?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The search for new nutritional alternatives that favor human health is related to one of the world’s tendencies in science and food technology nowadays: research on food with functional properties (antioxidant activity, prebiotic activity, intestinal motility, among others), mainly regions’ autochthonous products, from which the productive sector can benefit thanks to its transformation and added value generation. In this review, the importance of four Andean food items with the potential to be explored and maximized by obtaining functional products is described. Because of the fact that blackberry, yacón, açaí and yellow pitahaya are promissory Andean foods with exceptional qualities for consumers’ health, information was gathered about studies and possible effects in treatment of diseases, and the most used methods for the product and therefore for their biocompounds. It was concluded that this kind of food items represents important alternatives for the transformation and extraction of biocompounds (pigments, antioxidants, fructo-oligosaccharides, fiber, among others), in which non-thermal technologies play a fundamental role in conserving their functional properties, and at the same time, strengthen rural agro-industry and the exploitation of autochthonous products for strengthening region’s and world’s economy.
1. Introduction
In the past few years, the issue of malnutrition has increased, due to the inappropriate eating habits associated with the unbalanced intake of nutrients, caused by the accelerated life style and excessive intake of processed foods with high amounts of additives and artificial preservatives (WHO Citation2015).
It has been reported that the main causes of death in the world are attributed to non-communicable diseases (NCD) (38 million people a year), from which 75% occur in countries with low – and middle-income (World Health Organization, Citationn.d.) (Plummer et al., Citation2016). According to the World Health Organization (World Health Organization, Citationn.d.) among the most common NCDs are: cardiovascular and gastrointestinal diseases, irritable bowel syndrome, arthritis, diabetes, hypercholesterolemia and cancer.
Due to the above, the new research trends in food, agriculture and health areas have focused on developing functional foods, to prevent and/or reduce the cases of NCD, in which 30% are due to food and behavioral factors (Plummer et al., Citation2016).
Currently, there are many food items classified as functional; among them, we can find fruits, vegetables, cereals and tubers, and although there is still no normalized definition of a functional food item, any food can be considered as such, if it is satisfactorily proven that it provides a beneficial action in one or more body functions, beyond its nutritional effects, in a way that it turns out relevant whether to improve health and well-being or to reduce the risk of disease how cardiovascular diseases, cancer and diabetes (Quintin et al., Citation2019; Roberfroid, Citation2000).
On the other hand, Andean crops that were historically part of the population’s diet and in many cases used as medicinal plants, they are now being underutilized, losing these foods’ potential to get innovative products with excellent nutritional and sensory properties (Jacobsen et al., Citation2003). All of this, thanks to their biocompounds, these crops can act as functional foods, that might supply the population’s needs and produce an added value.
The implementation of these crops has positive social and economic effects, since the food and agriculture sector is facing multiple challenges, because it must produce more food for a growing population in order to guarantee food sovereignty; according to The Six Pillars of Food Sovereignty, developed at The Nyeleni International Steering Committee (Citation2007), there are based on six pillars, where Andean crops and agro-industry play a fundamental role, given that the need for food is prioritized, classified as something else than just a commodity. Food Sovereignty respects producers’ work stimulating sustainable development, in a way that, control is established over production and territory, avoiding the privatization of natural resources and promoting both knowledge and traditional skills such as scientific research, with the purpose of developing new technologies to optimize and make the most of these crops’ potentialities in the inclusion of the worlds’ new tendencies, like a competitive advantage for regions. The same way, as the availability of food increases, imports decrease and exports increase, encouraging small and medium-sized producers (Jacobsen et al., Citation2003).
Additionally, the use of autochthonous crops and wild plants has an environmental impact, because it favors biodiversity, through the preservation of natural resources, avoiding monoculture sowing methods with fewer agrochemicals (Jacobsen et al., Citation2003), therefore a reduction in the genetic erosion for future Andean generations.
Therefore, this review provides information about the importance of Andean foods (such as blackberry, yacón, açai and yellow pitahaya), and the physicochemical and functional properties of some of them, through the collection of studies on possible effects on the treatment and/or prevention of diseases (Campos et al., Citation2012; Da Silveira et al., Citation2019; Diaz et al., Citation2017; Gowd et al., Citation2019, Citation2018; Tibério de Jesus et al., Citation2019; De Souza et al., Citation2016; Oliveira-Barbosa et al., Citation2016; Sousa et al., Citation2015; Torres et al., Citation2017; Van de Velde et al., Citation2018). In addition, it brings together different stabilization techniques for its biocompounds, as well as advances in its application in the food sector.
2. Andean Food, physicochemical and functional properties
The Andean countries in terms of biodiversity have comparative and competitive advantages over other regions, thanks to their geographical location that attributes them ecological factors such as luminosity, temperature and humidity, which are conducive to the growth of native and tropical crops; with essential characteristics, as an alternative to nutrition problems in much of the world (Gordillo and Obed, Citation2013; Jacobsen et al., Citation2003).
In the last few decades, the food and pharmaceutical industries have focused on the exploitation of these crops to prevent and/or treat diseases through scientific research, since the market is in search of healthy foods of plant origin that meet the nutritional requirements ideal for human development with the possibility of supplying or fortifying foods that are currently of industrial production (Rojas-Llanes et al., Citation2014; The Nyéleni, Citation2007; International Steering Committee, Citation2007).
The importance of Andean crops in the agri-food sector is that the variety of food is increased through the use of natural resources available in the region, improving the nutritional status of the daily diet with a greater and better contribution of nutrients and/or biocompounds like proteins, carbohydrates, vitamins, minerals, fiber and antioxidants (Quintin et al., Citation2019); in the case of blackberry, as a Andes berry or “mora de Castilla” is highly consumed in Colombia where annual production surpassing 1000 tonnes (Osorio et al., Citation2012); the major productor of açaí is Brazil, although the US, Japanese and European markets have an interest in açaí (Neri-Numa et al., Citation2018). In addition, many of these crops are resistant to adverse environmental conditions, avoiding periods of seasonal scarcity, or pests and diseases, acting as a biological barrier for other plants, helping to conserve the soil and increasing fertility (Rojas et al., Citation2010).
Based on the above, the Andean foods studied in the review are blackberry, yacon, açaí and yellow pitahaya, which have different potentialities that make them excellent sources of beneficial compounds for the organism.
2.1. Blackberry
Blackberry, a kind of fruit belonging to the genus Rubus (Rosaceae family, Rubus genus) (Gowd et al., Citation2018) is a worldwide-distributed fruit (Zia-ul-haq et al., Citation2014); both blackberries’ native species and improved cultivars are harvested (Clark and Finn, Citation2014). Andean blackberry, or Castilla blackberry (Rubus glaucus Benth.), is native from tropical zones in America, mainly Colombia and Ecuador (Arozarena et al., Citation2012), the fruits consist of many small drupes on a receptacle about 1–2.5 cm long and is distinguished by its dark reddish color and bitter taste () (Osorio et al., Citation2012; Rojas-Llanes et al., Citation2014).
Castilla blackberry is a very coveted fruit in the national and international market (Bowen-Forbes et al., Citation2010; Sánchez, Citation2012), because it has a high nutritional content of dietary fiber, folic acid, antioxidants, vitamins (vitamin C, vitamin K) and minerals (calcium, iron, potassium) (Kaume et al., Citation2012; Rodríguez-Barona et al., Citation2015; Sariburun et al., Citation2010); Marques et al. (Citation2010) report berries have rich source of an antioxidant such as ascorbate, beta-carotene, glutathione, alpha tocopherol, anthocyanins and phenolic compounds (Marques et al., Citation2010).
However, it has critical points after its harvest, since it is highly perishable and has a very fragile structure; these factors lead to a shelf life of three to five days in environmental conditions because during the storage and transportation processes a big amount of product is lost (Franceschinis et al., Citation2014). Blackberries are very complex when it comes to genetic background, growth characteristics, and number of species; and they’re of special interest compared to other fruits, due to the fact that it has been proven that they contain a big amount of antioxidants, such as phenolic components (ellagic acid, tannins, quercetin, gallic acid and anthocyanins) (Dai et al., Citation2009; Franceschinis et al., Citation2014; Huang et al., Citation2012; Kaume et al., Citation2012); on the other hand, the stability of anthocyanins depends on numerous factors such as temperature, light, oxygen, pH and the presence of co-pigments (Weber et al., Citation2017). Studies developed by de Souza and others (Citation2014) state that their intake has a positive impact on human health, it would also prevent some diseases as shown in , which compiles some research done by different authors who evaluated the physicochemical and functional properties of blackberry, as the presence of phenolic compounds with antioxidant capacity.
Table 1. Functional properties of biocompounds found in blackberries
There are many authors that studied the effects of blackberry and its biocompounds on models and line cells. Gomes, et al. (Citation2019) evaluated the effects of blackberry juice supplementation on cisplatin-induced renal pathophysiology in mice, and found protection against histopathological alterations through decreased in urea and creatinine levels and modulation of catalase enzyme activity. Meireles, et al. (Citation2015) analyzed the supplementation with blackberry extract in a context of high-fat or standard diet, in Wistar rats; finding a slightly improved glucose metabolism and significantly decreased levels of lactate, independently of diet. Serino et al. (Citation2020) obtained positive Nox1 expression and atherosclerosis results in male mice that were fed low-fat, high-fat or high-fat supplemented with 2% freeze-dried blackberry powder diets for 5 weeks. Regarding behavioral parameters, oxidative stress and inflammatory markers in a model of mania, Chaves et al. (Citation2020) found that the treatment with blackberry extract was able to prevent hyperlocomotion and oxidative damage in the cerebral cortex, hippocampus, and striatum.
