citation1959; Liu et al., Citation2012), and analgesic (Perinayagam et al., Citation2004) .
The different cultivars of Indian gooseberry, commercially cultivated in India are ‘Banarasi’, ‘Francis’, ‘Chakaiya’, ‘Kanchan (NA-4)’, ‘NA6ʹ and ‘NA7ʹ (Pathak, Citation2003; Scartezzini et al., Citation2006). In Pakistan, ‘Desi’, ‘Shisha’, and ‘Banarasi’ cultivars are available; while in China ‘Langen’, ‘Fen’gan’, ‘Liuyuebai’, ‘Bian’gan’, ‘Quibai’ and ‘Shan’gan’ cultivars are cultivated. Indian gooseberry can be processed into pickles, candy, Murabba (whole fruit preserve), juices, mouth fresheners, fruit leathers, etc. (Bhattacherjee et al., Citation2011; Daniel and Dudhade, Citation2010; Nath and Sharma, Citation1998), which have real economic importance. Fruits of Indian gooseberry form the base ingredient for Chyavanprash, an Ayurvedic preparation. Presently in India Chyavanprash is processed by some large companies and some small-scale producers as well; the total Chyavanprash industry in the country is worth Rs 200 crore. In fact, because of their sour and astringent taste, the fruits are consumed more in processed form than raw.
However, ripe fruits of Indian gooseberry have a low shelf-life of 5–6 days and are highly perishable (Pathak et al., Citation2009). The fruit is susceptible to number of postharvest fungal pathogens among them most prevalent are blue and black mold. Thom (Citation1930) reported annual yield loss up to 50% due to fruit rot of Indian gooseberry caused by blue mold. The fungal diseases deteriorate the fruit quality and lower its marketability which affects the fruit production severely. Taking into consideration its enormous health benefits as well as economic importance, it is necessary that the post-harvest losses of Indian gooseberry be curtailed. This review focuses on the different postharvest diseases of Indian gooseberry and the management strategies adopted to tackle those diseases.
Postharvest Diseases of Indian Gooseberry
Numerous postharvest diseases caused by fungal pathogens have been reported in Indian gooseberry. Some of these diseases along with their respective causal organisms and symptoms have been presented in and .
Figure 1. Postharvest fungal diseases of Indian gooseberry: (I) Anthracnose by Colletotrichum gloeosporioides. (II) Fruit rot by Penicillium digitatum, (III) Soft rot by Phomopsis Phyllanthus. (IV) Fruit rot by Aspergillus niger.
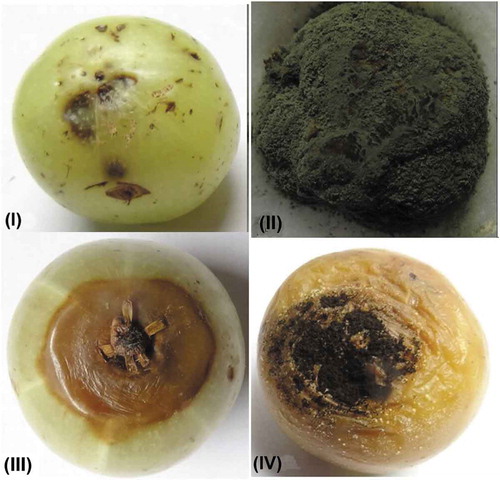
Table 1. Fungal diseases of Indian gooseberry.
Strategies for Postharvest Management
A report by the Indian Council of Agricultural Research (ICAR) – Central Institute of Post-Harvest Engineering and Technology (CIPHET) in Ludhiana (2015) showed that postharvest loss was maximum (4.58–15.88%) in fruits and vegetables among other crops. Therefore, effective strategies need to be introduced in order to reduce the losses incurred through the postharvest damage to agricultural produce. In the following section, the different methods for postharvest disease management of Indian gooseberry have been discussed.
