Abstract
Background: Although intravenous lipid emulsion (ILE) was first used to treat life-threatening local anesthetic (LA) toxicity, its use has expanded to include both non-local anesthetic (non-LA) poisoning and less severe manifestations of toxicity. A collaborative workgroup appraised the literature and provides evidence-based recommendations for the use of ILE in poisoning.
Methods: Following a systematic review of the literature, data were summarized in four publications: LA and non-LA poisoning efficacy, adverse effects, and analytical interferences. Twenty-two toxins or toxin categories and three clinical situations were selected for voting. Voting statements were proposed using a predetermined format. A two-round modified Delphi method was used to reach consensus on the voting statements. Disagreement was quantified using RAND/UCLA Appropriateness Method.
Results: For the management of cardiac arrest, we recommend using ILE with bupivacaine toxicity, while our recommendations are neutral regarding its use for all other toxins. For the management of life-threatening toxicity, (1) as first line therapy, we suggest not to use ILE with toxicity from amitriptyline, non-lipid soluble beta receptor antagonists, bupropion, calcium channel blockers, cocaine, diphenhydramine, lamotrigine, malathion but are neutral for other toxins, (2) as part of treatment modalities, we suggest using ILE in bupivacaine toxicity if other therapies fail, but are neutral for other toxins, (3) if other therapies fail, we recommend ILE for bupivacaine toxicity and we suggest using ILE for toxicity due to other LAs, amitriptyline, and bupropion, but our recommendations are neutral for all other toxins. In the treatment of non-life-threatening toxicity, recommendations are variable according to the balance of expected risks and benefits for each toxin.
For LA-toxicity we suggest the use of Intralipid® 20% as it is the formulation the most often reported. There is no evidence to support a recommendation for the best formulation of ILE for non-LAs. The voting panel is neutral regarding ILE dosing and infusion duration due to insufficient data for non-LAs. All recommendations were based on very low quality of evidence.
Conclusion: Clinical recommendations regarding the use of ILE in poisoning were only possible in a small number of scenarios and were based mainly on very low quality of evidence, balance of expected risks and benefits, adverse effects, laboratory interferences as well as related costs and resources. The workgroup emphasizes that dose-finding and controlled studies reflecting human poisoning scenarios are required to advance knowledge of limitations, indications, adverse effects, effectiveness, and best regimen for ILE treatment.
Introduction
The lipid emulsion workgroup was established as a collaborative effort among the American Academy of Clinical Toxicology, the European Association of Poison Centres and Clinical Toxicologists, the American College of Medical Toxicology, the Asia Pacific Association of Medical Toxicology, the American Association of Poison Control Centers, and the Canadian Association of Poison Control Centers. This article presents the workgroup’s recommendations regarding the use of intravenous lipid emulsion therapy in poisoning for a preselected set of toxins. These recommendations are based on the results of four systematic reviews,[Citation1–4] derived from a comprehensive analysis of the published evidence and further followed by an expert consensus.
Methods
The detailed methodology for the workgroup’s process was previously published.[Citation5] Each association selected clinical experts to serve on this committee and additional selections were made for their specific expertise in related fields. Two medical librarians assisted the workgroup in the design of the search strategies, article retrieval, and management of citations but did not vote on the recommendations. For the published systematic reviews, the following databases were searched from inception to 15 December 2014: BIOSIS Previews (via Ovid), CINAHL (via EBSCO), the Cochrane Library/DARE, Embase (via Ovid), Medline (Ovid), PubMed, Scopus, and Web of Science. The literature review was updated in December 2015 as described later. No language restrictions were applied. Articles in languages other than English were professionally translated. A methodologist with expertise in systematic reviews and guideline development oversaw the process. The workgroup considered four systematic reviews that summarized the evidence pertaining to potential benefits and harms of the use of lipid emulsion in poisoning, which were published prior to finalizing the recommendations, and an international survey evaluating ILE availability and cost.[Citation1–4,Citation6]
The voting panel decided to evaluate only those toxins or categories of toxins for which a minimum of three human cases were reported in the literature. The 22 toxins or categories were selected as the following: amitriptyline, class 1 antidysrhythmics, baclofen, bupivacaine, bupropion, lipid-soluble beta-receptor antagonists (defined as a positive log D), non-lipid-soluble beta-receptor antagonists, cocaine, non-dihydropyridine calcium channel blockers (diltiazem and verapamil), dihydropyridine calcium channel blockers, diphenhydramine, ivermectin, lamotrigine, malathion, olanzapine, selective serotonin receptor inhibitors, other cyclic antidepressants, other antihistamines, other antipsychotics, other insecticides, other local anesthetics (LAs), and other pesticides.
The workgroup determined clinical situations in which lipid emulsion could be indicated. These were categorized as (1) cardiac arrest, (2) life-threatening toxicity, or (3) non-life-threatening toxicity (). Life-threatening toxicity was defined as the presence of any of the following: dysrhythmias such as ventricular tachycardia with compromised organ perfusion, ventricular fibrillation, status epilepticus, and/or hypotension with organ compromise. Shock or end-organ compromise was defined as the presence of cellular ischemia as evidenced by increased lactate concentration, acute kidney injury as defined by the Kidney Disease Improving Global Outcomes (KDIGO) guideline,[Citation7] increased troponin, altered mental status, or decreased capillary refill. Hypotension was defined as a low blood pressure as per age-related defined standards. Non-life-threatening toxicity was defined as clinical situations without immediate threat to life such as coma, altered mental status, simple seizure, hypotension without organ compromise, and dysrhythmias such as sinus tachycardia or other stable dysrhythmias. Altered mental status was defined as the impairment in one of the spheres of brain function: cognition, alertness or orientation, and coma a deep state of unconsciousness as per the American Academy of Neurology.[Citation8]
Table 1. Intravenous lipid emulsion (ILE) in poisoning: clinical situations.
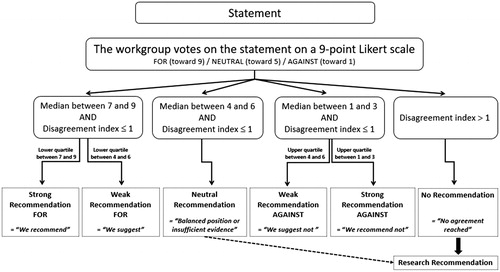
A generic format of voting statements was developed during several conference calls in order to refine the final wording and ensure generalizability to all toxins (Appendix 1). A two-round modified Delphi method was utilized to reach a consensus on clinical recommendations. After considering the balance between desirable and undesirable outcomes, the quality of evidence for outcomes as well as costs and resource use, members of the voting panel cast their votes on a 9-point Likert scale for each proposed statement for each toxin/category of toxins included. The RAND/UCLA Appropriateness Method was used to quantify disagreement between the votes cast by the panel. The median values, the lower/upper quartiles, and the disagreement indexes were calculated for each of the two rounds of votes. Median values ranging from 7 to 9 reflected that the workgroup was in favor of the proposed statement, 4 to 6 reflected a neutral position, and 1 to 3 reflected that the workgroup was against the statement. The disagreement index describes the dispersion of ratings and values less than or equal to 1 indicate agreement. A second round of voting determined the final strength of recommendations (). A strong recommendation in favor (level 1) was defined as a median value between 7 and 9 with a lower quartile between 7 and 9 and a disagreement index ≤1 (similarly, a strong recommendation against was defined as a median value between 1 and 3 with an upper quartile between 1 and 3 and a disagreement index ≤1). A weak/conditional recommendation in favor (level 2) was defined as a median value between 7 and 9 with a lower quartile between 4 and 6 and a disagreement index ≤1 (similarly, a weak/conditional recommendation against was defined as a median value between 1 and 3 with an upper quartile between 4 and 6 and a disagreement index ≤1). A neutral position was resulted when the median value was between 4 and 6 with a disagreement index ≤1. To better understand the reason for a neutral vote, members could specify if their position was neutral due to major uncertainties in the evidence or to a balance between the desirable and undesirable effects of adherence to the proposed statement. When the disagreement index exceeded 1, no recommendation resulted as this illustrated an inability of the voting panel to reach consensus. All recommendations are followed by the strength of recommendations (1 or 2) and the grading of the level of evidence (A to D) (), in accordance with the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation).[Citation9]
Table 2. Strength of recommendation and level of evidence.