On the other hand, extracts rich in antioxidants have also been studied by various authors. Feresin, et al. (Citation2016) investigated the role of blackberry polyphenol extracts in attenuating angiotensin II–induced senescence in vascular smooth muscle cells (VSMCs). They observed an increment in superoxide dismutase 1 expression, attenuation of the up-regulation of Nox1 expression and the phosphorylation induced by Ang II, and reduced senescence in response to Nox1 overexpression. Gowd et al. (Citation2019) found that gut metabolites of blackberry anthocyanins improved the glucose consumption and glycogen content significantly in HepG2 cells.
Human research has also been carried out as reported by Solverson et al. (Citation2018). The effects of berry intake on energy substrate use and glucoregulation in volunteers consuming a high-fat diet were evaluated. This treatment resulted in a significant reduction in average 24 h respiratory quotient, indicating increased fat oxidation; and the insulin AUC was significantly lower compared to the control.
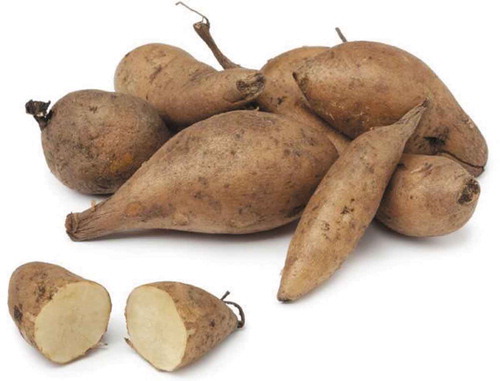
2.2. Yacon
Yacon (Smallanthus sonchifolius) is an autochthonous tubber from the Andean region (Castro et al., Citation2017) (), it is studied and under-used, and it belongs to the Compositae family (Caetano et al., Citation2016). It is a productive crop that grows at a 1000–3200 M.A.S.L. altitude, under heat or cold conditions and it doesn’t have any diseases or plagues-related problems due to the protective effects of its di and sesquiterpenes (Sousa et al., Citation2015). For these reasons yacon can be considered an interesting and promissory crop with high economic and environmental value (Sousa et al., Citation2015). Although production has developed in a traditional way since pre-Hispanic cultures, it is in Peru where production takes place in the north and south with approximately 600 ha planting areas and the market has grown in recent years, developing agroindustrial products with added value such as syrup, flakes, flour, chips that generate income of importance to farmers from 2771 kg of gross volume in 2001 to 44217 kg of gross volume in 2012 where the largest item in the destination market is the United States (Comisión CODEX Alimentarius, Citation2012).
It is a new herbaceous evergreen plant that has been expanded to many countries such as Japan, New Zealand, Czech Republic and Brazil (Brites et al., Citation2016; Oliveira et al., Citation2013), however, it is native from the Andean region, where its tuberous roots are eaten as food because of its juiciness and sweet taste (Caetano et al., Citation2016; Habib et al., Citation2011). Since most of the roots’ biomass is composed of water (86%-90% of fresh weight), the energetic value is low; besides, it contains between 60% and 70% of the dry weight in carbohydrates, mainly β-(2,1) fructo-oligosaccharides (FOS), with a low polymerization (3–10 fructans) (Castro et al., Citation2017). For this reason, yacon’s root has been considered a functional food item (Brites et al., Citation2016; Habib et al., Citation2011). It is also rich in phenolic compounds, especially chlorogenic acid, ferulic acid and some other caffeic acid derivatives (Simonovska et al., Citation2003); it has a long history of safe use in South America and in other places with possible beneficial properties for health, including prebiotic, antidiabetic, antioxidant and antimicrobial effect (Campos et al., Citation2016). The former being said, it is highly important to explore its most relevant biological properties in order to encourage the industry to value its richness (Sousa et al., Citation2015).
FOS are sugars naturally found in many edible plants as onions and garlic, but never in such high concentrations as yacon roots. The rise in the use of FOS as food ingredient has started researches on their possible effects on health (Caetano et al., Citation2016; Habib et al., Citation2011).
Due to the previous, it is extremely important to explore its most relevant biological properties in order to encourage the industry to value its richness (Sousa et al., Citation2015), since yacon’s consumption is related to the promotion of human health benefits (See ), such as hypoglycemic effects, chemopreventive potential agent against colon cancer, and improved gastrointestinal function (Caetano et al., Citation2016; Genta et al., Citation2009; Sousa et al., Citation2015).
Table 2. Functional properties of the parts/biocompounds found in yacon
Many preparations of yacon demonstrated effects in many health disruptions with clinical or in vivo trials in yacon syrup, Adriano et al. (Citation2019) founded has a postprandial decreasing effect glucose and insulin concentrations in adult, the study was performed randomized, crossover, double-blind clinical intervention with 40 women, consumed breakfast+ 40 g of yacon syrup with 14 f of fructooligosacharides (FOS), in blood measured postprandial glucose and insulin.
In yacon flour Habib et al. (Citation2011) found beneficial effects on diabetes-associated hyperlipidemia in Wistar rats with the administration of flour yacon was in tablets containing level of FOS (340 or 6800 mg/kg body weight per day); Vaz-Tostes et al. (Citation2014) improved intestinal immune response increased the serum levels of IL-4 and fecal sIgA in preschool children received yacon flour in preparations for 18 week (0.14 g FOS/kg of body per day); Grancieri et al. (Citation2017) observed that beneficial effects on the intestinal health of animals with induced colorectal cancer induced in Wistar rats, the animals that received supplementation with 7.5% FOS of yacon flour for 8 weeks.
In yacon-based product was evaluated for Marcon et al. (Citation2019) improves satiety and mucosal integrity, and possibly favor anti-inflammatory immune responses in the colon with increased the number of regulatory T cells, and regulated the expression of RORγt transcription evaluated in BALB/c male mice supplemented with 6.0% FOS + inulin for 8 weeks.
The leaf, Martinez-Oliveira et al. (Citation2018) mentioned how leaf present effective neuroprotection than the root evaluated in Wistar rats induced neurotoxicity (- amyloid (A
) and supplemented with extracts of the leaves or roots for 14 days measured memory tests and oxidative stress parameters and biochemical parameters regarding toxicogenetic in leaves, Moreira Szokalo et al. (Citation2020) evaluating the in vitro effect of aqueous extract was prepared to that commonly used in popular medicine, 2 g placed in boiling water and left/20 minutes, the extract was performed in thin layer chromatography and high-performance liquid chromatography to identify the main compounds, other test to determine the range of doses and the cytochalasine B-blocked micronucleus (cytome assay) for geneotoxicity, the results to determine safe consumption as a tea infusion of 2% and the inability of the metabolic system to counteract the genetic instability.
2.3. Açaí
Açaí (Euterpe oleracea Mart.) belongs to the Arecaceae family and is a South America native palm tree especially from the Amazonian plain (El Morsy et al., 2015; Xie et al., Citation2011; Yamaguchi et al., Citation2015).
Açaí is an economically significant fruit in the Amazonian region of Brazil and it is exported to other regions worldwide () (Garzón et al., Citation2017); it is used to make drinks and has been used for years in native Amazon communities in countries such as Brazil, Venezuela, Ecuador, Suriname and Colombia; It is sold fresh in the international market to countries such as the United States, Europe, Japan; Brazil being the largest producer and exporter (Alberto Rojano et al., Citation2011). It is rounded and weighs 2 grams approximately, 17% is edible because the seed is the remaining 83%, and its color when it’s ripe is dark almost black purple (De Souza et al., Citation2012). It contains around 13% protein, 48% lipids and 1.5% total sugars; it is rich in phenolic compounds, such as anthocyanins (mainly cyanidin-3-O-glycoside and cyanidin-3-rutinoside), epicatechin, velutina and catechin (Brito et al., Citation2016; Fernandes de Oliveira et al., Citation2016), mono and polysaturated fatty acids, phytosterols and dietary fiber, plus it is a good source of potassium, magnesium, calcium, phosphorus, sodium and vitamins E and B1 (De Souza et al., Citation2012; Lucas et al., Citation2018; Schauss et al., Citation2006).
Açaí’s pulp has gotten plenty of attention in the last few years being one of the new “superfruits”, due to its potential antioxidant and anti-inflammatory properties (Brito et al., Citation2016; Yamaguchi et al., Citation2015), and it has gained participation and popularity in the export markets as an ingredient in functional food, because it can be eaten fresh or in a variety of beverage or food preparations with functional contributions to the diet, beyond its basic nutritional composition (Fragoso et al., Citation2013; Xie et al., Citation2011), as different authors have reported it (see ).
Table 3. Functional properties of biocompounds found in açaí
As mentioned above, the composition of açaí allows it to have some health benefits, classifying it as a functional food. This is why various authors have sought to demonstrate these effects using cell lines or animal models, by ingesting this fruit in different ways. Monge – Fuentes, et al. (Citation2017) used açaí oil in nanoemulsion (NanoA) as a novel photosensitizer for photodynamic therapy (PDT) used to treat melanoma in in vitro and in vivo experimental models. They found that Tumor-bearing C57BL/6 mice treated five times with PDT using açaí oil in nanoemulsion showed tumor volume reduction of 82% in comparison to control/tumor group. Marques et al. (Citation2016) also evaluated the açaí oil, finding that, according to the cytogenetic tests carried out, this product presented no significant genotoxic effects in the analyzed cells, at the three tested doses (30, 100 and 300 mg/kg).
