Abstract
Introduction
Novel psychoactive substances (NPS) have been increasingly reported in the last 15–20 years. We aimed to describe presentations to the emergency department (ED) with acute recreational drug toxicity involving NPS.
Methods
Data were extracted from the European Drug Emergencies Network (Euro-DEN) Plus database for all presentations to ED (36 EDs in 24 European countries) with acute toxicity between January 2014 and December 2019. Patient demographics, agents involved, and clinical outcomes were described and the subgroup of presentations involving NPS was compared with the rest of the cohort.
Results
Out of 43,633 Euro-DEN Plus presentations, 3304 (7.6%) involved at least one NPS. Agents were identified mainly based on self-report or clinical presentation, with analytical confirmation being performed only in 17.9% of NPS presentations. The proportion of NPS presentations varied by centre (0–48.8%). For centres where data were available for all 6 years, NPS-related presentations peaked in 2015 (11.9%). In 2014, 78.4% of NPS agents reported were cathinones, while only 3.4% were synthetic cannabinoids (SCs); conversely, in 2019 only 11.6% of NPS agents reported were cathinones, while 72.2% were SCs. NPS-related presentations involved younger patients (median 30 (23–37) vs. 32 (25–40) years, p < 0.001) and more males (84.8 vs. 75.8%, p < 0.001) compared with the rest of the cohort. Patients presenting to ED after using NPS were more likely to self-discharge (22.8 vs. 15.1%), less likely to be admitted to critical care (3.6 vs. 6.1%) but had a longer length of stay in hospital (median 5.1 (2.7–18.7) vs. 4.7 (2.5–9.2) h, p < 0.001). Death occurred in 0.5% of all presentations involving NPS and in 0.4% of non-NPS presentations.
Conclusions
This large multicentre series of NPS presentations to European EDs showed marked geographical variation and changes over time in the proportion of presentations to ED involving NPS, as well as the proportion of NPS subgroups.
Introduction
Use of recreational drugs is common: in 2020, approximately 96 million or 29% of adults in Europe (aged 15–64) are estimated to have used illicit drugs at least once in their lifetime. Additionally, an estimated 20 million young adults (aged 15–34) have used drugs in the last year (17%), with about twice as many males (21%) as females (12%) reporting doing so [Citation1].
Novel psychoactive substances (NPS) have been increasingly reported in the last 15–20 years [Citation1]. They are designed to mimic traditional drugs of misuse, but their chemical structure is modified to avoid existing drug laws. An increasing number of European countries, however, have now changed their legislation to control generic groups of NPS [Citation2–4]. They are usually categorised based either on their chemical structure or their psychoactive effects. An increasing number of countries have been reporting NPS seizures and concerns have been growing over the harm caused by their use [Citation5].
Over 800 different NPS have emerged worldwide in the last two decades and there are 40–50 new NPS – previously undetected on the European market – reported every year to the Early Warning System (EWS) of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). In addition to these new NPS, around 400 previously reported NPS are detected in Europe each year [Citation1]. Because of the rapid emergence of a large number of different compounds, reliable data to guide management of patients presenting with toxicity due to these substances are limited [Citation6]. The pattern of acute toxicity seen with NPS use varies from mild self-limiting features to more severe effects including extreme agitation, seizures, cardiotoxicity, and death [Citation7,Citation8]. The lack of data and the potential for severe toxicity from NPS have led to a significant burden on emergency departments (EDs), with healthcare professionals reporting that they are less confident in managing acute toxicity associated with NPS compared with classical recreational drug toxicity [Citation9].
As individuals experiencing acute toxicity following drug use often present to EDs, these visits provide key epidemiological data on harm in complement to information gathered via other means [Citation1]. Evaluation of healthcare resource utilisation related to acute drug toxicity generally relies on coded hospital admission data which can significantly underestimate numbers [Citation10,Citation11]. In addition, systematic collection of data on ED presentations of drug-related toxicity in Europe is scarce [Citation12]. To address this important public health gap, the European Drug Emergencies Network (Euro-DEN) was formed in 2013 [Citation13,Citation14] aiming to provide a snapshot of the epidemiology of ED presentations related to acute recreational drug toxicity by gathering systematic and structured surveillance data in sentinel centres with toxicological expertise across Europe. The initial Euro-DEN has expanded after the first year to the Euro-DEN Plus project to incorporate more sentinel centres across more European and neighbouring countries.