A first vote occurred in October 2014 and results were discussed in a face-to-face meeting in the same month. A second vote in September 2015 determined the final recommendations. Results were discussed in a second face-to-face meeting in October 2015. An update of the literature for publications through 31 December 2015 was performed in January 2016 using the same search strategy previously mentioned and is presented in Appendices 2–4. The literature update was summarized and presented to the members of the voting panel. Members were given an opportunity to update their votes in March of 2016.
Clinical recommendations
In the discussions that follow if there is no specific mention of a scenario, it is implied that the voting was neutral. The complete voting results for each statement for each toxin/category of toxins are presented in Appendix 5. An executive summary of the recommendations for all toxins/categories of toxins is presented in .
Table 3. Executive summary of indications regarding the use of ILE in poisoning.
Recommendations for local anesthetics
Indications:
In cardiac arrest due to toxicity of bupivacaine, we recommend using ILE after standard ACLS is started (1D), while our recommendation is neutral regarding its use in cardiac arrest due to other local anesthetics.
Rationale: The voting panel noted that bupivacaine is the LA for which the most data exist with results supporting the efficacy of ILE. However, controlled data from animal experiments suffer from several methodological shortcomings. These include: reporting a statistical difference for short experimental time frames not directly relevant to clinical situations, the failure to perform autopsies to search for potential adverse effects, and the lack of reporting of acidosis and hypoxia in study animals, both of which are common in human poisonings and can affect outcome. Human case reports are too heterogeneous and patients received concurrent multiple other medications making it impossible to definitively attribute any positive outcome to ILE alone.
However, while the level of evidence is very low, the risk/benefit ratio in cardiac arrest favors the use of ILE with bupivacaine and the voting panel had strong agreement for this indication. There are no data to allow an informed decision on which resuscitative medication to use first among sodium bicarbonate, epinephrine, or ILE. Some members pointed out that if the total dose administered is within the known therapeutic range and the route of exposure is clearly not intravascular, consideration of an allergic reaction to LA rather than LA systemic toxicity probably warrants the use of epinephrine first. Because of a lack of evidence for the use of ILE with other LAs, the voting panel was unable to provide a firm recommendation in the setting of cardiac arrest. However, several members noted that harm appears to be low and there was a strong agreement for a neutral vote. Concerns about reports of an unclear interaction with concurrent administration of epinephrine or other resuscitative medications with ILE reported mainly in the local anesthetics literature may explain the voting panel’s reticence to advise on ILE administration during cardiac arrest associated with non-bupivacaine LA toxicity.[Citation10–13] Data were insufficient to make an evidence-based recommendation for the use of ILE with other LAs. In comparison with bupivacaine, both the lipophilicity and toxicity profiles of the other LAs vary considerably, thereby invalidating recommendations made by analogy rather than data.
In life-threatening toxicity due to bupivacaine, we suggest using ILE as part of treatment modalities (2D) and we recommend its use if other therapies fail/in last resort (1D).
Rationale: The voting panel had strong agreement in concluding that there are enough data to support the use of ILE as a part of treatment modalities for patients with life-threatening bupivacaine toxicity. Moreover, while the risk/benefit ratio of this therapy is warranted if all other treatment modalities fail, the lack of data to guide the sequence of administration of resuscitative therapies made it impossible to decide on whether ILE should be the first-line treatment. Some members of the voting panel were concerned that waiting for other therapies to fail may decrease the potential efficacy of ILE and thus it should be given relatively early, while there was consensus to use ILE if a patient was already unresponsive to other treatments.
In life-threatening toxicity due to other LAs, we suggest using ILE if other therapies fail/in last resort (2D).
Rationale: There is a lack of convincing data for efficacy of ILE with other LAs. Despite this, there is a relatively favorable risk/benefit ratio in cases of prolonged toxicity in patients with a pulse but unresponsive to other treatments. As a result, the voting panel agreed that ILE could be used if other therapies fail or as a last resort. However, it was noted that in only a minority of reported cases ILE was used as sole treatment.
In non-life-threatening toxicity due to bupivacaine or other LAs, our recommendation is neutral regarding the use of ILE.
Rationale: The voting panel agreed that, in this situation, there is equipoise between risk and benefits. There are not enough data reported to make an evidence-based decision.
Lipid regimen
1. ILE formulation:
When ILE is indicated for bupivacaine and other LAs toxicity, we suggest using the brand Intralipid® 20% (2D).
Rationale: Most of the data reported used this specific ILE formulation. The voting panel agreed that there were insufficient data to discuss other formulations in human poisonings until such time as comparative studies are reported.
2. ILE dosing:
When ILE is indicated for bupivacaine and other LAs toxicity, our recommendation is neutral regarding the choice of ILE dosing.
Rationale: Although the voting panel agreement was for a neutral position, there was a slight preference for the most commonly reported dosing regimen: a bolus of 1.5 mL/kg and an infusion of 0.25 mL/kg/min of 20% ILE. Data are lacking with regards to ILE dose–response relationships for treating any human toxicity. No studies evaluated the benefit of an infusion after bolus vs. a bolus alone for toxins with a rapid endogenous clearance compared with most other toxins. The literature reports a varied range of bolus doses, infusion rates, and durations that make analysis of the optimal dosing regimen impossible.
3. ILE cessation:
When ILE is indicated for bupivacaine and other LAs toxicity, our recommendation is neutral regarding which endpoints to use to stop ILE administration (maximum dose or maximum duration).
Rationale: The voting panel attempted to define endpoints for ILE treatment either with a maximum dose administered, a maximum duration of administration, or with resolution of toxicity. Members expressed opinions that a maximum dose should not be exceeded due to concerns about adverse effects from lipid overload. There were no strong data to inform the threshold amount of ILE that results in lipid toxicity. To avoid lipid overload, it was suggested from the voting panel that ILE doses should be limited to a maximum of 10% of total blood volume to limit possible complications arising from increased triglyceride concentrations in excess of 15 mmol/L (glycerol-blanked method) reported when ILE represented more than 10% of test tube volumes.[Citation14] This is also to avoid fluid overload, which is an increasing concern in resuscitation as patients receiving ILE will likely have received other fluid solutions.[Citation15] This is particularly of concern when administering ILE to obese patients, neonates, or young children. Assuming ILE remains mostly in the intravascular compartment, this rationale would indicate a total ILE dose of 560 mL for a 70 kg adult patient with an estimated blood volume of 5.6 L.
Also, given the pharmacokinetics of LAs and the lack of historical data to indicate a recurrence of toxicity once clinical improvement occurs, resolution of toxicity may be considered as an appropriate endpoint. The risk of prolonged ILE therapy on immune function or other organ function is as yet undefined for infusions administered for less than 24 h. However, some members were of the opinion that waiting to see a sustained improvement in the clinical status would be inadvisable, until such a time that studies report a benefit for continuation of therapy beyond the point of clinical resolution.
There is no strong evidence guiding the maximum duration of ILE therapy that could be safely administered if clinical improvement does not occur. However, the voting panel commented that clinical protocols for the use of ILE should recommend a specific duration and maximal volume of therapy to avoid administration of high doses of ILE over indefinite periods. This should take into consideration the adverse effect profile and the maximum duration of ILE recommended during parenteral nutrition.[Citation16] As most articles reported use of ILE for 20–30 min, it seems reasonable to limit the duration of infusion to this time period until controlled experiments are published assessing specific treatment durations. Thus, the voting panel concluded that not enough evidence exists to inform on when to stop ILE.