Physical Methods
The fruits of E. officinalis are highly susceptible to microbial decay at the postharvest stage which can be effectively controlled by various physical methods. Dehydration is the oldest and a very low-cost technique to prolong the shelf life of fruits and vegetables (Doymaz, Citation2007). Microbes need enough moisture to grow and cause spoilage. Dehydration or drying is the method of removing moisture from fruits and vegetables which in turn prevents the microbial growth and enzyme activities, minimizing many moisture mediated deterioration reactions (Boyer and Huff, Citation2008; Krokida and Marinos-Kouris, Citation2003). Different drying techniques like osmo-air drying, direct sun drying, indirect solar drying, and oven drying are used in present times to preserve the fruit and prolong its shelf-life. Direct sun drying was more prevalent in earlier times, mainly because of the low operational costs. Nevertheless, this method had a number of disadvantages-quality of fruits dropped due to direct exposure to UV rays, contamination by dust and insects (Ertekin and Valdiz, Citation2004). Osmo-air drying has lately garnered more of attention because it allows minimum thermal degradation of nutrients in the products (Shi et al., Citation1997). It is essentially a two-step dehydration process. In the first stage, water is removed from the fruit by dipping in 40% sucrose solution for 12 h (Sagar and Kumar, Citation2010). In the second stage, the drained products are dried in a hot vacuum drier to the desired moisture level. Recently an LPG-based CRIDA drier has been developed in Hyderabad which successfully dehydrated blanched Indian gooseberry segments (Gudapaty et al., Citation2010). Dahiya and Dhawan (Citation2003) have shown that osmo-air drying helps in better retention of nutrients like ascorbic acid and sugars of Indian gooseberry fruit than other conventional methods of drying (Banerjee and Ghosh, Citation2015). Apart from dehydration techniques, hot water treatments have also been employed in a number of cases to curb postharvest losses (Gramaje et al., Citation2009; Kim et al., Citation2009; Olesen et al., Citation2004). Dipping of Indian gooseberry fruits in hot water (60°C for 2min) protected the fruits from Aspergillus niger infection, causing 100% inhibition of the spore germination (Sharma and Srivastava, Citation2014). Application of high electric field (HEF) to Indian gooseberry fruits has shown promising results in terms of shelf-life extension; fruits treated with alternating current (AC) HEF showed a better degree of freshness, as compared to untreated fruits (Bajgai et al., Citation2006). Indian gooseberry fruits, when treated with UV-C light, showed resistance towards A. niger infection; UV-C could induce resistance in fruits against the pathogen, thereby extending its shelf-life (Sharma and Srivastava, Citation2014).
Fruits can be stored for an extended period of time in refrigerated condition (Kore et al., Citation2013). Appropriate packaging methods and storage can reduce the postharvest losses by up to 30% (Goyal et al., Citation2008). Packaging of Indian gooseberry fruits in low-density polyethylene pouches reported minimum fruit decay (Singh and Kumar, Citation1987) compared to other treatments like storage at room temperature or at evaporative cooling conditions (Singh and Kumar, Citation1987). Storage in corrugated fiber boxes with newspaper liners prolonged the shelf life of Indian gooseberry fruits to 11 days (Singh et al., Citation2009). HDPE gauge polythene proved to be another effective packing material for Indian gooseberry fruits, showing an increase in shelf-life to 15 days along with retention of total sugar and ascorbic acid content (Vishwakarma et al., Citation2012).
Chemical Methods
Application of pesticides and fungicides is the usual methods of protection of the crops against infectious diseases. Pre-harvest application of several fungicides has resulted in reduction of post-harvest decay as well as increase in shelf-life of several crops. Dipping of Indian gooseberry fruits with Carbendazim at 500 and 1000 ppm, respectively, showed maximum resistance against Penicillium funiculosum when compared to treatments with other synthetic fungicides like benomyl, metallic copper, Captan, Mancozeb, etc. (Yadav et al., Citation2012a). Fungicides are often combined as co-formulations because they are capable of improved disease control, and resistance management. Mixture of Calcium Chloride, Bavistin, and Bayleton, when applied to leaves, was found to enhance the shelf–life of Indian gooseberry (Gupta and Singh, Citation2016). Many organic and inorganic compounds also enhance nutrient content of fruits, increase their shelf life as well as antagonists against different phytopathogens. Treatment of Indian gooseberry fruits with 1% Calcium nitrate helped in reducing physiological weight loss, retention of total sugar and ascorbic acid content along with increasing the shelf–life (Gangwar et al., Citation2012; Nath et al., Citation1992). GA3 can work efficiently with Calcium nitrate, thereby resulting in minimum spoilage of Indian gooseberry fruits (Singh et al., Citation2014). Foliar spray of Zinc sulfate, Magnesium sulfate and Copper sulfate (0.5% each) resulted in a better quality of fruits with a simultaneous rise in fruit yield (Chandra and Singh, Citation2015).