The main objective of this study was to describe NPS-related presentations to EDs participating in the Euro-DEN Plus project over 6 years, in terms of demographics as well as variations over time and geographical locations. Second, patient demographics and clinical outcomes were compared between presentations involving an NPS and the rest of the cohort.
Materials and methods
Euro-DEN plus project and database
Euro-DEN was created in 2013 and was initially funded by a grant from the European Commission (JUST/2012/DPIP/AG/3591). Since 2014 the network has expanded to form the Euro-DEN Plus project. The case definition for an ED presentation to be included is: i) the patient and/or accompanying person reports the recreational use of psychoactive drug(s) related to the patient presentation to ED; and/or ii) the attending physician records that the presentation is consistent with use of such drug(s); and/or iii) such drug(s) are confirmed via analytical testing [Citation14]. In accordance with usual clinical practice, toxicological analyses were not routinely performed as management was based primarily on clinical features of toxicity and the drugs reported or suspected [Citation15]. Presentations associated with prescription or over-the-counter medication were included if these drugs were used for recreational purposes but not if the presentation was related to an adverse effect or deliberate/accidental self-poisoning of a prescribed or over-the-counter medication. Presentations were also excluded if they were related to lone ethanol toxicity, not directly related to acute recreational drug toxicity (e.g., trauma, infection, and withdrawal), or associated with self-harm. A standardised minimum dataset of key demographic, clinical and outcome variables was purposely designed and collected for all consecutive presentations with acute recreational drug toxicity to participating EDs [Citation13]. Data were handled in compliance with relevant national legislation and local ethical approval.
Overall, presentations were included from 36 centres over 24 countries during the study period (data availability detailed in Table S1).
Study design
All presentations in the Euro-DEN Plus database between 1 January 2014 and 31 December 2019 (72 months) were included. The following variables were extracted from the database for each presentation: (i) demographic data (date of presentation, age, and sex); (ii) drugs reported including co-ingestion of ethanol; (iii) presence/absence of cardiac arrest on presentation; and (iv) outcome data (disposition from ED, length of hospital stay, and death within hospital stay). Overall frequency of NPS-related presentations and their geographical distribution were defined. Focusing on centres reporting cases for all 6 years, annual trends of NPS-related presentations were evaluated. Patient demographics of the whole cohort were analysed. When two or more agents were identified in a single presentation, either by self-report or by analytical confirmation, all agents were considered in their respective groups. NPS were further classified into subgroups according to the European Database on New Drugs (EDND) from the EMCDDA [Citation16]. Patient demographics and outcomes were compared between presentations involving NPS and the rest of the series. The whole cohort was chosen as comparator group because a wide range of pharmacological mechanisms are involved in NPS toxicity and drug classification is arbitrary.
Statistical analysis
Categorical variables were summarised as frequency (percentage) and continuous variables as median (interquartile range, and IQR), except as stated otherwise. To compare NPS presentations and non-NPS presentations, a Mann–Whitney U test was used to for continuous variables while a chi-squared test was used for categorical variables. Where the categorical variable had more than 2 possible values, post-hoc pairwise comparisons were performed using multiple z-tests of two proportions with a Bonferroni correction. The level of significance for all statistical tests was set at p < 0.05. Statistical analysis was performed using SPSS Statistics version 25 (IBM Corp., Armonk, NY).
Results
Between 1 January 2014 and 31 December 2019, there were 43,633 presentations with acute recreational drug toxicity to Euro-DEN Plus centres. NPS use was reported in 3304 (7.6%) presentations, including 70 (0.2%) presentations where multiple NPS were reported. In total, 65,003 agents were reported including 3384 (5.2%) NPS. NPS were the third most common group reported after established illicit drugs (45,517, 70.0%) and prescription only medications (13,443, 20.7%). The most common NPS subgroups reported were synthetic cannabinoids (SCs) (1520, 44.9%), followed by cathinones (1133, 33.5%). Mephedrone represented 66.5% (754) of the cathinones reported. Analytical testing was used in 593 (17.9%) of presentations involving an NPS and in 10,076 (25.0%) of presentations not involving an NPS.