Regarding the extract, more research has been carried out. Machado, et al. (Citation2019) evaluated the anti-inflammatory effect of açaí extract and its mechanism, in macrophages exposed to phytohemagglutinin to induce inflammation; the extract decreased NLRP3 inflammasome levels and reduced pro-inflammatory cytokines, and caused cell cycle arresting and decreased proliferation. Coelho da Mota et al. (Citation2018) demonstrated that the hydroalcoholic extract of açaí seeds treatment had a protective effect against ischemic damage to TRAM flaps in hamsters, improving microvascular blood flow and increasing the survival of flap zones contralateral to the vascular pedicle. Do Carmo et al. (Citation2017) found that the hydroethanolic extracts from six açaí genotypes have a protective effect (13–62%) on SH-SY5Y cells insulted by H2O2 at a concentration of 50 ug/mL by DCFH-DA assay.
On the other hand, some authors have investigated the dehydrated fruit. Choi et al. (Citation2017) supplied pellets containing 5% açaí powder, which reduced the incidences of both colonic adenoma and cancer. In the açaí-treated mice, the myeloperoxidase and proinflammatory cytokines levels in the colon were significantly down-regulated. Fragoso et al. (Citation2018) evaluated the possible protective effects of lyophilized açaí pulp in a colitis-associated carcinogenesis rat model, finding a reduction in the total number of aberrant crypt foci (ACF), ACF multiplicity, tumor cell proliferation and incidence of tumors with high-grade dysplasia.
2.4. Yellow pitahaya
Yellow Pitahaya (Selenicereus megalanthus) belongs to the Cactaceae family and it is a crop originating in the tropical and subtropical regions of America () (Ayala-Aponte et al., Citation2014; Torres Grisales et al., Citation2017), specifically in the northern part of South America (Nerd and Mizrahi, Citation1999); nonetheless, it has been harvested in Vietnam for over 100 years after its introduction by the French (Nerd and Mizrahi, Citation1999); The yellow pitahaya has 20 species (Selenicereusspp). That occur in Bolivia, Peru, Ecuador, Colombia and Venezuela; being the largest producers in Colombia with 43.7% and Israel with approximately 20.8%, exports are made in fresh fruit marketed in specialty delicatessens as well as in hotels in Europe (Montesinos et al., Citation2015; Mosquera et al., Citation2011).
The fruit is a medium-sized berry (around 180–250 g), with a yellow skin having nibs and thorns that detach during the ripening process. Its flesh is white, sweet, soft and slightly fibrous and it contains many small digestible black seeds (Nerd and Mizrahi, Citation1999).
Yellow Pitahaya is an exotic fruit very desired for its consumption in many parts of the world, not only for its delicious taste and lush color and shape, but also for its nutritional content and well-known functional and medicinal properties (Ayala et al., Citation2010; Baquero et al., Citation2005; Diaz et al., Citation2017). It has a high content of phenolic compounds and ascorbic acid and it has also been reported to be a β-carotene, lycopene and vitamin E source, with average concentrations of 1.4, 3.4 and 0.26 µg/100 g of the edible part, respectively (Wichienchot et al., Citation2010). It can act as a functional food since you can find beneficial properties in it from the skin to its stem (Diaz et al., Citation2017); however, as observed in , there are not enough studies about its benefits on health or its impact in reducing chronic diseases (De Mello et al., Citation2014; Serna-Cock et al., Citation2013).
Table 4. Functional properties of biocompounds found in the yellow pitahaya
Besides, when in changing temperature storage and in commercialization, it has been found that browning, necrosis and skin’s softening appear as the main factors of damage, which is one of the main causes of post-harvest loss (Baquero et al., Citation2005; Vásquez et al., Citation2016), representing approximately 15% of handling inconvenient. Therefore, it is necessary to employ post-harvest techniques to minimize these losses, to extend the product’s shelf life, or extract the biocompounds of interest in the fruit, so as to produce a higher added value to the sector.
As mentioned, in yellow pitahaya there are numerous studies evaluating the content of antioxidants, vitamins, fatty acids, polyphenols and other compounds with functional potential; however, there are few studies on Selenicereus megalanthus, in vivo studies that demonstrate this effectiveness.
Jauregui and Leon (Citation2018) have evaluated the laxative effect and know in which concentration of the hydroalcoholic extract of the exocarp of the fruit (25%, 50% and 75%) in albino mice; the results, show the laxative effect that the higher concentration of extract, the greater the laxative effect and mentioned to this effect by the presence of active metabolites such as anthraquinones, tannins, mucilages and glycosides; in the other hand, Torres Grisales et al. (Citation2017) evaluated the peristalsis acceleration by measuring feces in biomodels (golden hamster) fed with various parts of this fruit (stem, seed, peel and pulp), found to the seed and pulp show increase of feces in 55.13% and 35.01% respectively. Other author mentioned the effect to the dragon fruit species are Hylocereus and Selenicereus in glycemic control on type 2 diabetes, anticancer property, cardiovascular disease, hypocholestrolemic, prebiotic effect and antimicrobial (Choo, Yian Koh and Pick, Citation2016; Dasaesamoh et al., Citation2016; Ibrahim, et al., Citation2018; Kim et al., Citation2011; Kumar et al., Citation2018)
3. Biocompounds stabilization
Sensorial perception (appearance/color, texture, and flavor) and bioactive compounds’ deterioration limits the shelf life of commercial products and restrain the use of biocompounds for certain applications (Carocho et al., Citation2018; Chung et al., Citation2016). Overall quality and shelf life can be reduced by several factors including microbial growth, water loss, enzymatic browning, lipid oxidation, off-flavor, texture deterioration, rise in respiration rate and senescence process, among others. For this reason, many researches have been carried out to improve their stability to be used thoroughly in food and beverages (Chung et al., Citation2016; Quirós-Sauceda et al., Citation2014; Raybaudi-Massilia and Mosqueda-Melgar, Citation2012). Furthermore, efficacy of functional products in the prevention of diseases depends on the preservation of the bioavailability of active ingredients (Fang and Bhandari, Citation2010). In addition, as discussed by Weber et al. (Citation2017) in their research on the stability of spray-dried blackberry anthocyanins decreasing and controlling moisture favors the conservation and stability of functional and physico-chemical characteristics; additionally, the physical chemical behavior as to the retention capacity of biocompounds depends on the material being selected. This is how the use of arabic gum and maltodextrin has been reported as materials that in addition to being economical in scale processes favor the protection of biocompounds (Busch et al., Citation2017; Diaz et al., Citation2017; Di Battista et al., Citation2015; Faridi et al., Citation2016; Gonçalves et al., Citation2014; Jia et al., Citation2016; Mazumder and Ranganathan, Citation2020); as well as spray drying is the technology that is used most because the scaling and energy expenditure can be lower compared to other technologies ().
Table 5. Technologies applied to the biocompounds found in Andean food
The conventional thermal processing used in the food industry is still the most taken technology for the extension of the shelf life and products’ preservation (Tiwari et al., Citation2009a); nevertheless, thermal processing particularly under severe conditions can induce many chemical and physical changes which damage the organoleptic properties and can reduce the content or bioavailability of some bioactive compounds (Qin et al., Citation2019; Rawson et al., Citation2011) and sensory parameters such as color consistency and flavor (Laura Tibério et al., Citation2019). Al-juhaimi et al. (Citation2018) in their study collected different investigations where they show that heat treatments can cause various changes in their physicochemical properties, specifically in the activity of the bioactive compounds present in different fruits and vegetables, like strawberry, tomatoes, spinach, shallots, kale, swamp cabbage, and cabbage; Tembo et al. (Citation2017) mentioned degradation ascorbic acid includes dehydroascorbic acid, 2-furoic acid, 2-furaldehyde, 2,3-diketogulonic acid, 3-deoxypentosone, and low molecular weight compounds; dehydroascorbic acid formation is reversible and compounds have no vitamin C activity and may contribute to change in flavor and odor and is possible to form colored compounds including 5-hydroxymethylfurfural in anaerobic conditions.
The thermal and kinetic characteristics of various types of fruits have been conducted to understand the phenomena that occur during thermal degradation how to mentioned Rueda-Ordóñez et al. (Citation2019) the thermal decomposition to exposure biomass can be divided into three stages: moisture evaporation, volatile release and carbonization, thus, depend on the temperature and time (Laura Qin et al., Citation2019; Rueda-Ordóñez et al., Citation2019; Tibério et al., Citation2019). However, some authors report in particular cooking methods (boiling, microwaving) in mushrooms, bitter melon, broccoli, bitter gourd and water convolvulus preserve or improve the nutritional quality like antioxidant activity, L-ascorbic acid ((NG et al., Citation2011; Ng et al., Citation2019; NG and Rosman, Citation2019; Ng and Tan, Citation2017). Likewise, authors such as Tembo et al. (Citation2017), showed that thermal pasteurization (72 °C, 15 s) retained vitamin C which further showed extended half-life under refrigeration temperature (6 °C).
Hence, consumers’ demand for food and nutritious beverages, that are minimally and naturally treated, has led to the interest in non-thermal technologies. Non-thermal technologies are preservation treatments are effective at environmental or sub lethal temperatures, this way minimizing the negative thermal effects in nutritional parameters and food quality (Qin et al., Citation2019; Tiwari et al., Citation2009). The growing interest in these technologies is not only with the purpose of getting high-quality food with “fresh” characteristics, but also to provide food items with improved functionalities (Rawson et al., Citation2011), as evidenced in the results reported from the use of different technologies by different authors in .