Recommendations for non-local anesthetics
Amitriptyline and other tricyclic antidepressants
Indications:
In cardiac arrest due to either amitriptyline or any other tricyclic antidepressants toxicity, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to amitriptyline, we suggest using ILE if other therapies fail/in last resort (2D), but we suggest not using ILE as first-line therapy (2D).
In life-threatening toxicity due to other tricyclic antidepressants, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to amitriptyline, we recommend not using ILE as first-line therapy (1D) and furthermore we suggest not using ILE as part of treatment modalities (2D).
In non-life-threatening toxicity due to other tricyclic antidepressants, we suggest not using ILE in any circumstances (2D).
Rationale: One human RCT (published only in abstract and not specifying which TCAs were involved) failed to show a benefit of ILE on the duration of cardiotoxicity when patients were randomized to standard treatment with bicarbonate or ILE.[Citation17] This explains why ILE therapy is discouraged either as first-line therapy either in life-threatening toxicity or with non-life-threatening toxicity. However, the situation of whether ILE might be beneficial in cases refractory to standard therapy, including epinephrine and bicarbonate therapy, was not explored. Many human case reports exist with inherent publication bias. However, the voting panel noted that some animal experiments, including one with an orogastric-poisoning model most similar to the clinical poisoning reported no benefit and possibly even harm.[Citation18] Decades of published evidence for the efficacy of bicarbonate therapy exist. Thus, the panel voted for the use of ILE only in life-threatening toxicity from amitriptyline after failure of standard therapies, with moderate agreement.
Beta-receptor antagonists
Indications:
In cardiac arrest due to toxicity of both lipid soluble and non-lipid soluble beta-receptor antagonists, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to lipid soluble beta-receptor antagonists, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to non-lipid soluble beta-receptor antagonists, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to lipid soluble beta-receptor antagonists, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to non-lipid soluble beta-receptor antagonists, we suggest not using ILE as first-line therapy nor as part of treatment modalities (2D).
Rationale: The voting panel had consistent agreement in their votes. Reasons cited for the results are the balance existing between risks and expected benefit of using ILE, the evidence for the safety and efficacy of high dose insulin euglycemia therapy and the possible use of extracorporeal assist devices reporting problems with concurrent ILE use. Moreover, the distinction between lipid-soluble and non-lipid soluble drugs, which were initially divided into two distinct categories to account for differences in log D did not influence the voting panel’s evaluation, except in cases of life-threatening toxicity where benefits were not considered to outweigh risk for non-lipid soluble beta receptor antagonist toxicity as a first-line therapy or in non-life-threatening toxicity as part of treatment modalities.
Bupropion
Indications:
In cardiac arrest due to bupropion toxicity, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to bupropion, we suggest using ILE if other therapies fail/in last resort (2D), but we suggested not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to bupropion, we suggest not using ILE as first-line therapy (2D).
Rationale: Few case reports exist with survival outcome and it is unclear if the patients would have survived without ILE. However, the voting panel mentioned the likelihood of publication bias. Also, most cases of bupropion toxicity do well with non-specific therapies aimed at maintaining vital functions. Several case reports demonstrate improvement with bicarbonate therapy. It is unclear whether higher doses of bicarbonate, a medication with an established safety profile, would yield similar outcomes to ILE. More controlled data are needed to inform on whether or not ILE interferes with the efficacy of standard therapies such as benzodiazepines or barbiturates for seizures. The concurrent use of ILE and other therapies has not been studied in any detail. However, in the situation of prolonged and refractory status epilepticus, a trial of ILE seems reasonable. Hence, a 2D recommendation was made for cases with life-threatening toxicity if other therapies fail. However, in pulseless cardiac arrest, the voting panel felt ILE was not indicated given the reported interference it has on the effect of epinephrine or extracorporeal treatments. Hence, there was not a favorable enough risk/benefit ratio. Once ROSC (return of spontaneous circulation) is achieved, then ILE is suggested if life-threatening toxicity persists.
Calcium channel blockers
Indications:
In cardiac arrest due to toxicity from calcium channel blockers (including diltiazem, verapamil and dihydropyridines), our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to diltiazem, verapamil, or dihydropyridine calcium channel blockers, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to diltiazem verapamil, or dihydropyridine calcium channel blockers, we suggest not using ILE as first-line therapy (2D).
Rationale: Due to the inconsistent outcomes reported, ranging from sudden death to immediate response, in both animal experiments and human case reports, no clear recommendation can be made. Some members felt cardiac arrest presents a situation where little harm seems to exist for a “trial” of ILE. However, as noted above, other members expressed their concerns that ILE can enhance intestinal absorption of toxins as is demonstrated in oral drug poisoning models.[Citation19] In addition, problems associated with the concurrent use of ILE with extracorporeal assist devices and the potential for interference with resuscitative medications with evidence of benefit, such as vasopressors and insulin–glucose, were also highlighted. A single study that mimicked an oral overdose of verapamil demonstrated worse outcomes when ILE was given. Animal studies showed no clinical benefit or benefit at dose requirements exceeding a dose of 12 mL/kg of 20% ILE.[Citation19,Citation20] No studies comparing the current standard of care with vasopressors or insulin–glucose therapy are available in a model consistent with human clinical poisoning. Considering the lack of information on dose, potential adverse effects and especially interference with extracorporeal assist devices or interference with medications known to be effective, the voting panel determined that the benefits were probably equal to the risks and thus, a neutral position resulted in the presence of cardiac arrest.
Unless organ perfusion is compromised, the voting panel felt that there was not enough information to make a decision and at the very least a balance exists between risks and potential benefits of ILE. Thus, there is a question as to whether ILE should be a part of the treatment modalities or used after standard therapy fails (last resort) in life-threatening toxicity for all calcium channel blockers.
Furthermore, the reason for not suggesting lipid emulsion if other therapies fail, in cases of non-life-threatening toxicity due to diltiazem and verapamil, and not suggesting ILE in all other circumstances of non-life-threatening toxicity due dihydropyridines, is based on a risk/benefit analysis. Certain signs and symptoms of CCB toxicity, such as ileus or bradycardia and hypotension, may not respond or entirely correct with various therapies but only resolve with time and metabolism of the toxicant.
Cocaine
Indications:
In cardiac arrest due to cocaine toxicity, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to cocaine, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to cocaine, we suggest not using ILE as first-line therapy (2D) or as part of treatment modalities (2D).
Rationale: Too few case reports exist, all with varied outcomesto make a favorable recommendation. Several experimental studies with cocaine and ILE using pre-treatment animal models [Citation21,Citation22] were excluded a priori due to the lack of generalizability to the clinical setting of human cocaine poisoning. The voting panel was in agreement on a neutral recommendation concerning the use of ILE in cardiac arrest and commented there was a paucity of data on the efficacy of ILE to reverse signs and symptoms of cocaine toxicity. The body of evidence and published experience with other treatments such as sodium bicarbonate, and benzodiazepines is much greater than that for ILE therapy. More controlled data are needed to assess whether or not ILE interferes with the efficacy of the standard therapies.
Diphenhydramine
Indications:
In cardiac arrest due to diphenhydramine toxicity, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to diphenhydramine, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to diphenhydramine, we recommend not using ILE as first-line therapy (1D) and we suggest not using ILE otherwise (2D).
Rationale: Due to the efficacy of bicarbonate therapy and the lack of superiority of ILE over bicarbonate reported in one animal experiment, the voting panel concluded there was no scenario where ILE would be clearly indicated at this time. There are no data to inform on the best timing of ILE administration. Diphenhydramine possesses sodium channel blocking properties and a trial of ILE has clinical equipoise when considering the possible risks of an acute administration of ILE in cases otherwise unresponsive to repeated administration of sodium bicarbonate (e.g. more than 200 mEq [Citation23]). However, the role of ILE in non-life-threatening toxicity, such as anticholinergic delirium, was not reported in the literature at all.