Demographics
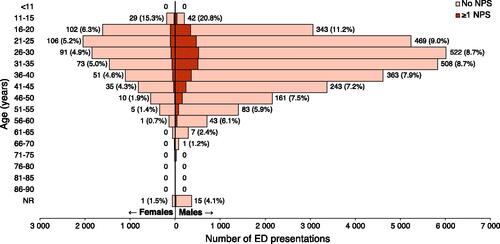
NPS-related presentations involved younger patients (30 (23–37) vs. 32 (25–40) years, p < 0.001) and a larger proportion of males (84.8 vs. 75.8%, p < 0.001) than presentations not involving NPS ().
Figure 1. Demographics of patients presenting to ED for acute drug toxicity. Histogram labels contain absolute number of ED presentations involving at least one NPS (percentage of all drug-related ED presentations). All centres reporting data were included. NR: not recorded; NPS: novel psychoactive substance; ED: emergency department.
Trends over time
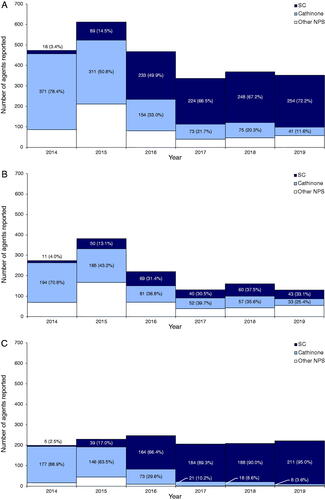
For the 14 Euro-Den Plus centres reporting data for all 6 years, the proportion of presentations involving an NPS peaked in 2015 (593, 11.9%) and thereafter fell to around 6% in 2017–2019 (). Looking at the same subset of centres, there was a significant change in NPS subgroups over the 6-year period (): in 2014, 371 (78.4%) of the 473 NPS agents reported were cathinones, while only 16 (3.4%) were SCs; conversely, in 2019 only 41 (11.6%) of the 352 NPS agents reported were cathinones, while 254 (72.2%) were SCs. This pattern of increase in SCs over time was significantly influenced by the centre reporting the most NPS presentations (STH, London, UK), with the remaining centres reporting a more modest increase in the proportion of SCs from 4.0% (11) to 33.1% (43) (). Among sentinel centres reporting cases for all 6 years, the median (IQR) age of patients reporting NPS use was 29 (24–34) years in 2014, 28 (22–35) years in 2015, 32 (25–38) years in 2016, 32 (26.3–40) years in 2017, 34 (26.8–42) years in 2018, and 34 (27–43) years in 2019.
Figure 2. Number of presentations to ED for acute drug toxicity by year. Histogram labels contain absolute number of ED presentations involving at least one NPS (percentage of all drug-related ED presentations). Only centres reporting data for all 6 years were included. NPS: novel psychoactive substance; ED: emergency department.
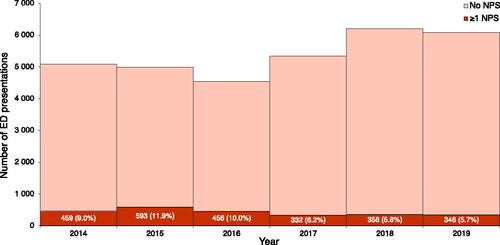
Figure 3. Number of NPS agents leading to ED presentations for acute drug toxicity reported by year. Histogram labels contain absolute number of NPS agents reported by year representing a specific subgroup (percentage of all NPS agents leading to ED presentations for acute drug toxicity). NPS denotes novel psychoactive substance, SC synthetic cannabinoids, ED: emergency department; “Other NPS” include: arylalkylamine, arylcyclohexylamine, indolalkylamine (tryptamine), novel benzodiazepine, novel opioid, phenylethylamine, piperazine, piperidine, plant, tryptamine, unknown or “branded” NPS. A: Centres reporting data for all 6 years only. B: Centres reporting data for all 6 years only, excluding St Thomas’ Hospital in London, UK. C: St Thomas’ Hospital in London, UK only.