In the region, research has been carried out with the incorporation of different technologies for both extraction and stabilization of these biocompounds in order to generate products with high added value that may have a market in the food, cosmetic, pharmaceutical industries; either as type products raw material or finished products; being spray drying and the use of encapsulation materials such as gum arabic and maltodextrin the most used raw materials for stabilization of biocompounds.
4. Conclusions
Nowadays the demand for natural products has increased due to the interest in nutritious, safe and environmentally responsible foods. Therefore, we can see that Andean foods and tropical fruits represent a potential source of natural food ingredients, creating a great opportunity for the agroindustry where consumers are looking for exotic characteristics and the presence of compounds able to prevent non-communicable chronic diseases, such as antioxidants, fructo-oligosaccharides and pectins; so the blackberry, the yacon, the açaí and the yellow pitahaya, are within the trend of functional foods. However, the short shelf life of these foods has limited their consumption making their inclusion within the new trends not completely explored yet. Nonetheless, these could have a greater application in the food industry by increasing their stability and that of their biocompounds. This way, the use of non-thermal processing and preservation technologies, as well as studies focused on bioactive compounds such as polyphenols and sugars, will probably grow exponentially, due to the higher consumers’ demand of healthy minimally processed food items. Moreover, the use of encapsulated biocompounds, can lead to improvements both on stability and on bioavailability in vivo and in vitro, and optimize paths for their management and exploitation; in the region, spray drying has been implemented and can continue to be implemented, because it is an economic technology of easy scaling and standardization compared to technologies such as lyophilization and freeze drying that involve high energy expenditure as well as expensive equipment in industrial applications that are difficult for agribusiness companies to access.
References
- Adriano, L.S., A.P. Dionísio, F.A.P. Abreu, A.A.F. De, Carioca, G.J. Zocolo, N.J. Wurlitzer, C. Oliveira, A. Cunha, and H.A. Sampaio. 2019. Yacon syrup reduces postprandial glycemic response to breakfast: A randomized, crossover, double-blind clinical trial. Food Research International. 126–108682. doi: org/10.1016/j.foodres.2019.108682.
- Albarici, T.R., and J.D.C. Pessoa. 2012. Effects of heat treatment and storage temperature on the use of açaí drink by nutraceutical and beverage industries. Food Science and Technology. 32(1):9–14. doi: 10.1590/S0101-20612012005000026..
- Alberto Rojano, B., C.Z. Vahos, I. Felipe Alzate, A. Arbeláez, A. Juleza Mosquera Martínez, F. Bernardo Cortés Correa, and L. Gamboa Carvajal. 2011. Polifenoles y Actividad Antioxidante del Fruto Liofilizado de Palma Naidi (açai Colombiano) (Euterpe oleracea Mart). Revista Facultad Nacional De Agronomia 64(2):6213–6220.
- Al-juhaimi, F., K. Ghafoor, M.M. Ozcan, M.H.A. Jahurul, E.E. Babiker, S. Jinap, F. Sahena, M.S. Sharifudin, and I.S.M. Zaidul. 2018. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. Journal of Food Science and Technology. 55:3872–3880. doi: 10.1007/s13197-018-3370-0.
- Alves Filho, E.G., P.J. Cullen, J.M. Frias, P. Bourke, B.K. Tiwari, E.S. Brito, S. Rodriguez, and F.A.N. Fernandes. 2016. Evaluation of plasma, high-pressure and ultrasound processing on the stability of fructooligosaccharides. International Journal of Food Science and Technology. 51(9):2034–2040. doi: 10.1111/ijfs.13175..
- Aranha, L.N., M.G. Silva, S.K. Uehara, R.R. Luiz, J.F. Nogueira Neto, G. Rosa, and G.M. Moraes de Oliveira. 2019. Effects of a hypoenergetic diet associated with açaí (Euterpe oleracea Mart.) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, dyslipidemic individuals. Clinical Nutrition. doi: 10.1016/J.CLNU.2019.06.008..
- Arozarena, I., J. Ortiz, I. Hermosin-Gutierrez, I. Urretavizcaya, S. Salvatierra, I. Cordova, M.R. Marín-Arroyo, M.J. Noriega, and M. Navarro. 2012. Color, ellagitannins, anthocyanins, and antioxidant activity of Andean blackberry (Rubus glaucus Benth.) wines. Journal of Agricultural and Food Chemistry. 60(30):7463–7473. doi: 10.1021/jf300924z..
- Ayala, A., L. Serna, and E. Mosquera. 2010. Liofilizacion de pitahaya amarilla (Selenicereus megalanthus). Vitae 17(2):121–127.
- Ayala Aponte, A., C.J. Giraldo Cuartas, and L. Serna Cock. 2010. Cinéticas de deshidratación osmótica de pitahaya amarilla (Selenicereus megalanthus). Interciencia. 35:539–544.
- Ayala-Aponte, A., L. Serna-Cock, J. Libreros-Triana, C. Prieto, and K. Di Scala. 2014. Influence of osmotic pre-treatment on convective drying of yellow pitahaya. Dyna. 81(188):145–151. Retrieved from doi:10.15446/dyna.v81n188.41321
- Aybar, M.J., A.N. Sánchez Riera, A. Grau, and S.S. Sánchez. 2001. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. Journal of Ethnopharmacology. 74(2):125–132. Retrieved from doi:10.1016/S0378-8741(00)00351-2
- Baquero, L.E., J.A. Castro, and C.E. Narváez. 2005. Catalase, peroxidase and polyphenoloxidase from pitaya amarilla (Acanthocereus pitajaya) fruits: Ripening and Senescense. Acta Biológica Colombiana 10(2):49–60. Retrieved fromhttp://www.scielo.org.co/scielo.php?script=sci_arttextandpid=S0120-548X2005000200004
- Baroni, S., F. Suzuki-Kemmelmeier, S.M. Caparroz-Assef, R.K. Nakamura, and C.A. Bersani-Amado. 2008. Effect of crude extracts of leaves of Smallanthus sonchifolius (yacon) on glycemia in diabetic rats. Revista Brasileira De Ciências Farmacêuticas. 44(3):521–530. doi: 10.1590/S1516-93322008000300024.
- Bickford, P.C., T. Gould, L. Briederick, K. Chadman, A. Pollock, D. Young, B. Shukitt-Hale, and J. Joseph. 2000. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Research. 866(1–2):211–217. doi: 10.1016/s0006-8993(00)02280-0..
- Bowen-Forbes, C.S., Y. Zhang, and M.G. Nair. 2010. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. Journal of Food Composition and Analysis. 23(6):554–560. doi: 10.1016/J.JFCA.2009.08.012..
- Brites, M.L., S.M. Meira, A. Brandelli, and C.Z. Noreña. 2016. Characterization of powder from the permeate of yacon extract by ultrafiltration and dehydrated by spray drying Caracterização de pó do permeado do extrato de yacon obtido por ultrafiltração e desidratado por atomização. Ciência E Agrotecnologia. 40(5):585–595. doi: 10.1590/1413-70542016405013916..
- Brito, C., A.T. Stavroullakis, A.C. Ferreira, K. Li, T. Oliveira, G. Nogueira-Filho, and A. Prakki. 2016. Extract of acai-berry inhibits osteoclast differentiation and activity. Archives of Oral Biology. 68:29–34. doi: 10.1016/j.archoralbio.2016.03.016..
- Busch, V.M., A. Pereyra-Gonzalez, N. Segatin, P.R. Santagapita, N. Poklar Ulrih, and M.P. Buera. 2017. Propolis encapsulation by spray drying: Characterization and stability. LWT - Food Science and Technology. 75:227–235. doi: 10.1016/j.lwt.2016.08.055..
- Caetano, B.F.R., N.A. de Moura, A.P.S. Almeida, M.C. Dias, K. Sivieri, and L.F. Barbisan. 2016. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients. 8(7):1–13. doi: 10.3390/nu8070436.
- Campos, D., A. Aguilar-Galvez, and R. Pedreschi. 2016. Stability of fructooligosaccharides, sugars and colour of yacon (Smallanthus sonchifolius) roots during blanching and drying. International Journal of Food Science and Technology. 51:1177–1185. doi: 10.1111/ijfs.13074..
- Campos, D., I. Betalleluz-Pallardel, R. Chirinos, A. Aguilar-Galvez, G. Noratto, and R. Pedreschi. 2012. Prebiotic effects of yacon (Smallanthus sonchifolius), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chemistry. 135(3):1592–1599. doi: 10.1016/j.foodchem.2012.05.088..
- Carocho, M., P. Morales, and I.C. Ferreira. 2018. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends in Food Science and Technology. 71:107–120. doi: 10.1016/j.tifs.2017.11.008.
- Carvalho Lago, C., and C.P. Zapata Noreña. 2016. Polydextrose as wall material for microencapsulation of yacon juice by spray drying. Food and Bioprocess Technology. 9(12):2103–2113. doi: 10.1007/s11947-016-1797-8..
- Castro, A., F. Vilaplana, and L. Nilsson. 2017. Characterization of a water soluble, hyperbranched arabinogalactan from yacon (Smallanthus sonchifolius) roots. Food Chemistry. 223:76–81. doi: 10.1016/j.foodchem.2016.12.019..