A comment was made regarding the use of ILE with “other antihistamines”. It was noted that if the lipid sink or conduit theories proves to be valid, there might be a theoretical benefit for ILE in severe toxicity from sedating antihistamines. In particular, those agents with a log D value of 2 or 3 when other therapies have failed. However, more than 70% of the panel voted that there were insufficient data to consider recommendations in the category “other antihistamines”. This made it impossible to make a statement about any particular antihistamine, as described in the methodology.
Lamotrigine
Indications:
In cardiac arrest due to lamotrigine toxicity, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to lamotrigine, we suggest not using ILE as first-line therapy (2D).
In non-life-threatening toxicity due to lamotrigine, we suggest not using ILE as first-line therapy (2D) nor as part of treatment modalities (2D).
Rationale: The voting panel determined that too few case reports exist with survival outcome reported. It is also unclear if these patients would have survived without ILE. Additionally, the voting panel mentioned publication bias and the fact that most cases of lamotrigine toxicity do well with supportive care. More controlled data are needed to inform clinicians on whether or not ILE interferes with the efficacy of the standard therapies such as benzodiazepines and sodium bicarbonate to reverse the proconvulsant and sodium channel blocking properties of lamotrigine. Severe lamotrigine poisoning can also result in metabolic acidosis and acute kidney injury, which may require dialysis. The concurrent use of ILE and other therapies has not been studied in enough detail to provide comment. Of note, as described in our adverse effect review article,[Citation5] the use of ILE with any extracorporeal circuit such as occlusion of the circulation.
Other toxins
This section includes the other toxins and categories of toxins for which the voting results were similar. Therefore, rather than lengthy individual statements, the results are discussed in aggregate. Once again the readers are referred to the Appendices and tables for a complete record of the voting.
In cardiac arrest due to toxicity of Class 1 Vaughan–Williams antidysrhythmics, baclofen, ivermectin and other insecticides, malathion and other pesticides, olanzapine and other antipsychotics, and selective serotonin reuptake inhibitors, our recommendation is neutral regarding the use of ILE.
In life-threatening toxicity due to other insecticides, malathion and other pesticides, olanzapine, and other antipsychotics, we suggest not using ILE as first-line therapy (2D).
In life-threatening toxicity due to Class 1 Vaughan–Williams antidysrhythmics, baclofen, ivermectin, and selective serotonin reuptake inhibitors, our recommendation is neutral regarding the use of ILE.
In non-life-threatening toxicity due to Class 1 Vaughan–Williams antidysrhythmics, baclofen, ivermectin, and other insecticides, malathion and other pesticides, olanzapine and other antipsychotics, and selective serotonin reuptake inhibitors, we suggest not using ILE as first-line therapy (2D).
Rationale: Even though some of these toxins are lipid-soluble (defined by their log D) due to the paucity of data, the panel primarily considered possible benefit, possible risks, and the availability of other alternative treatments in their votes.
Animal data provide conflicting results for the pesticide and insecticide groups. Thus, adverse effects and potential interferences were a major consideration influencing the voting. Moreover, these toxins represent a heterogeneous group of chemicals that are not amenable to a common statement on treatment with ILE. Antipsychotics rarely cause significant cardiovascular mortality and status epilepticus is uncommon. Class 1 antidysrhythmics produce their toxic effect by blocking sodium channels and no studies have compared bicarbonate to ILE for these drugs. The controversy surrounding efficacy, the adverse effect profile, and the potential interferences with essential laboratory testing question the inclusion of ILE in the care of these poisonings. Clinically relevant studies with clear meaningful endpoints and drug concentration measurements to assess the toxic burden are required before stronger recommendations can be made for these toxins. Thus, the panel vote resulted in a neutral recommendation, leaving the decision to the clinician to weigh the pros and cons of ILE in these situations.
Lipid regimen
The panel chose to vote on a preferred lipid regimen for each category of toxins. However, the results were not significantly different from one toxin to another. In all cases, the vote was neutral with the median ranging from 4 to 6 and the disagreement index always below 1, reflecting the consensus among panel members.
1. ILE formulation:
When ILE is indicated in non-LAs toxicity, our recommendation is neutral regarding the formulation of ILE.
Rationale: Not enough data exist comparing various formulations of ILE. Most articles do not report the brand of ILE used and simply list a concentration of 20%. There is no evidence to support a recommendation for the best formulation of ILE for non-LAs, even though the most common formulation used was Intralipid® 20%. While there is experimental evidence suggesting that one lipid formulation might be preferable to another, it is unclear if the relationship is true for all toxins or is applicable to human poisonings.[Citation24]
2. ILE dosing:
When ILE is indicated in non-LAs toxicity, our recommendation is neutral regarding the dosing of ILE.
Rationale: In the only ILE dose-finding study, the greatest benefit to survival in a rodent model of IV verapamil toxicity occurred with an ILE dose of 18.6 mL/kg.[Citation20] However, the greatest benefit to HR, MAP occurred at 24.8 mL/kg ILE. It is unknown how these doses would translate to human poisonings. Additional concern was expressed over the IV model of poisoning and the risk for lipid-induced increase in gastrointestinal absorption, as noted above. Nevertheless, the finding that increased survival occurred in the group treated with a lower dose of ILE suggests that a higher dose, although associated with greater hemodynamic improvement, may not be necessary in all cases.
Members expressed opinions that until the adverse effect profile for acute ILE administration is properly defined, the lowest possible dose should be used. ILE dosing should be guided by clinically significant physiological endpoints, rather than arbitrary hemodynamic parameters. Importantly, analytical interferences are likely to be shorter in duration or less significant if a lower blood concentration of ILE is achieved.[Citation1] The maximum safe dose of ILE is unknown. Moreover, the reported ILE regimens may vary significantly from the commonly recommended regimen of 1.5 mL/kg bolus of ILE 20% followed by 0.25–0.5 mL/kg/min.[Citation25,Citation26] For a 70 kg person, this dose translates to more than 1 L of lipid emulsion in the first hour at the lowest rate and more than 2 L if the highest infusion rate (0.5 mL/kg/min) is administered. Parenteral nutrition guidelines limit the daily amount of ILE to between 500 and 1200 mL per 24-h period or 10 mL per kg of a 20% formulation.[Citation27] Several reports cite a single rodent study assessing the apparent safety and LD50 of ILE in nine rats.[Citation28] The LD50 is an imperfect measure of safety. Also, this study only included one ILE dose within the range currently used in the clinical setting. No studies have assessed varying doses and infusion rates or their combinations in humans.
The panel agreed that a maximum dose (per kg body weight) should be specified and the rate of infusion kept low. Termination of the infusion should be considered when there is sustained clinical improvement or the maximum dose has been reached. This is speculative and dose-finding studies are much needed. A recent publication called into question cases where exceedingly large volumes of ILE were used and created a pharmacokinetic–pharmacodynamic model to predict an infusion rate that would only produce “modestly lipemic plasma”. Although, based on their model, these authors recommend a regimen of 1.5 mL/kg followed by 0.25 mL/kg/min for 3 min and then an infusion of 0.025 mL/kg/min for up 6.5 h,[Citation29] this regimen should be validated for safety and efficacy before it can be routinely recommended.
3. ILE cessation:
When ILE is indicated in non-LAs toxicity, our recommendation is neutral regarding which endpoints to use to stop ILE administration (maximum dose or maximum duration).