Biological Methods
Biological methods involve primarily the introduction of microbial antagonists like different fungal and bacterial species in controlling several plant diseases. These antagonists have various modes of action that include antibiosis and/or competition for nutrients and space (Wisniewski and Wilson, Citation1992). Few reports of the application of microbial antagonists to prevent postharvest diseases in Indian gooseberry have come up lately. Pre-harvest treatment with Lactobacillus acidophilus showed a reduction in pathogenesis caused by P. funiculosum in Indian gooseberry fruits (Sharma and Sumbali, Citation2009). Again, Trichoderma harzianum and Pseudomonas fluorescens have been reported to reduce the disease severity caused by P. funiculosum in Indian gooseberry (Yadav et al., Citation2012b). An in vitro evaluation of microbial antagonists against the fruit rot complex of Indian gooseberry has also been reported, where P. fluorescens recorded the maximum growth inhibition against A. niger and P. funiculosum (Wakade et al., Citation2015). Numerous other postharvest pathogens of Indian gooseberry like P. digitatum, Rhizopus stolonifer, A. niger, Colletotrichum gloeosporioides have been reported to be controlled by microbial antagonists in many other crops. Penicillium digitatum was shown to be controlled by Bacillus pumilus, and Saccharomycopsis schoenii (Huang et al., Citation1992; Pimenta et al., Citation2008). Rhizopus rot on apple, peach, and tomato is effectively controlled by the application of T. harzianum (Batta, Citation2007; El-Katatny and Emam, Citation2012). Aspergillus niger infection in lemons is controlled by B. subtilis (Manjula et al., Citation2004). A combination of Brevundimonas diminuta, Stenotrophomonas maltophilia, a member of Enterobacteriaceae and Candida membranifaciens has been successful in reducing the anthracnose disease of mango caused by C. gloeosporioides. However, publications regarding the biological control of the aforesaid pathogens in Indian gooseberry are lacking. Nevertheless, the reports that were obtained from these above mentioned crops can provide scope for further research on the various microbial antagonists that will curb the postharvest diseases of Indian gooseberry.
Use of Botanicals for Disease Management
Application of 6% onion extract (aqueous) has been reported to be useful in enhancing the shelf-life of Indian gooseberry (Kumar et al., Citation2009). Edible oils like mustard oil and linseed oil were successful in reducing the Penicillium rot of Indian gooseberry fruit; while among medicinal oil, neem oil was most effective (Baghel et al., Citation2009). Reduction of P. funiculosum rot in Indian gooseberry was achieved by the application of aqueous extracts of garlic bulbs (Goswami and Sumbali, Citation2010). Application of Azadirachta indica extract was also reported to inhibit P. funiculosum growth in Indian gooseberry (Yadav et al., Citation2013). Aspergillus and blue mold fruit rots are effectively controlled by the application of Curcuma longa rhizome extract (5%), A. indica leaf extract (5%), and Carbendazim (0.1%) as pre- and post-inoculation treatments. In pre inoculation treatment fruits were first dipped in the treatment solution for about 7–8 min then inoculated while in post-inoculation treatment fruits were inoculated first and then treated with the treatment solution. In both the cases, the interval between inoculation and treatment or vice versa was 12 h (Jat et al., Citation2013).
Conclusion
There is a pressing need to introduce effective strategies to control post-harvest diseases which will pose minimum risk to human health and the environment as a whole. Biological control of pests is being introduced lately in agricultural practices in place of fungicides, because they are economically viable and environment friendly. The main aim of this review was to discuss the various post-harvest disease management strategies of Indian gooseberry that included physical, chemical and ultimately biological methods. Reports regarding the biological control of pathogen involving a microbial antagonist in Indian gooseberry are rather limited. Therefore, in the future, there is scope for research in this sector of biological control practices as well as for other novel strategies that might come up to combat post-harvest diseases.