- Cervantes-Elizarrarás, A., J. Piloni-Martini, E. Ramírez-Moreno, E. Alanís-García, N. Güemes-Vera, C.A. Gómez-Aldapa, Q.Y. Zafra-Rojas, and N.D.S. Cruz-Cansino. 2017. Enzymatic inactivation and antioxidant properties of blackberry juice after thermoultrasound: Optimization using response surface methodology. Ultrasonics Sonochemistry. 34:371–379. doi: 10.1016/j.ultsonch.2016.06.009..
- Chaves, V.C., M.S.P. Soares, L. Spohr, F. Teixeira, A. Vieira, L.S. Constantino, F. Dal Pizzol, C.L. Lencina, R.M. Spanevello, M.P. Freitas, et al. 2020. Blackberry extract improves behavioral and neurochemical dysfunctions in a ketamine-induced rat model of mania. Neuroscience Letters. 714:134566. doi: 10.1016/j.neulet.2019.134566.
- Chen, W., Y. Xu, L. Zhang, H. Su, and X. Zheng. 2016. Blackberry subjected to in vitro gastrointestinal digestion affords protection against Ethyl Carbamate-induced cytotoxicity. Food Chemistry. 212:620–627. doi: 10.1016/j.foodchem.2016.06.031..
- Choi, Y.J., Y.J. Choi, N. Kim, R.H. Nam, S. Lee, H.S. Lee, H. Lee, Y.J. Surh, and D.H. Lee. 2017. Açaí Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice. Gut and Liver. 11(2):243–252. doi: 10.5009/gnl16068.
- Choo, J.C., R. Yian, and A. Pick. 2016. Medicinal properties of pitaya: A review. Spatula DD. 6(2):69–76. doi: 10.5455/spatula.20160413015353.
- Choque Delgado, G.T., R. Thomé, D.L. Gabriel, W.M.S.C. Tamashiro, and G.M. Pastore. 2012. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutrition Research. 32(11):884–892. doi: 10.1016/j.nutres.2012.09.012..
- Chung, C., T. Rojanasasithara, W. Mutilangi, and D.J. McClements. 2016. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chemistry. 212:596–603. doi: 10.1016/j.foodchem.2016.06.025..
- Clark, J.R., and C.E. Finn. 2014. Blackberry cultivation in the world. Revista Brasileira De Fruticultura. 36(1):46–57. doi: 10.1590/0100-2945-445/13..
- Comisión CODEX Alimentarius. 2012. Propuesta de nuevo trabajo para una norma regional del codex para el yacón (Smallanthus sonchifolius). Retrieved from http://www.fao.org/tempref/codex/Meetings/CCLAC/CCLAC18/la18_15s.pdf
- Costa, R.G., K. Andreola, R. de Andrade Mattietto, L.J.G. de Faria, and O.P. Taranto. 2015. Effect of operating conditions on the yield and quality of açai (Euterpe oleracea Mart.) powder produced in spouted bed. LWT - Food Science and Technology. 64(2):1196–1203. doi: 10.1016/J.LWT.2015.07.027..
- da Mota, C., D.S. Sicuro, F.L. Resende, A.C. De Moura, R.S. Bottino, and E. Bouskela. 2018. Effects of açaí and cilostazol on skin microcirculation and viability of TRAM flaps in hamsters. Journal of Surgical Research. 228:253–262. doi: 10.1016/j.jss.2018.03.014.
- Da Silveira, T.F.F., M. Cristianini, G.G. Kuhnle, A.B. Ribeiro, J.T. Filho, and H.T. Godoy. 2019. Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization. Innovative Food Science and Emerging Technologies. 55:88–96. doi: 10.1016/J.IFSET.2019.05.001..
- Dai, J., A. Gupte, L. Gates, and R.J. Mumper. 2009. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food and Chemical Toxicology. 47(4):837–847. doi: 10.1016/j.fct.2009.01.016..
- Dasaesamoh, R., W. Youravong, and S. Wichienchot. 2016. Digestibility, fecal fermentation and anti-cancer of dragon fruit oligosaccharides. International Food Research Journal. 23(6):2581–2587. Retrieved fromhttp://www.ifrj.upm.edu.my/23(06) 2016/(38).pdf
- de Gomes, M.G., L. Del Fabbro, A.T. Rossito Goes, L.C. Souza, F. Donato, S.P. Boeira, M. Prigol, and C.R. Jesse. 2019. Blackberry juice anthocyanidins limit cisplatin-induced renal pathophysiology in mice. Pathophysiology. 26(2):137–143. doi: 10.1016/j.pathophys.2019.04.004.
- De Mello, F.R., C. Bernardo, C.O. Dias, L.C. Bosmuler Züge, J.L. Meira Silveira, E.R. Amante, and L.M. Bileski Candido. 2014. Evaluation of the chemical characteristics and rheological behavior of pitaya (Hylocereus undatus) peel. Fruits. 69(5):381–390. doi: 10.1051/fruits/2014028..
- De Mendonça, K.S., J.L. Gomes Corrêa, J.R. de Jesus Junqueira, M.C. de Angelis Pereira, and M. Barbosa Vilela. 2015. Optimization of osmotic dehydration of yacon slices. Drying Technology: An International Journal. 34(4):386–394. doi: 10.1080/07373937.2015.1054511..
- De Souza, L., E.D. Forville, L.E. Neves, A.D.C. Tais, A.M. Chupel, D. Granato, and M.L. Masson. 2016. Evaluation of dried yacon (Smallanthus sonchifolius) as an efficient probiotic carrier of Lactobacillus casei LC-01. LWT - Food Science and Technology. 75:220–226. doi: 10.1016/j.lwt.2016.08.027..
- De Souza, M.O., L. Souza E Silva, C.L. de Brito Magalhães, B.B. de Figueiredo, D.C. Costa, M.E. Silva, and M.L. Pedrosa. 2012. The hypocholesterolemic activity of açaí (Euterpe oleracea Mart.) is mediated by the enhanced expression of the ATP-binding cassette, subfamily G transporters 5 and 8 and low-density lipoprotein receptor genes in the rat. Nutrition Research 32(12):976–984. doi: 10.1016/j.nutres.2012.10.001..
- De Souza, V.R., P.A.P. Pereira, T.L.T. Da Silva, L.C. De Oliveira Lima, R. Pio, and F. Queiroz. 2014. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chemistry. 156:362–368. doi: 10.1016/j.foodchem.2014.01.125..
- Di Battista, C.A., D. Constenla, M.V. Ramírez-Rigo, and J. Piña. 2015. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technology. 286:193–201. doi: 10.1016/j.powtec.2015.08.016..
- Diaz, Y.L., L.S. Torres-Valenzuela, J.A. Serna-Jiménez, and L.I. Sotelo. 2017. Encapsulation effect on spray drying of yellow pitahaya biocomponents of functional interest. Informacion Tecnologica. 28(6):23–34. doi: 10.4067/S0718-07642017000600004..
- Do Carmo, M.R.T., A.B. Von Sulzback, A.V. Carvalho, A. Jablonski, T.K. Rabelo, M.J.C. Fonseca, G.D. Pens, S.F. Hickmann, A.P. Rossini, and R.A. de Oliveira. 2017. Hydroethanolic extracts from different genotypes of açaí (Euterpe oleracea) presented antioxidant potential and protected human neuron-like cells (SH-SY5Y). Food Chemistry. 222:94–104. doi: 10.1016/j.foodchem.2016.12.006.
- Duffy, K.B., E.L. Spangler, B.D. Devan, Z. Guo, J.L. Bowker, A.M. Janas, A. Hagepanos, R.K. Minor, R. DeCabo, P.R. Mouton, et al. 2008. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiology of Aging. 29(11):1680–1689. doi:10.1016/j.neurobiolaging.2007.04.002..
- Elisia, I., C. Hu, D.G. Popovich, and D.D. Kitts. 2007. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chemistry. 101(3):1052–1058. doi: 10.1016/J.FOODCHEM.2006.02.060..
- Fang, Z., and B. Bhandari. 2010. Encapsulation of polyphenols - A review. Trends in Food Science and Technology. 21(10):510–523. doi: 10.1016/j.tifs.2010.08.003..
- Faridi Esfanjani, A., and S. Mahdi Jafari. 2016. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids and Surfaces B: Biointerfaces. 146:532–543. doi: 10.1016/j.colsurfb.2016.06.053..
- Favacho, H.A.S., B.R. Oliveira, K.C. Santos, B.J.L. Medeiros, P.J.C. Sousa, F.F. Perazzo, and J.C.T. Carvalho. 2011. Anti-inflammatory and antinociceptive activities of Euterpe oleracea Mart., Arecaceae, oil. Revista Brasileira De Farmacognosia. 21(1):105–114. doi: 10.1590/S0102-695X2011005000007..
- Feresin, R.G., J. Huang, D.S. Klarich, Y. Zhao, S. Pourafshar, B.H. Arjmandi, and G. Salazar. 2016. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food and Function. 7(10):4175–4187. doi: 10.1039/c6fo00743k.
- Fernandes de Oliveira, L., J.L. Gomes Corrêa, M.C. de Angelis Pereira, A.D.L. Souza Ramos, and M. Barbosa Vilela. 2016. Osmotic dehydration of yacon (Smallanthus sonchifolius): Optimization for fructan retention. LWT - Food Science and Technology. 71:77–87. doi: 10.1016/j.lwt.2016.03.028..