Rationale: The group reached consensus that there is insufficient information to determine when ILE should be stopped. The literature is heterogeneous in the endpoints to therapy from resolution of symptoms to an arbitrary decrease of serum TCA concentration resulting in duration of therapy of up to several days.[Citation30] No clinical studies exploring different endpoints to therapy have been published to date. The workgroup suggests that clinical resolution is a desirable endpoint if toxicity is unlikely to recur, but if this endpoint takes time, consideration should be made not to exceed the rate of endogenous triglyceride metabolism as discussed above. The group noted that in many of the reports ILE was started along with other therapies and continued for hours or days even though the effect of ILE was unclear. The workgroup could not find evidence to suggest a specific endpoint. However, adverse effects seem to be associated with higher volume and faster infusion rates of ILE.[Citation3] Analytical interferences and inability to measure several biochemical parameters and the unknown effect of ILE on other medications may justify lower doses and a shorter duration of infusion.[Citation1]
Limitations
Despite the fact that our combined search strategies were exhaustive and not limited by language, it is possible that some publications were missed; especially abstracts not yet published as full articles. The literature infrequently reports the concentration of the toxin, precise timing of interventions with regards to clinical improvement or important information on the formulation, or total amount of ILE given. Also, in most case reports, multiple therapies were administered simultaneously, making the specific efficacy of lipid emulsion difficult to ascertain. Case reports are known to be subject to publication bias. We also noticed the majority of publications failed to mention the presence or absence of adverse effects and when events occurred there was a tendency to attribute them to the toxin rather than the therapy. Our clinical appraisal for the non-LA toxins was limited because most of the controlled studies occurred in animals and the majority were not performed with models bearing any resemblance to clinical poisonings, which usually involve ingestion of the toxin. The greatest limitation of these recommendations may be their reliance on published data which is often of poor quality, may have significant publication delay of up to several years, and cannot capture the vast numbers of positive and negative outcomes of ILE that remain unreported. Additionally, it should be noted that the Delphi method is itself imperfect, with the greatest criticism being its tendency to force “middle-of-the-road” consensus.[Citation31] We feel we have addressed this concern in three ways: limiting the number of rounds of voting to two, in order not to artificially force consensus by repeated regression to the mean, using a disagreement index to demonstrate the true strength of the recommendations, and the inclusion of minority opinions in the comments and rationale when they were provided by the workgroup members. Also, patients’ views and preferences were not directly sought in the development of the clinical recommendations due to the highly heterogeneous target population under study. Finally, as the study and clinical use of ILE continues to evolve, we recognize additional information may emerge to alter this analysis.
Discussion
Despite some early enthusiasm for the use of ILE in the treatment of acute poisoning, the voting panel found an absence of evidence to recommend its use in most poisonings and clinical scenarios where its use is previously reported. Thus the preponderance of neutral votes likely represents the workgroups’ caution to make recommendations for or against a therapy where so little moderate- or high-quality human data exist. Furthermore, it is worth reiterating that the neutral recommendations result from a balance between pro and con assessments (rather than a lack of data which would result in no recommendation at all) but rather; based on disagreement index, represent a strong consensus around neutrality.
Moreover, many specific aspects of ILE therapy have not been validated in the clinical setting. These include the optimal dose of ILE for clinical efficacy, the threshold dose for adverse effects, and the minimum or maximum duration of therapy. It is acknowledged that clinicians may have personal preferences for indication, dose, and duration of ILE treatment. Given that there is a lack of evidence to support any particular approach, the workgroup felt that it reasonable to comment that the most common formulation reported was Intralipid® 20%, and that the most common regimen was a bolus of 1.5 mL/kg of ILE 20% followed by an infusion of 0.25 mL/kg/min, in order to provide some guidance in situations were favorable recommendations or suggestions were made. Additionally, the workgroup recognized the lack of data on which to guide the duration of therapy, and, therefore, some members proposed a maximum dose of 10% of blood volume based on safety concerns but this position was not officially voted upon. This is somewhat in keeping with parenteral nutrition recommendations to limit the maximum dosage of ILE between 10 and 12.5 mL/kg/d of 20% ILE [Citation32] as well reported increased triglyceride concentrations in excess of 15 mmol/L (glycerol-blanked method) when ILE represented more than 10% of test tubes volumes,[Citation14] which for a 70 kg individual with a blood volume of 80 mL/kg would yield 8.0 mL/kg. Indications in which it is easier to measure the risk and benefit of ILE therapy, such as cardiac arrest or systemic toxicity with no other treatment alternatives, may warrant a “trial” of ILE therapy. Conversely, ILE should not be considered in clinical scenarios where the risk of death or organ damage is small, or when there are other accepted treatments, which have not been used first. Concern was expressed as to the lack of data regarding the impact of ILE on the effectiveness of other resuscitative medications or antidotes, or the ability of ILE to interfere with the biochemical and drug assay testing. In addition, outcome data from animal models may not be directly translatable to clinical practice. Notably, the majority of animal studies of ILE efficacy administered toxins intravenously, whereas the majority of clinical poisonings (except for local anesthetics) are the result of oral exposure.
Future research questions
The voting panel is hopeful that randomized controlled trials will be undertaken to enable a more informed evaluation of the role of ILE in select poisonings. Efforts in animals should be directed at designing controlled studies evaluating the timing of administration, using orogastric administration of the toxin. In particular, studying the dose–response relationship for the loading–dose and the infusion is important, while clearly reporting on the presence or absence of adverse effects. Ideally, these studies would also focus on determining the optimal endpoint for ILE therapy. In vitro studies may be sufficient to evaluate the potential interferences of ILE on assays of common co-ingestants or binding affinity with other medications. Moreover, the efficacy of commercially available lipid emulsions should be compared in order to determine the relative effectiveness of the commercially available products.
Conclusion
ILE is a recent therapy for which there is an incomplete understanding of its efficacy, mechanisms of action, safety, and associated analytical interferences. Clinical recommendations regarding the use of ILE in poisoning were only possible in a small number of scenarios based on a very low quality of evidence, balance of risks and benefits, and resource use. The workgroup emphasizes that human dose-finding and randomized controlled studies are required to advance knowledge of limitations, indications, adverse effects, effectiveness, and best regimen for ILE treatment.
External review
The American Academy of Clinical Toxicology, the American College of Medical Toxicology, the American Association of Poison Control Centers, the Asia Pacific Association of Medical Toxicology, the European Association of Poison Centres and Clinical Toxicologists, and the Canadian Association of Poison Control Centers endorsed these recommendations prior to publication.
Applicability
The recommendations will be circulated to the membership of each association, published in a participating association’s sponsored-journal and presented at international conferences.
Planned update
These recommendations are to be updated in 5 years unless new data warrants an earlier review.
Acknowledgements
The workgroup gratefully acknowledge the work of the AACT staff for the arrangement of meetings and conference calls and Marielle Carpenter for copy editing.
Disclosure statement
All members completed a conflict of interest form for the AACT and received no honoraria. Webcast conference and rooms for meeting were provided by the AACT. No member with a financial or academic conflict of interest preventing an objective assessment of the literature participated in the reviews or the elaboration of the recommendations (i.e. no committee member’s livelihood or academic career is depending on a grant studying lipid emulsion in poisoning).
Funding
Dr. Turgeon is a recipient of a New Investigator Award from the Canadian Institutes of Health Research (CIHR). Dr. Lavergne is a recipient of a salary support award from the Fonds de la Recherche du Québec – Santé (FRQS).
References
- Grunbaum AM, Gilfix BM, Hoffman RS, et al. Review of the effect of intravenous lipid emulsion on laboratory analyses. Clin Toxicol. 2016;54:92–102.
- Hoegberg LC, Bania TC, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol. 2016;54:167–193.
- Hayes BD, Gosselin S, Calello DP, et al. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin Toxicol. 2016;54:365–404.
- Levine M, Hoffman RS, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol. 2016;54:194–221.
- Gosselin S, Morris M, Miller-Nesbitt A, et al. Methodology for AACT evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol. 2015;53:557–564.
- Cormier M, Gosselin S. Cost and availability of intravenous lipid emulsion therapy: a worldwide survey. [Abstract] 36th International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 24–27 May, 2016, Madrid, Spain. Clin Toxicol (Formerly J Toxicol: Clin Toxicol). 2016;54:495–496.
- Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84:622–623.
- American Academy of Neurology. Coma [Internet] [cited 2016 Jun 15]. [Available from: https://patients.aan.com/disorders/index.cfm?event=view&disorder_id=893.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. 2008;336:924–926.
- Hiller DB, Gregorio GD, Ripper R, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111:498–505.