Literature cited
- Akhund, S., M. Suhail, I. Rani, F.I. Memon, and H. Abro. 2010. Fruit borne mycoflora of Amla (Phyllanthus Emblica L.). Pak. J. Bot. 42(6):4229–4233.
- Baghel, A., R.K. Dantre, and K.P. Verma. 2009. Efficacy of different edible oils, medicinal oils and the concentrations of effective oil used against fruit rot of aonla (Penicillium citrinum). J. Interacademicia. 13(2):142–147.
- Bajgai, T.R., G.V. Raghavan, F. Hashinaga, and M.O. Ngadi. 2006. Electrohydrodynamic drying—A concise overview. Drying Technol. 24:905–910. doi: 10.1080/07373930600734091.
- Bajpai, P.N., and H.S. Shukla. 2002. Aonla, p. 527–528. In: T.K. Bose, S.K. Mitra and D. Sanyal (eds.). Fruits: Tropical and subtropical. Vol. II, Naya Udyog, Calcutta, West Bengal, India.
- Banerjee, I., and U. Ghosh. 2015. Effect of pretreatment and concentration of sugar solution on retention of nutritional parameters of osmodried whole amla (Phyllanthus emblica L). Int. J. Latest Trends Eng. Tech 5(3):64–67.
- Barrett, M.F. 1956. Common exotic trees of South Florida (Dicotyledons). University of Florida Press, Gainesville.
- Batta, Y.A. 2007. Control of postharvest diseases of fruit with an invert emulsion formulation of Trichoderma harzianum Rifai. Postharvest Biol. Technol. 43:143–150. doi: 10.1016/j.postharvbio.2006.07.010.
- Benthal, A.P. 1946. Trees of Calcutta and its neighbourhood. Thacker Spink & Co. Ltd, Calcutta.
- Bhattacharya, A., A. Chatterjee, S. Ghosal, and S.K. Bhattacharya. 1999. Antioxidant activity of active tannoid principles of Emblica officinalis (amla). Indian J. Exp. Biol. 37(7):676–680.
- Bhattacherjee, A.K., D.K. Tandon, A. Dikshit, and S. Kumar. 2011. Effect of pasteurisation temperature on quality of aonla juice during storage. J. Food Sci. Technol. 48(3):269–273. doi: 10.1007/s13197-010-0171-5.
- Boyer, R., and K. Huff. 2008. Using dehydration to preserve fruits, vegetables, and meats. Vol. 348. Virginia Cooperative Extension, VCE Publications, Petersburg, VA, United States. p.348–597.
- Chandra, R., and K.K. Singh. 2015. Foliar application of zinc sulphate, magnesium sulphate and copper sulphate on the yield and quality of aonla (Emblica officinallis Gaerth) cv. “NA-7” under Garhwal Himalaya. J. Med. Pl. Stud. 3(5):42–45.
- Cort, W.M. 1982. Antioxidant properties of ascorbic acid in foods, p. 153–178. In: P.A. Seib and B.M. Tolbert (eds.). Ascorbic acid: Chemistry, metabolism, and uses. Advances in Chemistry Series, 200. American Chemical Society, Washington, DC, USA.
- Dahiya, P.S., and S.S. Dhawan. 2003. Effects of drying methods on nutritional composition of dehydrated aonla fruit (Emblica officinalis G.) during storage. Plant Foods Hum. Nutr. 58:1–9.
- Daniel, N.J., and P.A. Dudhade. 2010. Analysis of economic characteristics of value chains of three underutilised fruits of India. Icuc 3:1–26.
- Doymaz, I. 2007. Air drying characteristics of tomatoes. J. Food Sci. Technol. 78:1291–1297.
- El-Katatny, M.H., and A.S. Emam. 2012. Control of postharvest tomato rot by spore suspension and antifungal metabolites of Trichoderma harzianum. J. Microbiol. Biotech. Food Sci. 1:1505–1528.
- Ertekin, C., and O. Valdiz. 2004. Drying of eggplant and selection of a suitable thin layer drying model. J. Food Eng. 63:349–359. doi: 10.1016/j.jfoodeng.2003.08.007.