Geographic distribution
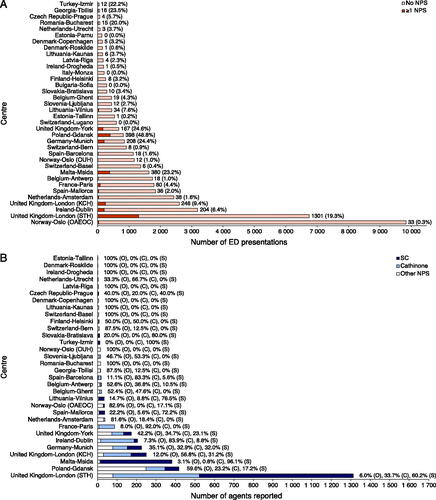
There was significant geographical variation in total number of presentations to ED for acute recreational drug toxicity reported, in the proportion of presentations involving an NPS (), and in the proportions of NPS subgroups reported (). The five hospitals in London (United Kingdom), Oslo (Norway), and Amsterdam (Netherlands) accounted for 52.2% (22,782) of all presentations to ED for acute recreational drug toxicity. Four centres reported no presentations involving an NPS, 20 centres reported less than 5% of their presentations involving an NPS, 6 centres reported more than 20% of their presentations involving an NPS, and Gdansk (Poland) reported 48.8% (418) of presentations involving an NPS (). Amongst centres reporting more than 100 NPS agents over the study period, Dublin (172, 83.9%) and London KCH (142, 56.8%) reported the most cathinone agents, while Msida (366, 96.1%) and London STH (791, 60.2%) reported the most SC agents ().
Figure 4. A: Number of presentations to ED for acute drug toxicity by centre. Histogram labels contain absolute number of ED presentations involving at least one NPS (percentage of all drug-related ED presentations). All centres reporting data were included and are labelled as country-city (centre). NPS: novel psychoactive substance; ED: emergency department; OUH: Oslo University Hospital; OAEOC: Oslo Accident and Emergency Outpatient Clinic; KCH: King’s College Hospital; STH: St Thomas’ Hospital. B: Number of NPS agents leading to ED presentations for acute drug toxicity reported by centre. Histogram labels contain the percentage of all NPS agents leading to ED presentations for acute drug toxicity reported by centre representing a specific subgroup (O) for “other NPS”, (C) for “cathinone” and (S) for “SC”. All centres reporting data were included and are labelled as country-city (centre). NPS: novel psychoactive substance; SC: synthetic cannabinoids; ED: emergency department; OUH: Oslo University Hospital; OAEOC: Oslo Accident and Emergency Outpatient Clinic; KCH: King’s College Hospital; STH: St Thomas’ Hospital.
Co-use of substances
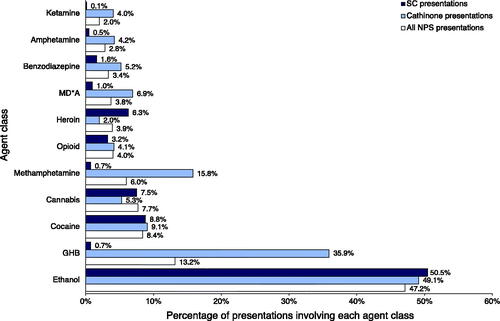
Other agents (excluding ethanol) were co-used in 40% (1319) of presentations involving an NPS, and the patterns differ between NPS subgroups (). Amongst 2388 presentations involving an NPS where information was recorded, 1126 (47.2%) reported ethanol use. GHB was co-used more frequently with cathinones than with SCs (400, 35.9 vs. 11, 0.7%), so was methamphetamine (176, 15.8 vs. 11, 0.7%). Cocaine was associated with 9.1% (101) of cathinone cases and 8.8% (134) of SC cases. Cannabis was reported in only 7.5% (114) of presentations involving SCs.