- Ferrari, C.C., S.P. Marconi, I.D. Alvim, and J.M. De Aguirre. 2013. Storage stability of spray-dried blackberry powder produced with maltodextrin or gum arabic. Drying Technology. 31(4):470–478. doi: 10.1080/07373937.2012.742103..
- Fragoso, M.F., G.R. Romualdo, D.A. Ribeiro, and L.F. Barbisan. 2013. Açai (Euterpe oleracea Mart. Feeding Attenuates Dimethylhydrazine-induced Rat Colon Carcinogenesis. Food and Chemical Toxicology. 58:68–76. doi: 10.1016/j.fct.2013.04.011..
- Fragoso, M.F., G.R. Romualdo, L.A. Vanderveer, J. Franco-Barraza, E. Cukierman, M.L. Clapper, F.C. Robson, and L.F. Barbisan. 2018. Lyophilized açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food and Chemical Toxicology. 121:237–245. doi: 10.1016/j.fct.2018.08.078.
- Franceschinis, L., D.M. Salvatori, N. Sosa, and C. Schebor. 2014. Physical and functional properties of blackberry freeze and spray-dried powders. Drying Technology. 32(2):197–207. doi: 10.1080/07373937.2013.814664..
- Garzón, G.A., C.-E.-E. Narváez-Cuenca, J.-P.-P. Vincken, and H. Gruppen. 2017. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chemistry 217(15):364–372. doi: 10.1016/j.foodchem.2016.08.107..
- Genta, S., W. Cabrera, N. Habib, J. Pons, I.M. Carillo, A. Grau, and S. Sánchez. 2009. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clinical Nutrition. 28(2):182–187. doi: 10.1016/j.clnu.2009.01.013..
- Genta, S.B., W.M. Cabrera, A. Grau, and S.S. Sánchez. 2005. Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food and Chemical Toxicology. 43(11):1657–1665. doi: 10.1016/j.fct.2005.05.007.
- Genta, S.B., W.M. Cabrera, M.I. Mercado, A. Grau, C.A. Catalán, and S.S. Sánchez. 2010. Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: Constituents of the most active fractions. Chemico-Biological Interactions. 185(2):143–152. doi: 10.1016/j.cbi.2010.03.004..
- Gonçalves, C., C.B. Dellinghausen, R.C. Zambiazi, J.R. Kuhn, S. Rickes, F.K. Döring, E.B. Valmir, and M.N. Ramos. 2014. Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT - Food Science and Technology. 58(2):527–533. doi: 10.1016/J.LWT.2014.03.042..
- Gordillo, G., and M. Obed J (FAO). 2013. Seguridad y Soberania Alimentaria. Retrieved 12 November 2019, from http://www.fao.org/3/a-ax736s.pdf.
- Gowd, V., T. Bao, L. Wang, Y. Huang, S. Chen, X. Zheng, S. Cui, and W. Chen. 2018. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chemistry. 269(15):618–627. doi: 10.1016/J.FOODCHEM.2018.07.020..
- Gowd, V., T. Bao, and W. Chen. 2019. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Research International. 120:523–533. doi: 10.1016/J.FOODRES.2018.11.001..
- Grancieri, M., N.M. Bruonoro, M. Vaz Tostes, G. das, D.S. de Oliveira, L.D.C. Nunes, L.D.N. Marcon, and M.L. Viana. 2017. Yacon flour (Smallanthus sonchifolius) attenuates intestinal morbidity in rats with colon cancer. Journal of Functional Foods. 37:666–675. doi: org/10.1016/j.jff.2017.08.039.
- Habib, N.C., S.M. Honoré, S.B. Genta, and S.S. Sánchez. 2011. Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: Biochemical approach. Chemico-Biological Interactions. 194(1):31–39. doi: 10.1016/j.cbi.2011.08.009..
- Huang, W., H. Zhang, W. Liu, and C. Li. 2012. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. Journal of Zhejiang University. Science. 13(2):94–102. doi: 10.1631/jzus.B1100137.
- Ibrahim, S. R. M., Mohamed, G. A., Khedr, A. I. M., Zayed, M. F., El-Kholy, A. A. S. 2018. Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance-A review. Journal of Food Biochemistry 42:e12491. https://doi.org/10.1111/jfbc.12491
- Jacobsen, S.E., A. Mujica, and R. Ortiz. 2003. La importancia de los cultivos andinos. Revista Venezolana De Sociología Y Antropología. 13(36):14–24. Retrieved fromhttps://www.redalyc.org/articulo.oa?id=70503603
- Jauregui, K.K., and M.I. Ramos. 2018. Efecto laxante del extracto hidroalcohólico del exocarpo del fruto de Hylocereus megalanthus (pitahaya) en ratones albinos (Universidad María Auxiliadora). Retrieved from. http://repositorio.uma.edu.pe/handle/UMA/167
- Jia, Z., M.J. Dumont, and V. Orsat. 2016. Encapsulation of phenolic compounds present in plants using protein matrices. Food Bioscience. 15:87–104. doi: 10.1016/j.fbio.2016.05.007..
- Kaume, L., L.R. Howard, and L. Devareddy. 2012. The Blackberry Fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. Journal of Agricultural and Food Chemistry. 60(23):5716–5727. doi: 10.1021/jf203318p.
- Khayat. 2005. Comportamento do risco para doença aterosclerótica coronária na população de Inhangapí cuja base alimentar é o fruto do açaí (Euterpe oleracea). Trabalho de conclusão de curso. (Graduação em Medicina) - Universidade Federal do ParáUniversidade Federal do Pará. Retrieved 12 November 2019, from http://repositorio.unicamp.br/jspui/handle/REPOSIP/91144
- Kim, H.J., H.K. Choi, J.Y. Moon, Y.S. Kim, A. Mosaddik, and S.K. Cho. 2011. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. Journal of Food Science. 76(1):C38–45. doi: 10.1111/j.1750-3841.2010.01908.x.
- Kopjar, M., K. Jakšić, and V. Piližota. 2012. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. Journal of Food Processing and Preservation. 36(6):545–552. doi: 10.1111/j.1745-4549.2011.00631.x..
- Kopjar, M., N.N. Tiban, V. Pilizota, and J. Babic. 2009. Stability of anthocyanins, phenols and free radical scavenging activity through sugar addition during frozen storage of blackberries. Journal of Food Processing and Preservation 33(1):1–11. doi: 10.1111/j.1745-4549.2008.00244.x..
- Kumar, S.B., R. Issac, and M. Lakshmi Prabha. 2018. Functional and health-promoting bioactivities of dragon fruit. Drug Invention Today. 10(3):3307–3310. Retrieved fromhttp://jprsolutions.info/files/final-file-5bfa3ac1b15519.09714920.pdf
- Lucas, B.F., R.C. Zambiazi, and J.A.V. Costa. 2018. Biocompounds and physical properties of açaí pulp dried by different methods. LWT. 98:335–340. doi: 10.1016/J.LWT.2018.08.058..
- Machado, A.K., F.C. Cadoná, C.E. Assmann, A.C. Andreazza, M.M.M.F. Duarte, B.C. Dos Santos, X. Zhou, D.V. de Souza, E.R. Esteves, and I.B.M. da Cruz. 2019. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflammasome modulation. Journal of Functional Foods. 56:364–371. doi: 10.1016/j.jff.2019.03.034.
- Machado, R.M.D., R.N. Haneda, B.P. Trevisan, and S.R. Fontes. 2012. Effect of enzymatic treatment on the cross-flow microfiltration of açaí pulp: Analysis of the fouling and recovery of phytochemicals. Journal of Food Engineering. 113(3):442–452. doi: 10.1016/J.JFOODENG.2012.06.022..
- Mahdavi, S.A., S.M. Jafari, M. Ghorbani, and E. Assadpoor. 2014. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Drying Technology: An International Journal. 32(5):509–518. doi: 10.1080/07373937.2013.839562..
- Marcon, L.D.N., L.F. de Sousa Moraes, B.C. Cruz, S. dos, M.D.D.O. Teixeira, T.C. Vidon Bruno, I.E. Ribeiro, A.C. Messias, C.L.F. Fortes, L.L. de Oliveira, et al. 2019. Yacon (Smallanthus sonchifolius)-based product increases fecal short-chain fatty acids and enhances regulatory T cells by downregulating RORγt in the colon of BALB/c mice. Journal of Functional Foods. 55:333–342. doi: org/10.1016/j.jff.2019.02.039.
- Marques, E.S., J.G. Froder, J.C.T. Carvalho, P.C.P. Rosa, F.F. Perazzo, and E.L. Maistro. 2016. Evaluation of the genotoxicity of Euterpe oleraceae Mart. (Arecaceae) fruit oil (açaí), in mammalian cells in vivo. Food and Chemical Toxicology. 93:13–19. doi: 10.1016/j.fct.2016.04.018..
- Marques, K.K., M.H. Renfroe, P.B. Brevard, R.E. Lee, and J.W. Gloeckner. 2010. Differences in antioxidant levels of fresh, frozen and freeze-dried strawberries and strawberry jam. International Journal of Food Sciences and Nutrition 61(8):759–769. doi: 10.3109/09637481003796306..
- Martinez-Oliveira, P., M.F. de Oliveira, N. Alves, R.P. Coelho, B.C. Pilar, A.A. Güllich, D.J. Ströher, A. Boligon, J.P. Escobar, P.B. Mello-Carpes, et al. 2018. Yacon leaf extract supplementation demonstrates neuroprotective effect against memory deficit related to β-amyloid-induced neurotoxicity. Journal of Functional Foods. 48:665–675. doi: org/10.1016/j.jff.2018.08.004.