- Gangwar, S., H.S. Shukla, D. Katiyar, and V. Pandey. 2012. Effect of calcium nitrate on physico-chemical changes and shelf-life of aonla (Emblica officinalis Gaertn) fruits. Hort. F. R. Spect. 1(3):253–258.
- Ghosal, S., V.K. Tripathi, and S. Chauhan. 1996. Active constituent of Emblica officinalis: Part 1st the chemistry and antioxidant effects of two new hydrolysable tannins, emblicanin A and B. Indian J. Chem. 35:941–948.
- Goswami, N., and G. Sumbali. 2010. Evaluation of plant bulb and rhizome extracts for management of Penicillium rot of Phyllanthus emblica. Ann. Plant Protect. Sci 18(2):415–419.
- Goyal, R.K., R.T. Patil, A.R.P. Kingsly, W. Himanshu, and K. Pradeep. 2008. Status of postharvest technology of aonla in India. A Rev. Am. J. Food Technol. 3:13–23. doi: 10.3923/ajft.2008.13.23.
- Gramaje, D., J. Armengol, D. Salazar, I. López-Cortés, and J. García-Jiménez. 2009. Effect of hot-water treatments above 50°C on grapevine viability and survival of petri disease pathogens. Crop Prot. 28(3):280–285. doi: 10.1016/j.cropro.2008.11.002.
- Gudapaty, P., S. Indavarapu, G.R. Korwar, A.K. Shankar, R.K.V. Adake, V. Bandi, and S.R. Kanchu. 2010. Effect of open air drying, LPG based and pre-treatments on the quality of Indian gooseberry (aonla). J. Food Sci. Technol. 47(5):541–548. doi: 10.1007/s13197-010-0114-1.
- Gupta, N., and V.B. Singh. 2016. Pre-harvest foliar application of calcium chloride, bavistin and bayleton on post-harvest life of emblica officinalis Gaertn. fruits. Bangladesh J. Bot. 45(1):211–219.
- Hassan, A., A.K. Sinha, and P.K. Mishra. 2014. Studies on ascorbic acid (Vitamin-C) content in different citrus fruits and its degradation during storage. Sci. Cult. 80(9–10):265–268.
- Hooper, D., and H. Field. 1937. Useful plants and drug, of Iran and Iraq. Publ. 387. Bot Ser. Vol 1, No.3. Field Museum of Natural History, Chicago.
- Huang, J.L., and Z.G. Zhong. 2011. Study of galic acid extracted from the leaves of phyllanthus emblica on apoptotic mechanism of human hepatocellular carcinoma cells BEL-7404. Zhong Yao Cai 34:246–249.
- Huang, Y., B.L. Wild, and S.C. Morris. 1992. Postharvest biological control of Penicillium digitatum decay on citrus fruit by Bacillus pumilus. Ann. Appl. Biol. 120:367–372. doi: 10.1111/j.1744-7348.1992.tb03433.x.
- Ihantola-Vormisto, A., J. Summanen, and H. Kankaanranta. 1997. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 63:518–524. doi: 10.1055/s-2006-957595.
- Jamaluddin, A. 1978. Cladosporium rot of fruits of Phyllanthus emblica caused by Phomopsis phylanthi punith and its chemical control. Nat. Accd. Sci. India. 5(6):183–185.
- Jamaluddin, A., M.P. Tandon, and R.N. Tandon. 1975. A fruit rot of aonla caused by phoma. Proc. Natl. Acad. Sci. India. 8(45):75–77.
- Jamwal, K.S., I.P. Sharma, and L. Chopra. 1959. Pharmacological investigations on the fruits of Emblica officinalis. J. Sci. Ind. Res. 18:180–181.
- Jat, R.G., S.K. Goyal, and S. Choudhary. 2013. Management of Aspergillus and blue mould rot of anola fruits. Asian J. Bio. Sci. 8(1):91–93.
- Kamthan, K.P., R. Misra, and A.K. Shukla. 1981. Nigrospora fruit rot of Emblica officinalis, a new disease record. Sci. Cult. 47:371–372.