Figure 5. Other agents most commonly reported in presentations to ED for acute drug toxicity involving at least one NPS. Histogram labels contain the percentage of presentations leading to ED presentations for acute NPS toxicity involving agents of each class. All centres reporting data were included. NPS: novel psychoactive substance; SC: synthetic cannabinoids; ED: emergency department. Agents were grouped as follows: ketamine (ketamine and esketamine); amphetamine (amphetamine); benzodiazepine (alprazolam, clonazepam, diazepam, etizolam, flurazepam, nitrazepam, oxazepam, and unknown benzodiazepine); MD*A (methylenedioxymethamphetamine, methylenedioxyamphetamine, and methylenedioxyethylamphetamine); heroin (heroin); opioid (buprenorphine, codeine, fentanyl, levomethadone, methadone, morphine, oxycodone, tramadol, unknown opioid); methamphetamine (methamphetamine); cannabis (cannabis and cannabidiol); cocaine (cocaine and crack cocaine); GHB (gamma-hydroxybutyric acid and gamma-butyrolactone); ethanol (ethanol). Note: co-ingestion of ethanol was not reported for 27.7% of all presentations involving at least one NPS.
Clinical outcomes
As shown in , patients presenting to ED after using NPS were more likely to self-discharge (22.8 vs. 15.1%) and to be admitted to a general ward (18.9 vs. 12.5%), but less likely to be admitted to critical care (3.6 vs. 6.1%). The length of hospital stay, defined as the time from ED presentation to discharge from hospital, is represented for both groups in . 66% of presentations involving an NPS were discharged within 12 h, 79% within 24 h, and 90% within 48 h. Comparatively, 80% of presentations not involving an NPS were discharged within 12 h, 90% within 24 h, and 95% within 48 h. Presentations involving an NPS had a slightly longer length of stay (5.1 (2.7–18.7) hours) than those not involving an NPS (4.7 (2.5–9.2) h, p < 0.001).
Figure 6. Length of hospital stays for presentations. Length of hospital stay is defined as the time from presentation to the emergency department to discharge from the hospital. In-hospital deaths were censored. All centres reporting data were included. NPS denotes novel psychoactive substance.
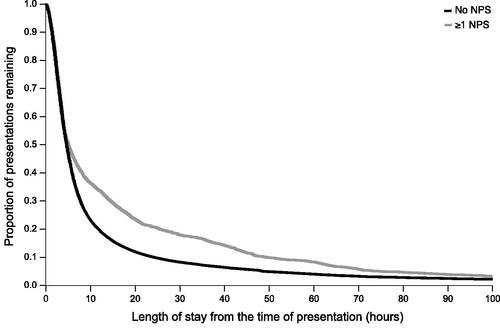
Table 1. Patient outcomes.
There were 15 deaths following presentations to ED reporting an NPS and before discharge from hospital (0.5% of all NPS presentations). Of those, 11 patients presented to ED in cardiac arrest. Six patients died in ED, one patient died within 72 h of presentation, and 8 died 72 h or more after hospital presentation. Mephedrone or methedrone was reported in 7 (46.7%) of these deaths while SCs were involved in 4 (26.7%). Notably, non-NPS agents were co-used in 11 (73.3%) deaths. Most of the deaths (13, 86.7%) were in males, and the median age at death was 34 (24.5–42.5) years.
Discussion
In this large multicentre, multinational series of consecutive presentations to European EDs related to acute toxicity following NPS use over the six-year period 2014–2019: i) the proportion of NPS presentations varied by centre and peaked in 2015; ii) there were proportionally more SCs and less cathinones reported over time; iii) patients presenting to ED after using NPS were less likely to be admitted to critical care but had a longer length of stay in hospital. This large series contributes important knowledge on the harms associated with NPS use in Europe. A few other national studies aim to overcome the limitations of self-report by pairing clinical data during ED presentations with analytical confirmation of biological samples [Citation17–23]. In the series from the STRIDA project [Citation20] (74% involving men), NPS cases peaked in 2014 before decreasing, and stimulants (mainly cathinones) as well as SCs each made up around 20% of NPS cases. Multiple other sources of data must however be considered to fully understand and monitor the use of these emerging drugs of concerns.