- Carreiro S, Blum J, Jay G, et al. Intravenous lipid emulsion alters the hemodynamic response to epinephrine in a rat model. J Med Toxicol: Off J Am Coll Med Toxicol. 2013;9:220–225.
- Hicks SD, Salcido DD, Logue ES, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacaine-induced cardiac arrest. Anesthesiology. 2009;111:138–146.
- Mayr VD, Mitterschiffthaler L, Neurauter A, et al. A comparison of the combination of epinephrine and vasopressin with lipid emulsion in a porcine model of asphyxial cardiac arrest after intravenous injection of bupivacaine. Anesth Analg. 2008;106:1566–1571.
- Grunbaum AM, Gilfix BM, Gosselin S, et al. Analytical interferences resulting from intravenous lipid emulsion. Clin Toxicol (Formerly J Toxicol: Clin Toxicol). 2012;50:812–817.
- Aditianingsih D, George YW. Guiding principles of fluid and volume therapy. Best Pract Res Clin Anaesthesiol. 2014;28:249–260.
- Intralipid [package insert]. Missisauga (ON): Fresenius Kabi; 2013.
- Kasnavieh SMH. Intravenous lipid emulsion for the treatment of tricyclic antidepressant toxicity a randomized controlled trial. VIIth Mediterranean Emergency Medicine Congress; 2013 September 8–11; Marseille, France.
- Perichon D, Turfus S, Gerostamoulos D, et al. An assessment of the in vivo effects of intravenous lipid emulsion on blood drug concentration and haemodynamics following oro-gastric amitriptyline overdose. Clin Toxicol. 2013;51:208–215.
- Perichon D, Turfus S, Graudins A. Intravenous lipid emulsion does not improve hemodynamics or survival in a rodent model of oral verapamil poisoning. Clin Toxicol. 2013;51:277.
- Perez E, Bania TC, Medlej K, et al. Determining the optimal dose of intravenous fat emulsion for the treatment of severe verapamil toxicity in a rodent model. Acad Emerg Med. 2008;15:1284–1289.
- Carreiro S, Blum J, Hack JB. Pretreatment with intravenous lipid emulsion reduces mortality from cocaine toxicity in a rat model. Ann Emerg Med. 2014;64:32–37.
- Heinonen JA, Litonius E, Backman JT, et al. Intravenous lipid emulsion entraps amitriptyline into plasma and can lower its brain concentration – an experimental intoxication study in pigs. Basic Clin Pharmacol Toxicol. 2013;113:193–200.
- Jang DH, Manini AF, Trueger NS, et al. Status epilepticus and wide-complex tachycardia secondary to diphenhydramine overdose. Clin Toxicol. 2010;48:945–948.
- Li Z, Xia Y, Dong X, et al. Lipid resuscitation of bupivacaine toxicity: long-chain triglyceride emulsion provides benefits over long- and medium-chain triglyceride emulsion. Anesthesiology. 2011;115:1219–1228.
- American College of Medical Toxicology Position Statement: interim guidance for the use of lipid resuscitation therapy. J Med Toxicol: Off J Am Coll Med Toxicol. 2011;7:81–82.
- American College of Medical ToxicologyPosition Statement: Guidance for the Use of Intravenous Lipid Emulsion. J Med Toxicol: Off J Am Coll Med Toxicol. 2016 [cited 2016 Apr 27].
- Mirtallo J, Canada T, Johnson D, et al. Safe practices for parenteral nutrition. JPEN J Parenter Enteral Nutr. 2004;28:S39–S70.
- Hiller DB, Di Gregorio G, Kelly K, et al. Safety of high volume lipid emulsion infusion: a first approximation of LD50 in rats. Reg Anesth Pain Med. 2010;35:140–144.
- Fettiplace MR, Akpa BS, Rubinstein I, et al. Confusion about infusion: rational volume limits for intravenous lipid emulsion during treatment of oral overdoses. Ann Emerg Med. 2015;66:185–188.
- Agarwala R, Ahmed SZ, Wiegand TJ. Prolonged use of intravenous lipid emulsion in a severe tricyclic antidepressant overdose. J Med Toxicol: Off J Am Coll Med Toxicol. 2014;10:210–214.
- Yousuf MI. Using experts’ opinions through Delphi technique. Pract Assess Res Eval. 2007;12:1–8.
- Mirtallo JM, Dasta JF, Kleinschmidt KC, et al. State of the art review: intravenous fat emulsions: current applications, safety profile, and clinical implications. Ann Pharmacother. 2010;44:688–700.
- Udelsmann A, Melo S. Hemodynamic changes with two lipid emulsions for treatment of bupivacaine toxicity in swines. Acta Cirurgica Brasileira/Sociedade Brasileira Para Desenvolvimento Pesquisa Em Cirurgia. 2015;30:87–93.
- Zaballos M, Sevilla R, Gonzalez J, et al. Analysis of the temporal regression of the QRS widening induced by bupivacaine after Intralipid administration. Study in an experimental porcine model. Revista Espanola Anestesiol Y Reanimacion. 2016;63:13–21.
- Bourque S, Panahi S, Mittal R, et al. Intralipid reverses propofol mediated hypotension in rats. FASEB J Conference: Exp Biol. 2015;29. 1 (Meeting Abstracts).
- Ceccherini G, Perondi F, Lippi I, et al. Intravenous lipid emulsion and dexmedetomidine for treatment of feline permethrin intoxication: a report from 4 cases. Open Vet J. 2015;5:113–121.
- Herring JM, McMichael MA, Corsi R, et al. Intravenous lipid emulsion therapy in three cases of canine naproxen overdose. J Vet Emerg Crit Care (San Antonio). 2015;25:672–678.
- Jourdan G, Boyer G, Raymond-Letron I, et al. Intravenous lipid emulsion therapy in 20 cats accidentally overdosed with ivermectin. J Vet Emerg Crit Care (San Antonio). 2015;25:667–671.
- Peacock RE, Hosgood G, Swindells KL, et al. A randomized, controlled clinical trial of intravenous lipid emulsion as an adjunctive treatment for permethrin toxicosis in cats. J Vet Emerg Crit Care (San Antonio). 2015;25:597–605.
- Saqib M, Abbas G, Mughal MN. Successful management of ivermectin-induced blindness in an African lion (Panthera leo) by intravenous administration of a lipid emulsion. BMC Vet Res. 2015;11:287.
- Seitz MA, Burkitt-Creedon JM. Persistent gross lipemia and suspected corneal lipidosis following intravenous lipid therapy in a cat with permethrin toxicosis. J Vet Emerg Crit Care (San Antonio). 2016.
- Varney SM, Bebarta VS, Boudreau SM, et al. Intravenous lipid emulsion therapy for severe diphenhydramine toxicity: a randomized, controlled pilot study in a swine model. Ann Emerg Med. 2016;67:196–205 e3.
- Williams K, Wells RJ, McLean MK. Suspected synthetic cannabinoid toxicosis in a dog. J Vet Emerg Crit Care (San Antonio). 2015;25:739–744.
- Glavas J, Flam D, Istvanic T, et al. Abstracts and Highlight Papers of the 34th Annual European Society of Regional Anaesthesia & Pain Therapy (ESRA) Congress 2015. Reg Anesth Pain Med. 2015;40:e1–e72. [https://doi.org/WorldCat]
- Goucher HC, Asher Y. The best-laid plans: a case report of an epidural anesthetic gone awry in a medically challenging patient. Anesth Analg. 2015;1:S471.
- Kamel I, Trehan G, Barnette R. Intralipid therapy for inadvertent peripheral nervous system blockade resulting from local anesthetic overdose. Case Rep Anesthesiol. 2015;2015:486543.
- Musielak M, McCall J. Lipid rescue in a pediatric burn patient. J Burn Care Res: Off Publ Am Burn Assoc. 2015.
- Procopio G, Gupta A, Hernandez M, et al. Lipid emulsion for inadvertent intrathecal administration of local anesthetics in the ED. Crit Care Med. 2015;1:309.