- Meireles, M., L.M. Rodríguez-Alcalá, C. Marques, S. Norberto, J. Freitas, I. Fernandes, N.A. Mateus, A. Gomes, and C. Calhau. 2016. Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes. Food and Function. 7(1):127–139. doi: 10.1039/c5fo00925a.
- Miao, L., and T. Wu. 2014. Açaí (Euterpe oleracea Mart.) Liquefied pulp for drinking and their antioxidant capacities during processing, p. 165–172. In: V. Preedy (ed.). Processing and impact on antioxidants in beverages. Academic press, Oxford. doi: 10.1016/B978-0-12-404738-9.00017-9..
- Monge-Fuentes, V., L.A. Muehlmann, J.P.F. Longo, J.R. Silva, M.L. Fascineli, P. de Souza, F. Faria, I.D. Anatolievich, A. Rodriguez, C.F. Pirani, et al. 2017. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. Journal of Photochemistry and Photobiology: Biology. 166:301–310. doi: 10.1016/j.jphotobiol.2016.12.002.
- Montesinos, J., L. Rodríguez-Larramendi, R. Ortiz-Pérez, M.D.L.Á. Fonseca-Flores, G. Ruíz, and F. Guevara-Hernández. 2015. Revisión bibliográfica Pitahaya (Hylocereus spp.) un recurso fitogenético con historia y futuro para el trópico seco Mexicano. Cultivos Tropicales. 36:67–76. Retrieved from https://ediciones.inca.edu.cu
- Moreira, S.R.A., F. Redko, J. Ulloa, S. Flor, M.S. Tulino, L. Muschietti, and M.A. Carballo. 2020. Toxicogenetic evaluation of Smallanthus sonchifolius (yacon) as a herbal medicine. Journal of Ethnopharmacology. 257:112854. doi: org/10.1016/j.jep.2020.112854.
- Mosquera, H.A., B. Betancourt, J.C. Castellanos, and L.E. Perdomo. 2011. Surveillance commercial of the production chain yellow pitahaya. Cuadermos De Administración 27(45):75–93. doi: 10.25100/cdea.v27i45.445.
- Nerd, A., and Y. Mizrahi. 1999. The effect of ripening stage on fruit quality after storage of yellow pitaya. Postharvest Biology and Technology. 15(2):99–105. doi: 10.1016/S0925-5214(98)00080-5..
- Neri-Numa, I.A., R.A. Soriano Sancho, A.P.A. Pereira, and G.M. Pastore. 2018. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Research International. 103:345–360. doi: 10.1016/J.FOODRES.2017.10.053.
- NG, Z.-X., J.-W. Chai, and U.R. Kuppusamy. 2011. Customized cooking method improves total antioxidant activity in selected vegetables. International Journal of Food Sciences and Nutrition. 62(2):158–163. doi: 10.3109/09637486.2010.526931..
- NG, Z.-X., and N.F. Rosman. 2019. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. Journal of Food Science and Technology. 56(2):865–877. doi: 10.1007/s13197-018-3547-6..
- Ng, Z.X., U.R. Kuppusamy, Z.X. Ng, and U.R. Kuppusamy. 2019. Effects of different heat treatments on the antioxidant activity and ascorbic acid content of bitter melon, Momordica charantia. Brazilian Journal of Food Technology. 22:e2018283. doi: 10.1590/1981-6723.28318..
- Ng, Z.X., and W.C. Tan. 2017. Impact of optimised cooking on the antioxidant activity in edible mushrooms. Journal of Food Science and Technology. 54(12):4100–4111. doi: 10.1007/s13197-017-2885-0..
- Oliveira, P., D. Pala, C. Teixeira, M. Oliveira, J. Ferreira, R.A. Lima, F.F. Andrezza, A.C. Pinheiro, and R.N. de Freitas. 2016. Açaí (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 32(6):674–680. doi: 10.1016/j.nut.2015.12.030..
- Oliveira, R.B., D.A. Chagas-Paula, A. Secatto, T.H. Gasparoto, L.H. Faccioli, A.P. Campanelli, and F.B. Da Costa. 2013. Topical anti-inflammatory activity of yacon leaf extracts. Revista Brasileira De Farmacognosia. 23(3):497–505. doi: 10.1590/S0102-695X2013005000032..
- Osorio, C., N. Hurtado, C. Dawid, T. Hofmann, F.J. Heredia-Mira, and A.L. Morales. 2012. Chemical characterisation of anthocyanins in tamarillo (Solanum betaceum Cav.) and Andes berry (Rubus glaucus Benth.) fruits. Food Chemistry. 132(4):1915–1921. doi: 10.1016/J.FOODCHEM.2011.12.026..
- Patras, A., N.P. Brunton, S. Da Pieve, and F. Butler. 2009. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innovative Food Science and Emerging Technologies. 10(3):308–313. doi: 10.1016/j.ifset.2008.12.004..
- Plummer, M., C. de Martel, J. Vignat, J. Ferlay, F. Bray, and S. Franceschi. 2016. Global burden of cancers attributable to infections in 2012: A synthetic analysis. The Lancet Global Health. 4(9):e609–e616. doi: 10.1016/S2214-109X(16)30143-7..
- Qin, Z., H.-M. Liu, X.-C. Cheng, and X.-D. Wang. 2019. Effect of drying pretreatment methods on structure and properties of pectins extracted from Chinese quince fruit. International Journal of Biological Macromolecules. 137:801–808. doi: org/10.1016/j.ijbiomac.2019.06.209.
- Quintin, D., P. Garcia-Gomez, M. Ayuso, and A.M. Sanmartin. 2019. Active biocompounds to improve food nutritional value. Trends in Food Science & Technology. 84:19–21. doi: 10.1016/J.TIFS.2018.03.024..
- Quirós-Sauceda, A.E., J.F. Ayala-Zavala, G.I. Olivas, and G.A. González-Aguilar. 2014. Edible coatings as encapsulating matrices for bioactive compounds: A review. Journal of Food Science and Technology. 51(9):1674–1685. doi: 10.1007/s13197-013-1246-x.
- Rahman Mazumder, M.A., and T.V. Ranganathan. 2020. Encapsulation of isoflavone with milk, maltodextrin and gum acacia improves its stability. Current Research in Food Science. 2:77–83. doi: 10.1016/J.CRFS.2019.12.003..
- Rawson, A., A. Patras, B.K. Tiwari, F. Noci, T. Koutchma, and N. Brunton. 2011. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Research International. 44(7):1875–1887. doi: 10.1016/j.foodres.2011.02.053..
- Raybaudi-Massilia, R.M., and J. Mosqueda-Melgar. 2012. Polysaccharides as Carriers and Protectors of Additives and Bioactive Compounds in Foods. The Complex World of Polysaccharides. InTech. doi:10.5772/50206
- Reis, F.R., M.K. Lenzi, and M.L. Masson. 2012. Effect of vacuum drying conditions on the quality of yacon (Smallanthus sonchifolius) slices: Process optimization toward color quality. Journal of Food Processing and Preservation. 36(1):67–73. doi: 10.1111/j.1745-4549.2011.00555.x..
- Roberfroid, M.B. 2000. Concepts and strategy of functional food science: The European perspective. The American Journal of Clinical Nutrition. 71(6):1660S–4S. doi:10.1093/ajcn/71.6.1660S.. discussion 1674S-5S.
- Rodríguez-Barona, S., G.I. Giraldo, and Y.P. Zuluaga. 2015. Evaluación de la incorporación de fibra prebiótica sobre la viabilidad de Lactobacillus casei Impregnado en matrices de mora (Rubus glaucus). Información Tecnológica. 26(5):25–34. doi: 10.4067/S0718-07642015000500005..
- Rojas, W., J.L. Soto, M. Pinto, M. Jäger, and S. Padulosi 2010. Granos Andinos: Avances, logros y experiencias desarrolladas en quinua, cañahua y amaranto en bolivia.
- Rojas-Llanes, J., J.R. Martínez, and E. Stanshenko. 2014. Content of phenolic compounds and antioxidant capacity of blackberry (Rubus glaucus Benth) extracts obtained under different conditions. Vitae 21(3):218–227. Retrieved fromhttp://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-40042014000300007&lng=en&nrm=iso&tlng=es
- Rossi, A., I. Serraino, P. Dugo, R. Di Paola, L. Mondello, T. Genovese, D. Morabito, G. Dugo, L. Sautebin, A.P. Caputi et al. 2003. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radical Research. 37(8): 891–900. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14567449.
- Rueda-Ordóñez, Y.J., C.J. Arias-Hernández, J.F. Manrique-Pinto, P. Gauthier-Maradei, and W.A. Bizzo. 2019. Assessment of the thermal decomposition kinetics of empty fruit bunch, kernel shell and their blend. Bioresource Technology. 292:121923. doi: org/10.1016/j.biortech.2019.121923.
- Sánchez, D.E. 2012. Estudio del potencial antioxidante de la mora (Rubus glaucus Benth) y sus cambios en función del proceso de maduración y bajo diferentes temperaturas de almacenamiento (Universidad Nacional de Colombia). Retrieved from http://bdigital.unal.edu.co/10581/.
- Sariburun, E., S. Şahin, C. Demir, C. Türkben, and V. Uylaşer. 2010. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. Journal of Food Science 75(4):C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x..