Institutions at the international level such as the Early Warning Advisory of the United Nations Office on Drugs and Crime (UNODC) [Citation24] and the EWS of the EMCDDA [Citation1] have adapted to rapidly detect, assess, and respond to health and social threats caused by NPS. Although availability remains high, the global quantities of synthetic NPS seized have been decreasing since 2012 [Citation24]. In Europe, quantities of NPS seized have stabilised or slowly declined since 2015, depending on the region [Citation1]. The rate at which NPS emerge has also decreased. This decrease in availability is likely a result of the introduction of new legislation in countries representing major NPS markets [Citation25,Citation26] as well as countries responsible for NPS production. Concomitantly, our data show a decrease in NPS-related presentations to European EDs since 2015, both as absolute numbers and as proportions of all drug-related presentations (). The almost year-by-year increase of the median age of NPS-related presentations in our data may represent a cohort of aging NPS users without recruitment of younger users.
Seizures of NPS are dominated by SCs and cathinones [Citation1,Citation24], which together accounted for 77% of all NPS seizures reported in 2018 in Europe [Citation1]. SCs represent the largest group of substances reported to the EWS: of the over 620 NPS monitored, 24% are SCs [Citation1]. Previous data from Euro-DEN Plus has shown that the most prevalent subgroups of NPS used have been cathinone derivatives, with mephedrone and methedrone being the most common [Citation27]. A trend previously reported in a single UK centre (i.e., decrease in cathinone presentations and increase in SC presentations) [Citation26] is corroborated in this study, with data heavily influenced by the same centre (). This trend may result from an increase in use of SCs among the socially marginalised, such as the prison and homeless subpopulations [Citation28–31] which may be impacted differently by legislation [Citation32]. More than 22 European countries have reported NPS use in prisons, with SCs representing the main challenge [Citation33].
In the UK, there has been a decrease in poison centre enquiries regarding NPS since 2014–2015. SCs have remained the commonest NPS subgroup triggering enquiries [Citation34]. NPS use has also been linked to fatal cases and post-mortem toxicological analyses provide key information on harm [Citation35,Citation36]. In 2019, NPS were involved in 2.1 deaths per million people in the UK, with SCs implicated in 45%. In our series, we report 0.45% of in-hospital deaths following presentation to ED related to NPS use (), with SCs implicated in 27%. Importantly, hospital and mortality data do not capture episodes of drug use resulting in little or no toxicity. In a US hospital survey, for example, 53% of individuals had used SCs in the last year but none admitted to seeking medical attention after taking these substances [Citation37]. In the UK in 2019–2020, NPS use was reported by 0.8% of all individuals in treatment at drug and alcohol services and by 1% of new individuals entering treatment, with predominantly cannabinoid NPS representing about half of these cases [Citation31]. The number of adults in treatment citing NPS use has been stable since 2016–2017, but citations of mephedrone have decreased since a peak in 2014–2015.
Only a few self-reporting surveys have been carried out to establish prevalence of NPS use in the community [Citation38]. Most studies reported that 3% or less of the general adult population reported recent (typically defined as within the last 12 months) NPS use. Prevalence data for England and Wales show a clear decline in the use of NPS from 0.9% (age 16–59 years) and 2.8% (age 16–24 years) in 2014–2015 to 0.3% (age 16–59 years) and 1.3% (age 16–24 years) in 2018–2019 [Citation39]. In contrast, certain surveys focused on specific sentinel groups. In 2019, the European School Survey Project on Alcohol and Other Drugs (ESPAD), which probed students in Europe aged 15–16 years over 35 countries, reported an average 3.4% of students having used NPS in their lifetime (3.1% for SCs and 1.1% for cathinones) vs. 2.5% in the last 12 months [Citation40]. The Global Drug Survey [Citation41] includes data from drug users in more than 25 countries (more than half of the sample being under 25 years old and going clubbing more than 4 times in a year). In this sample, 3.2% of responders reported seeking emergency medical care in the last 12 months following the use of SCs, and 2.8% following the use of “novel drugs” [Citation42]. In a study screening for classic recreational drugs and NPS and their metabolites in urine of former heroin addicts under methadone maintenance therapy, the authors detected cathinones and SCs in 4.4 and 1.3% of samples analysed, respectively [Citation43].
As drug users in the community may not however have accurate information on the exact substances they are consuming, complementary analytical methods can provide estimates of prevalence and geographical variation. These can target the general population through wastewater analysis [Citation44] or high-risk groups through syringe analysis [Citation45]; and ED data can be complimented with analytical confirmation as has been performed in the IONA (United Kingdom) [Citation17–19] and STRIDA (Sweden) [Citation20,Citation21] projects.