- Kim, Y., A.J. Lounds-Singleton, and S.T. Talcott. 2009. Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.). Food Chem 115:989–993. doi: 10.1016/j.foodchem.2009.01.019.
- Kore, V.T., H.L. Devi, and J. Kabir. 2013. Packaging, storage and value addition of aonla, an underutilised fruit, in India. Fruits 68(3):255–266. doi: 10.1051/fruits/2013064.
- Krokida, M.K., and D. Marinos-Kouris. 2003. Rehydration kinetics of dehydrated products. J. Food Eng. 57:1–7. doi: 10.1016/S0260-8774(02)00214-5.
- Kulkarni, S.N., and O.P. Sharma. 1971. Ascorbic acid status and macerating enzyme production in fruits of Emblica officinalis Gaertn. infected with Penicillium oxalicum Currie and Thom. JNKVV Res. J 5(2):131–132.
- Kumar, P.S., and V.R. Sagar. 2009. Influence of packaging materials and storage temperature on quality of osmo-vac dehydrated aonla segments. J. Food Sci. Technol. 46(3):259–262.
- Kumar, S., S. Kumar, J.K. Sandooja, M. Sharma, R. Lathar, and H. Mir. 2009. Studies on the post-harvest application of different botanicals on the shelf life of aonla (Emblica officinalis Gaertn) cv. Chakaiya. Haryana J. Hort. Sci. 38:40–42.
- Lal, B., A. Arya, and R.N. Rai. 1982. A new soft rot of aonla caused by Phomopsis phyllanthi Punith and its chemical control. Nat. Acad. Sci. Lett. 5(6):183–185.
- Liu, X., C. Cui, M. Zhao, J. Wang, W. Luo, B. Yang, and Y. Jiang. 2008. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem 109:909–915. doi: 10.1016/j.foodchem.2008.01.071.
- Liu, X., M. Zhao, K. Wu, X. Chai, H. Yu, and Z. Tao. 2012. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.). Food Chem 131:685–690. doi: 10.1016/j.foodchem.2011.09.063.
- Macmillan, H.F. 1943. Tropical gardening and planting with special reference to ceylon. 5th ed. Macmillan & Co., Ltd., London, UK.
- Mahata, S., A. Pandey, S. Shukla, A. Tyagi, S.A. Husain, and B.C. Das. 2013. Anticancer activity of Phyllanthus emblica Linn. (Amla): Inhibition of transcription factor AP-1 and HPV gene expression in cervical cancer cells. Nutr. Cancer. 65(Suppl. 1):88–97. doi: 10.1080/01635581.2013.785008.
- Manjula, K., G.K. Kishore, and A.R. Podile. 2004. Whole cells of Bacillus subtilis AF 1 proved more effective than cell-free and chitinase-based formulations in biological control of citrus fruit rot and groundnut rust. Can. J. Microbiol. 50(9):737–744. doi: 10.1139/w04-058.
- Mayes, S., F.J. Massawe, P.G. Alderson, J.A. Roberts, S.N. Azam-Ali, and M. Hermann. 2012. The potential for underutilized crops to improve security of food production. J. Exp. Bot. 63(3):1075–1079. doi: 10.1093/jxb/err396.
- Mishra, A., and A. Shivpuri. 1983. Anthracnose a new disease of aonla. Indian Phytopath. 36:406–407.
- Nath, V., I.S. Singhand, and S. Kumar. 1992. Evaluation of aonla cultivars for their shelf life at ambient temperature. Narendra Deva J. Agric. Res 7(1):117.
- Nath, V., and R.K. Sharma. 1998. Screening of aonla (Emblica officinalis Gaertn.) cultivars for processing. Prog. Hort. 30(2–1):67–77.
- Olesen, T., L. Nacey, N. Wiltshire, and S. O’Brien. 2004. Hot water treatments for the control of rots on harvested litchi (Litchi chinensis Sonn.) fruit. Postharvest Biol. Technol. 32:135–146. doi: 10.1016/j.postharvbio.2003.10.009.
- Pandey, G., B.D. Sharma, D.K. Hore, and N.V. Rao. 1993. Indigenous minor fruits genetic resources and their marketing status in north-eastern hill of India. J. Hill Res. 6(1):1–4.