- Sautebin, L., A. Rossi, I. Serraino, P. Dugo, R. Di Paola, L. Mondello, G. Tiziana, B. Domenico, P. Angelo, D. Giovanni, et al. 2004. Effect of anthocyanins contained in a blackberry extract on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Planta Medica. 70(8):745–752. doi:10.1055/s-2004-827206..
- Schauss, A.G., X. Wu, R.L. Prior, B. Ou, D. Patel, D. Huang, and J.P. Kababick. 2006. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (Açai). Journal of Agricultural and Food Chemistry 54(22):8598–8603. doi: 10.1021/jf060976g..
- Seeram, N.P., L.S. Adams, Y. Zhang, R. Lee, D. Sand, H.S. Scheuller, and D. Heber. 2006. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells In Vitro. Journal of Agricultural and Food Chemistry. 54(25):9329–9339. doi: 10.1021/jf061750g.
- Serino, A., Y. Zhao, J. Hwang, A. Cullen, C. Deeb, N. Akhavan, B. Arjmandi, and G. Salazar. 2020. Gender differences in the effect of blackberry supplementation in vascular senescence and atherosclerosis in ApoE−/− mice. Journal of Nutritional Biochemistry. 80:108375. doi: 10.1016/j.jnutbio.2020.108375.
- Serna, L., L. Torres, and A. Ayala. 2012. Effect of pre- and postharvest application of 1-methylcyclopropene on the maturation of yellow pitahaya (Selenicerus megalanthus Haw). Vitae 19(1):49–59. Retrieved fromhttp://www.scielo.org.co/scielo.php?script=sci_abstractandpid=S0121-40042012000100006
- Serna-Cock, L., L.S. Torres-Valenzuela, and A. Ayala-Aponte. 2013. Physical, chemical and sensory changes of refrigerated yellow pitahaya treated preharvest with 1-mcp. DYNA. 80(178):11–20.
- Shukitt-Hale, B., V. Cheng, and J.A. Joseph. 2009. Effects of blackberries on motor and cognitive function in aged rats. Nutritional Neuroscience. 12(3):135–140. doi: 10.1179/147683009X423292..
- Simonovska, B., I. Vovk, S. Andrensek, K. Valentová, and J. Ulrichová. 2003. Investigation of phenolic acids in yacon (Smallanthus sonchifolius) leaves and tubers. Journal of Chromatography. 1016(1):89–98. Retrieved from doi:10.1016/S0021-9673(03)01183-X
- Solverson, P.M., W.V. Rumpler, J.L. Leger, B.W. Redan, M.G. Ferruzzi, D.J. Baer, T.W. Castonguay, and J.A. Novotny. 2018. Blackberry feeding increases fat oxidation and improves insulin sensitivity in overweight and obese males. Nutrients. 10(8):1–16. doi: 10.3390/nu10081048.
- Sousa, S., J. Pinto, C. Pereira, F. Xavier Malcata, M.T. Bertoldo Pacheco, A.M. Gomes, and M. Pintado. 2015. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food and Bioproducts Processing. 95:96–105. doi: 10.1016/J.FBP.2015.04.003..
- Szlapak Franco, T., C.A. Perussello, L. Neves Ellendersen, and M.L. Masson. 2016. Effects of foam mat drying on physicochemical and microstructural properties of yacon juice powder. LWT - Food Science and Technology. 66:503–513. doi: 10.1016/j.lwt.2015.11.009..
- Tavares, L., I. Figueira, D. Macedo, G.J. McDougall, M.C. Leitão, H.L.A. Vieira, D. Stewart, P.M. Alves, R.B. Ferreira, and C.N. Santos. 2012. Neuroprotective effect of blackberry (Rubus sp.) polyphenols is potentiated after simulated gastrointestinal digestion. Food Chemistry. 131(4):1443–1452. doi: 10.1016/J.FOODCHEM.2011.10.025.
- Tembo, D.T., M.J. Holmes, and L.J. Marshall. 2017. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. Journal of Food Composition and Analysis. 58:40–51. doi: org/10.1016/j.jfca.2017.01.002..
- The Nyéleni. 2007. International Steering Committee. 2007. Forum for Food Sovereignty. doi:10.1007/bfb0115199
- Tibério, L.D.J., M. Cristianini, N. Medina Dos Santos, and M. Roberto Maróstica Júnior. 2019. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açaí (Euterpe oleracea Martius) pulp. Food Research International. 130:108856. doi: 10.1016/J.FOODRES.2019.108856..
- Tiwari, B.K., C.P. O’Donnell, and P.J. Cullen. 2009a. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends in Food Science and Technology. 20(3–4):137–145. doi: 10.1016/j.tifs.2009.01.058.
- Tiwari, B.K., C.P. O’Donnell, and P.J. Cullen. 2009b. Effect of sonication on retention of anthocyanins in blackberry juice. Journal of Food Engineering. 93(2):166–171. doi: 10.1016/J.JFOODENG.2009.01.027..
- Tonon, R.V., C. Brabet, and M.D. Hubinger. 2008. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering. 88(3):411–418. doi: 10.1016/J.JFOODENG.2008.02.029..
- Tonon, R.V., C. Brabet, and M.D. Hubinger. 2010. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Research International. 43(3):907–914. doi: 10.1016/J.FOODRES.2009.12.013..
- Torres Grisales, Y., D.V. Melo Sabogal, L.S. Torres-Valenzuela, J.A. Serna-Jiménez, and A. Sanín Villarreal. 2017. Evaluation of bioactive compounds with functional interest from yellow pitahaya (Selenicereus megalanthus haw). Revista Facultad Nacional De Agronomia. 70(3):8311–8318. doi: 10.15446/rfna.v70n3.66330..
- Ulbricht, C., A. Brigham, D. Burke, D. Costa, N. Giese, R. Iovin, R. Windsor, S. Tanguay-Colucci, W. Weer, and R. Windsor. 2012. An Evidence-Based Systematic Review of Acai (Euterpe oleracea) by the Natural Standard Research Collaboration. Journal of Dietary Supplements. 9(2):128–147. doi: 10.3109/19390211.2012.686347..
- Valentová, K., F. Šeršeň, and J. Ulrichová. 2005. Radical Scavenging and Anti-Lipoperoxidative Activities of Smallanthus sonchifolius Leaf Extracts. Journal of Agricultural and Food Chemistry. 53(14):5577–5582. doi: 10.1021/jf050403o..
- Van de Velde, F., M.E. Pirovani, and S.R. Drago. 2018. Bioaccessibility analysis of anthocyanins and ellagitannins from blackberry at simulated gastrointestinal and colonic levels. Journal of Food Composition and Analysis. 72:22–31. doi: 10.1016/J.JFCA.2018.05.007..
- Vásquez, W., K. Aguilar, R. Vilaplana, D P. Viteri, W. Viera, and S. Valencia-Chamorro. 2016. Calidad del fruto y pérdidas poscosecha de pitahaya amarilla (Selenicereus megalanthus Haw.) en Ecuador. Agronomia Colombiana 34(1):1081–1083. Retrieved fromhttp://repositorio.iniap.gob.ec/handle/41000/4860
- Vaz-Tostes, M., G. das, M.L. Viana, M. Grancieri, T.C. Luz, S. dos, H. Paula, R.G. De, Pedrosa, and N.M.B. Costa. 2014. Yacon effects in immune response and nutritional status of iron and zinc in preschool children. Nutrition 30(6):666–672. doi: org/10.1016/j.nut.2013.10.016.
- Weber, F., K. Boch, and A. Schieber. 2017. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT. 75:72–77. doi: 10.1016/J.LWT.2016.08.042..
- Wichienchot, S., M. Jatupornpipat, and R.A. Rastall. 2010. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chemistry. 120(3):850–857. doi: 10.1016/J.FOODCHEM.2009.11.026..
- Williams, C.M., M.A. El Mohsen, D. Vauzour, C. Rendeiro, L.T. Butler, J.A. Ellis, M. Whiteman, and J.P.E. Spencer. 2008. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Biology and Medicine. 45(3):295–305. doi: 10.1016/j.freeradbiomed.2008.04.008..
- Wong, D.Y.S., I.F. Musgrave, B.S. Harvey, and S.D. Smid. 2013. Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neuroscience Letters. 556:221–226. doi: 10.1016/j.neulet.2013.10.027..
- World Health Organization. (n.d.). Enfermedades no transmisibles. Retrieved 17 December 2018, from https://www.who.int/es/news-room/fact-sheets/detail/noncommunicable-diseases.
- World Health Organization. (2015). Cáncer: Datos y cifras. Retrieved 17 December 2018, from http://www.who.int/mediacentre/factsheets/fs297/es/.
- Xie, C., J. Kang, R. Burris, M.E. Ferguson, A.G. Schauss, S. Nagarajan, and X. Wu. 2011. Açaí juice attenuates atherosclerosis in ApoE deficient mice through antioxidant and anti-inflammatory activities. Atherosclerosis. 216(2):327–333. doi: 10.1016/j.atherosclerosis.2011.02.035..
- Yamaguchi, K.K., L.F. Pereira, C. Lamarão, E. Lima, and V.F. Veiga-Junior. 2015. Amazon açai: Chemistry and biological activities: A review. In Food Chemistry. 179(15):137–151. doi: 10.1016/j.foodchem.2015.01.055.
- Zia-ul-haq, M., M. Riaz, V. Feo, H.Z.E. De, Jaafar, and M. Moga. 2014. Rubus Fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules. 19:10998–11029. doi: 10.3390/molecules190810998.