- Riff C, Guilhaumou R, Dupouey J, et al. Amino-amides anesthetics systemic toxicity: about nine cases. Fundam Clin Pharmacol. 2015;29:48.
- Shih RD, Calello D. Complete spinal paralysis following a brachial plexus block treated with lipid infusion. Clin Toxicol. 2015;53:310–311.
- Stella FA, Panzavolta FG, Sesana F, et al. Intravenous lipid emulsion for treatment of local anesthetic systemic toxicity. Clin Toxicol. 2015;53:313.
- Agulnik A, Kelly D, Bruccoleri R, et al. Severe carbamazepine overdose treated with lipid emulsion therapy, hemodialysis, and plasmapheresis. Crit Care Med. 2015;43:154.
- Aksel G, Guneysel O, Tasyurek T, et al. Intravenous lipid emulsion therapy for acute synthetic cannabinoid intoxication: clinical experience in four cases. Case Rep Emerg Med. 2015;2015:180921.
- Barton CA, Johnson NB, Mah ND, et al. Successful treatment of a massive metoprolol overdose using intravenous lipid emulsion and hyperinsulinemia/euglycemia therapy. Pharmacotherapy. 2015;35:e56–e60.
- Baruah U, Sahni A, Sachdeva HC. Successful management of aluminium phosphide poisoning using intravenous lipid emulsion: report of two cases. Indian J. 2015;19:735–738.
- Bayram B, Kose I, Avci S, et al. Successful treatment of propafenone intoxication with intravenous lipid emulsion. Pharmacotherapy. 2015;35:e149–e152.
- Besserer F, Chuang R, Mink M, et al. Tilmicosin toxicity successfully treated with calcium, insulin, and lipid emulsion. Clin Toxicol. 2015;53:711–712.
- Brumfield E, Bernard KR, Kabrhel C. Life-threatening flecainide overdose treated with intralipid and extracorporeal membrane oxygenation. Am J Emerg Med. 2015;33:1840 e3–5.
- Christianson D, Vazquez-Grande G, Strumpher J, et al. Veno-arterial extracorporeal membrane oxygenation to treat cardiovascular collapse following a massive diltiazem overdose. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS; 2015. P. 191 (no pagination).
- Czerwonka E, Heim M. Lipid rescue therapy and hyperinsulinemia/euglycemia for atenolol and zolpidem overdose. Crit Care Med. 2015;43:316.
- Doepker B, Healy W, Cortez E, et al. High-dose insulin and intravenous lipid emulsion therapy for cardiogenic shock induced by intentional calcium-channel blocker and Beta-blocker overdose: a case series. J Emerg Med. 2014;46:486–490.
- Gerard L, Galloy AC, Capron A, et al. Mixed amlodipine/valsartan overdose treated by the molecular adsorbent recirculating system (MARS). Clin Toxicol. 2015;53:573–577.
- Hatten BW. QRS widening associated with quetiapine toxicity. Clin Toxicol. 2015;53:698–699.
- Johnson-Arbor K, Salinger L, Luczycki S. Prolonged laboratory interference after administration of intravenous lipid emulsion therapy. J Med Toxicol: Off J Am Coll Med Toxicol. 2015;11:223–226.
- Jovic-Stosic J, Putic V, Zivanovic D, et al. Failure of intravenous lipid emulsion in treatment of cardiotoxicity caused by mixed overdose including dihydropyridine calcium channel blockers. Vojnosanit Pregl. 2016;73:88–91.
- Khan A, Raman J, Lateef O. ECMO for shock due to drug overdose: timely transfer to ECMO capable center can save lives. Chest. 2015;148:197A.
- Laes JR, Williams DM, Cole JB. Improvement in hemodynamics after methylene blue administration in drug-induced vasodilatory shock: a case report. J Med Toxicol: Off J Am Coll Med Toxicol. 2015;11:460–463.
- Li K, Gugelmann H, Tabas JA. Young woman with seizures. Ann Emerg Med. 2015;66:363–367.
- Lookabill SK, Carpenter C, Ford MD. Accidental pediatric flecainide overdose treated with intravenous lipid emulsion and sodium bicarbonate. Clin Toxicol. 2015;53:701–702.
- Markota A, Hajdinjak E, Rupnik B, et al. Treatment of near-fatal beta blocker and calcium channel blocker intoxication with hyperinsulinemic euglycemia, intravenous lipid emulsions and high doses of norepinephrine. Signa Vitae. 2015;10:144–150.
- Matsumoto H, Ohnishi M, Takegawa R, et al. Effect of lipid emulsion during resuscitation of a patient with cardiac arrest after overdose of chlorpromazine and mirtazapine. Am J Emerg Med. 2015;33:1541 e1–2.
- McBeth PB, Missirlis PI, Brar H, et al. Novel therapies for myocardial irritability following extreme hydroxychloroquine toxicity. Case Rep Emerg Med. 2015;2015:692948.
- Montague AJ, Topeff JM, Katzung KG, et al. High dose insulin to treat propranolol toxicity in a 7-month-old infant. Clin Toxicol. 2015;53:740.
- Mukhtar O, Archer JR, Dargan PI, et al. Lesson of the month 1: acute flecainide overdose and the potential utility of lipid emulsion therapy. Clin Med (London, England). 2015;15:301–303.
- Ovakim DH, Gill GK, Kaila KS, et al. Near cardiovascular collapse following intentional European Yew (Taxus baccata) ingestion. Clin Toxicol. 2015;53:747–748.
- Ozcete E, Uz I, Kiyan S. Cardiac arrest due to a lethal dose of propafenone and first case to survive following treatment with intravenous fat emulsion. Nobel Med. 2015;11:89–92.
- Radley D, Droog S, Barragry J, et al. Survival following massive amitriptyline overdose: the use of intravenous lipid emulsion therapy and the occurrence of acute respiratory distress. J Intensive Care Soc. 2015;16:181–182.
- Reynolds JC, Judge BS. Successful treatment of flecainide-induced cardiac arrest with extracorporeal membrane oxygenation in the ED. Am J Emerg Med. 2015;33:1542 e1–1542.
- Sampson CS, Bedy SM. Lipid emulsion therapy given intraosseously in massive verapamil overdose. Am J Emerg Med. 2015;33:1844 e1.
- Schult R, Gorodetsky RM, Wiegand TJ. Intravenous lipid emulsion for amlodipine/benazepril toxicity: a case report. Clin Toxicol. 2015;53:251–252.
- Sebe A, Disel NR, Acikalin Akpinar A, et al. Role of intravenous lipid emulsions in the management of calcium channel blocker and beta-blocker overdose: 3 years experience of a university hospital. Postgrad Med. 2015;127:119–124.
- Su J. Early venoarterial extracorporeal membrane oxygenation for refractory shock secondary to amlodipine overdose. J Am Coll Cardiol. 2015;65:A627.
- Szadkowski M, Caravati EM, Gamboa D, et al. Treatment of flecainide toxicity in a 12-monthold child using intravenous lipid emulsion. Clin Toxicol. 2015;53:724.
- Tse CW, Chan YC, Lau FL. Intravenous lipid emulsion as antidote: experience in Hong Kong. Hong Kong J Emerg Med. 2015;22:100–107.
- Tse J, Ferguson K, Whitlow KS, et al. The use of intravenous lipid emulsion therapy in acute methamphetamine toxicity. Am J Emerg Med. 2016;34:1732.e3–4.
- Vodnala D, Rosman H, Shakir A. A wide complex rhythm, does it tell the whole story? J Am Coll Cardiol. 2015;65:A750.
- Watt P, Malik D, Dyson L. Gift of the glob – is it foolproof? Anaesthesia. 2009;64:1031–1033.
- Yesilbas O, Kihtir HS, Altiti M, et al. Acute severe organophosphate poisoning in a child who was successfully treated with therapeutic plasma exchange, high-volume hemodiafiltration, and lipid infusion. J Clin Apher. 2015. Epub 2015 Aug 14.