- Pandey, R.S., D.N. Shukla, D.V.S. Khati, and S.N. Bhargava. 1980. A new fruit rot of Phyllanthus emblica. Indian Phytopathol. 27:121–122.
- Pandey, R.S., S.N. Bhargave, D.N. Shukla, and D.K. Divedi. 1984. Two new fruit diseases of aonla caused by Alternaria sp. Int. J. Trop. Pl. Dis. 2:79–80.
- Pathak, P.K., D. Preeti, and S. Kumar. 2009. Effect of postharvest treatments on shelf life of aonla (Emblica officinalis) fruits damaged during harvesting. J. Food Sci. Technol. 46:283–285.
- Pathak, R.K. 2003. Status report on genetic resources of amla – aonla (Emblica officinalis Gaertn.) in South and Southeast Asia. M.V., Bhag, R. Rao, and R.K. Arora (Eds). IPGRI Office for South Asia, New Delhi, India.
- Perianayagam, J.B., S. Narayan, G. Gnanasekar, A. Pandurangan, S. Raja, K. Rajagopal, R. Rajesh, P. Vijayarakumar, and S.G. Vijaykumar. 2005. Evaluation of antidiarrheal potential of Emblica officinalis. Pharm. Biol. 43(4):373–377. doi: 10.1080/13880200590951856.
- Perinayagam, J.B., S.K. Sharma, A. Joseph, and A.J. Christina. 2004. Evaluation of antipyretic and analgesic activity of Embelica officinalis. J. ethnopharmacol. 95(1):83–85. doi: 10.1016/j.jep.2004.06.020.
- Pimenta, R.S., F.L. Silva, J.F.M. Silva, P.B. Morais, D.T. Braga, C.A. Rosa, and A. Corrêa Jr. 2008. Biological control of Penicillium italicum, P. digitatum and P. expansum by the predacious yeast Saccharomycopsis schoenii on oranges. Braz. J. Microbiol. 39(1):33–38. doi: 10.1590/S1517-83822008000100020.
- Rathod, G.M. 2010. Survey of post-harvest fungal diseases of some fruits from Marathwada Regions of Maharashtra, India. J. Ecobiotechnol. 2:7–10.
- Rawat, R.B.S., and R.C. Uniyal. 2003. Aonla – An important species for Indian systems of medicine. National seminar on Production and utilization of Aonla, August, 8–10, Salem, Tamil Nadu, India.
- Reddy, V.D., P. Padmavathi, M. Paramahamsa, and N.C. Varadacharyulu. 2010. Amelioration of alcohol-induced oxidative stress by Emblica officinalis (amla) in rats. Indian J. Biochem. Biophys. 47:20–25.
- Sagar, V.R., and S.R. Kumar. 2010. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 47:15–26. doi: 10.1007/s13197-010-0114-1.
- Scartezzini, P., F. Antognoni, and M.A. Raggi. 2006. Vitamin C content and antioxidant activity of the fruit and of the ayurvedic preparation of Emblica officinalis Gaertn. J. Ethnopharmacol. 104:113–118. doi: 10.1016/j.jep.2005.08.065.
- Setty, K.G.H. 1959. Blue mould of aonla fruits. Curr. Sci. 28:27–28.
- Sharma, N., and S. Srivastava. 2014. Hot water and UV C as methods of physical control in postharvest losses of Emblica officinalis Gaertn. Int. J. Curr. Microbiol. App. Sci 3(1):487–493.
- Sharma, R., and G. Sumbali. 2009. Bio-efficacy of Lactobacillus acidophilus against aonla fruit rot incited by Penicillium funiculosum. Ann. Pl. Prot. Sci. 17(1):266–268.
- Shi, J.X., M.L. Maguer, S.L. Wang, and A. Liptay. 1997. Application of osmotic treatment in tomato processing-effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res. Int. 30(9):669–674. doi: 10.1016/S0963-9969(98)00031-3.
- Singh, B.K., S. Singh, and S.M. Yadav. 2014. Packaging, post harvest treatments, storage and protection of aonla and their role in health care: an overview. Research Journal Of Microbiology 9:216-220. doi:10.3923/jm.2014.216.220.
- Singh, R., and S. Kumar. 1987. Studies on the effect of different storage conditions on decay loss of aonla var. Chaikaiya. Haryana J. Hort. Sci. 26:9–12.
- Singh, S., A.K. Singh, H.K. Josh, B.G. Bagle, and D.G. Dhandar. 2009. Evaluation of packages for transportation and storability of aonla (Emblica officinalis) under semi arid environment of western India. J. Food Sci. Technol. 46:127–131.
- Srivastava, M.P., S. Chandra, and R.N. Tandon. 1964. Postharvest diseases of some fruits and vegetables. Proc. Natl. Acad. Sci. India. 34(4):339–342.
- Sturrock, D. 1959. Fruits for southern. Florida. Southeastern Printing Co., Stuart, Florida.
- Tandon, R.N., and M.P. Shrivastava. 1964. Fruit rot of aonla caused by Pestalotia cruenta Syd. in India. Curr. Sci. 33(3):86–87.
- Tarwadi, K.V., and V. Agte. 2007. Antioxidant and micronutrient potential of common fruits available in the Indian subcontinent. Int. J. Food Sci. Nutr. 58(5):341–349. doi: 10.1080/09637480701243905.
- Tasduq, S.A., P. Kaisar, D.K. Gupta, B.K. Kapahi, H.S. Maheshwari, and S. Jyotsna. 2005. Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother. Res. 19:193–197. doi: 10.1002/ptr.1631.
- Thakur, C.P., and K. Mandal. 1984. Effect of Emblica officinalis on cholesterol-induced atherosclerosis in rabbits. Indian J. Med. Res. 79:142–146.
- Thakur, R.S., H.S. Puri, and A. Husain. 1989. Major medicinal plants of India. Central Institute of Medicinal and Aromatic Plants, Lucknow, India.
- Thom, C. 1930. Blue mold of aonla (Phyllanthus emblica L.). Mycologia 6:210–211.
- Vishwakarma, S.K., H.K. Shukla, B.K. Singh, and A.K. Singh. 2012. Effect of different gauge polythene and newspaper on storage life of kanchan cultivar of aonla (Emblica officinalis Gaertn. Sy. Phyllanthus emblica L.). Environ. Ecol. 30(2):424–426.
- Wakade, P.S., S. Yeturi, and G.D. Mate. 2015. In vitro evaluation of bioagents and fungicides against fruit rot complex of aonla (Emblica officinalis). Environ Ecol 33(3A):1391–1395.
- Webster, G.L. 1956. A monographic study of the West Indian species of Phyllanthus (continued). J. Arnold. Arbor. 37(4):340–359.
- Wisniewski, M.E., and C.L. Wilson. 1992. Biological control of postharvest diseases of fruits and vegetables: Recent advances. Hort. Sci. 27:94–98.
- Yadav, S.M., R.K. Patil, and D.L. Yadav. 2012b. Biological control of Penicillium fruit rot (Penicillium funiculosum Thom.) of aonla. Green Farming. 3(1):78–79.
- Yadav, S.M., R.K. Patil, S. Singh, L.P. Balai, and R.A. Kumar. 2012a. Eco-chemical management of a new fungal rot (Penicillium Funiculosum Thom.) of aonla. Bioscan. 7(4):649–651.
- Yadav, S.M., R.K. Patil, S. Singh, L.P. Balai, and R.A. Kumar. 2013. Use of botanical products for breakdown the parasitize of new recorded Penicillium funiculosum of Aonla. Plant Pathol. J. 12(2):120–123. doi: 10.3923/ppj.2013.120.123.
- Yang, C.J., C.S. Wang, J.Y. Hung, H.W. Huang, Y.C. Chia, and P.H. Wang. 2009. Pyrogallol induces G2-M arrest in human lung cancer cells and inhibits tumor growth in an animal model. Lung Cancer 66:162–168. doi: 10.1016/j.lungcan.2009.01.016.
- Yokozawa, T., H.Y. Kim, H.J. Kim, T. Okubo, D.C. Chu, and L.R. Juneja. 2007. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br. J. Nutr. 97(6):1187–1195. doi: 10.1017/S0007114507691971.