Appendix 1: Voting statements
General statement
Lipid emulsion is indicated in the treatment of XYZ toxicity.
Specific indications
Lipid emulsion is indicated in the treatment of XYZ toxicity:
In the presence of cardiac arrest, after Standard ACLS (CPR, airways) has been started
In the presence of LIFE-THREATENING toxicity
Lipid emulsion should be administered as first line therapy
Lipid emulsion be administered as part of treatment modalities
Lipid emulsion should be administered if other therapies fail (last resort)
In the presence of NON LIFE-THREATENING toxicity
Lipid emulsion should be administered as first line therapy
Lipid emulsion be administered as part of treatment modalities
Lipid emulsion should be administered if other therapies fail (last resort)
Types of ILE
The type of ILE to be used is…
Intralipid®10%
Intralipid® 20%
Intralipid® 30%
ClinOleic® 20%
Lipofundin® 20%
Other
If using a bolus of ILE the dose of the bolus indicated is…
0.25 mL/kg
0.50 mL/kg
0.75 mL/kg
1.0 mL/kg
1.25 mL/kg
1.5 mL/kg
1.75 mL/kg
2.0 mL/kg
Other
If using an infusion of ILE the dose of the infusion indicated is…
0.25 mL/kg/min
0.5 mL/kg/min
0.75 mL/kg/min
1.0 mL/kg/min
Other
Cessation of ILE
The decision to terminate the ILE treatment is indicated based on:
Total (maximum) duration of the infusion regardless of dose or clinical improvement status
Total (maximum) dose administered regardless of duration of infusion or clinical improvement status
Clinical improvement regardless of dose or duration administered
Other
In considering the total duration of the infusion as a criterion, lipid emulsion cessation is indicated, regardless of other factors such as clinical improvement or dose after…
10–20 min
21–30 min
31–40 min
41–50 min
51–60 min
Other
In considering the maximum dose of lipid emulsion administered as a criterion, lipid emulsion cessation is indicated, regardless of clinical improvement or duration after…
8 mL/kg or less
8.1–10.0 mL/kg
10.1–12.0 mL/kg
12.1–14.0 mL/kg
14.1–16.0 mL/kg
16.1–18.0 mL/kg
18.1–20.0 mL/kg
Other
In considering the clinical improvement as a criterion, lipid emulsion cessation is indicated, regardless of dose or duration after…
As soon as symptoms resolution occurred
After resolution of symptoms for 15–30 min
After resolution of symptoms for 31–45 min
After resolution of symptoms for 46–60 min
Other
Appendix 2
Literature update
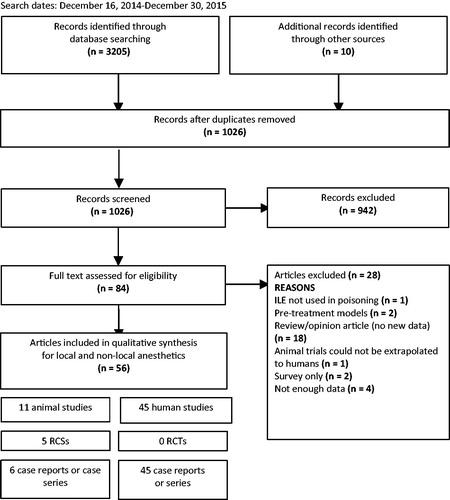
Search results for the year 2015 update: 1026 citations were reviewed to identify studies reporting the use of ILE in poisoning (). Of those, 942 were excluded after reviewing abstracts since the ILE administration was not related to poisoning. Of the 84 remaining articles, 28 were further excluded after reviewing complete manuscripts: one was excluded because ILE was not used for the treatment of poisoning, two were pre-treatment studies, one was an animal experiment that was not generalizable to humans, 18 were review articles without original data, two only reported survey information, and four did not present sufficient data for evaluation. A total of 56 studies were included in the update, from which eleven were animal reports, two regarding LA and nine non-LA poisonings.[Citation33–43]. Forty-five human reports, eight regarding LA and 37 non-LA were included.[Citation44–88] A summary of the new included articles is presented in Appendices 3 and 4.
Local anaesthetics
No new clinical trials were found in humans. Seven case reports and one case series reported a total of twelve cases.[Citation44–51] The LAs reported were lidocaine (n = 2), levobupivacaine (n = 1), bupivacaine (n = 3), ropivacaine (n = 3), combination of bupivacaine and ropivacaine (n = 1), and ropivacaine and lidocaine (n = 2). Patients experienced LA toxicity from different routes of exposure: nerve block (n = 7), intravenous (n = 4), epidural (n = 1), subcutaneous (n = 1), and intrathecal (n = 1) use. Two reports included two routes of LA exposure, intravenous plus epidural and intravenous plus subcutaneous. The toxic effects from LAs included a variety of cardiovascular and central nervous system symptoms including hypotension, cardiac arrest, agitation and coma. The concentration of ILE used was reported as 20% (n = 5) or not reported (n = 7). Dosing regimens and other treatments received were often not reported. In eight cases, authors noted that the symptoms resolved following ILE dosing. Details are provided in Appendix 3.
Two controlled animal experiments in swine assessed the response of bupivacaine toxicity to ILE. In one trial, 12 swine were administered 4–6 mg/kg of bupivacaine until the QRS complex duration was 150% of baseline value. Six swine were then given ILE 1.5 mL/kg followed by 0.25 mL/kg/min and six were given the same volume of 0.9% saline as controls. All animals survived. QRS duration decreased from 184 ± 62% ms to 132 ± 65% ms in the ILE-treated group, while there was no change in QRS duration (230 ± 56% ms) in the control animals (p = 0.03).[Citation34] The other trial included 30 swine, 10 serving as control, 10 receiving ILE as long-chain triglycerides (LCT) and 10 receiving ILE as 50% LCT and 50% medium-chain triglycerides (MCT). All animals were administered 5 mg/kg of bupivacaine followed by the study drug while monitoring hemodynamic parameters. A dose of 4 mL/kg of either ILE preparation or saline was administered to each group one minute after bupivacaine dosing. Both ILE groups had a similar improvement in the majority of the hemodynamic parameters compared to control.[Citation33] Details are reported in Appendix 4.
There were no new LA case reports described in animals.
Non-local anesthetics
No new clinical trials in humans were identified. Thirty-seven case reports or case series with miscellaneous toxins were found.[Citation52–88] The summary of human case reports is presented in Appendix 3.
Three animal experiments studied ILE for non-LA toxicity.[Citation35,Citation39,Citation42] In one trial, 20 cats with 14 additional controls were exposed dermally to permethrin. ILE was administered to the treatment group at 15 mL/kg as a continuous intravenous infusion over 60 minutes. A grading system for neurologic effects detected a decrease in the duration and severity of poisoning in the treatment group reported as a decrease in the duration and severity of poisoning in the treatment group.[Citation39] Another randomized experiment in swine sought to determine a difference in mean arterial blood pressure (MAP), QRS duration and survival after diphenhydramine poisoning. Twenty-four animals were equally divided into two groups. One group received ILE (7 mL/kg) and the other sodium bicarbonate. Diphenhydramine was administered intravenously until MAP fell by 50%. No differences were found between groups in the measured parameters.[Citation42] In the third study, rats received intravenous propofol 10 mg/kg to achieve a 55% ±2% drop in MAP. The rats were not randomised but the study included a control group receiving saline. The authors reported that propofol-mediated hypotension was completely reversed by ILE, and the effects on the anaesthetic potency of the drug were minimal.[Citation35] Of note, propofol was not chosen as a toxin to be evaluated for clinical recommendations by the workgroup because of the lack of human data. A summary of the animal case reports is available in Appendix 4.
Appendix 3. Summary of the 72 patients included in the 2015 update on the efficacy of ILE in poisoning.
Appendix 4. Summary of the 11 animal studies included in the 2015 update.
Appendix 5. Vote results