Limitations
It is important to consider that the choice of which agents to include under the NPS classification is arbitrary and varies significantly in the literature (e.g., inclusion of novel benzodiazepines, novel opioids, hallucinogens, GHB, ketamine, etc.) [Citation7]. Meaningful comparisons across studies and institutions are thus challenging. Additionally, according to the United Nations definition [Citation24], NPS are defined as substances of abuse that are not under control by international legislation. This definition therefore changes as new legislation is introduced and trends over time are difficult to interpret. In our study, agents were classified according to the EDND from the EMCDDA in order to standardise definitions across centres and over time.
Case identification was handled by each centre independently which may have resulted in different strategies and potentially different proportions of ED presentations related to acute drug toxicity captured.
Although our series is large, each country is represented by 1–3 centres only. These centres voluntarily contribute data to the Euro-DEN Plus network and are primarily located in large cities with different capacities and catchment areas [Citation46] which may therefore not represent the whole spectrum of European EDs. Indeed, while the two Spanish centres in the Euro-DEN Plus registry reported NPS in 1.6 and 2.0% of all drug-related ED presentations, this percentage was 0.3% in a registry of 11 Spanish EDs [Citation47]. These factors, along with city and country, likely contributed to the differences observed in the geographical distribution of NPS presentations. Furthermore, the 4 centres reporting the highest number of presentations represent more than half of presentations across all 36 centres in our series (), thereby limiting generalisability of results.
Agents are identified based on patient’s and witness’ self-report or clinical presentation, with analytical confirmation being performed in a minority of presentations. In our database, although there was generally a high degree of agreement between self-reported drugs and analytical toxicology results for commonly used illicit drugs (e.g., heroin and cocaine), NPS were reported more frequently in centres where mass spectrometry was available than in centres that performing immunoassays only [Citation48]. Another study from 2 Norwegian centres participating in Euro-DEN Plus reported that NPS were found in 8% of screened cases, though they were suspected in none [Citation49]. It is therefore likely that the prevalence of NPS is clinically underestimated in our study. Moreover, even in cases where patients self-report a specific agent, drug names can be misinterpreted (e.g., Mephedrone and Methedrone), and the chemical composition of products obtained may not be as advertised [Citation50–53].
Finally, data was entered into the database prospectively by each centre based on information available in patients’ clinical records, which resulted in a proportion of missing data for certain variables (as seen in and ). Presentations with missing data were not deleted in a listwise fashion to avoid introducing bias.
Conclusions
This large multicentre and multinational series of presentations to EDs following acute NPS toxicity showed significant variation over centres and over time, with a peak in presentations in 2015 and proportionally more SCs and less cathinones reported over time. Patients presenting with NPS toxicity are predominantly male and young, although median age seemed to increase over time. The data presented here are important to understand harms related to NPS use and healthcare resource utilisation. Triangulation of this data with complementary sources, such as drug seizures, poison centre enquiries, self-report surveys, and drug-related deaths will enable a greater understanding of the public health implications of NPS use in Europe.
Supplemental Material
Download Zip (35.1 KB)Acknowledgements
Euro-DEN Research Group corporate authors: Lukasz Anand, Kurt Anseeuw, Robertas Badaras, Jeffrey Bonnici, Miran Brvar , Blazena Caganova, Feriyde Calýskan, Alessandro Ceschi, Karam Chamoun, Laurence Daveloose, Miguel Galicia, Stefanie Geith, Ketevan Gorozia, Damjan Grenc, Femke Gresnigt, Laura Hondebrink , Gesche Jürgens, Jutta Konstari, Soso Kutubidze, Gabija Laubner, Evangelia Liakoni, Viesturs Liguts, Cathelijne Lyphout, Thomas MacMahon, Bruno Mégarbane , Adrian Moughty, Gabriela Viorela Nitescu, Roberta Noseda , Niall O'Connor, Raido Paasma, Juan Ortega Perez, Marius Perminas, Per Sverre Persett, Kristiina Põld, Jordi Puiguriguer, Julia Radenkova-Saeva, Jan Rulisek, Yasmin Schmid, Irene Scholz, Roberts Stašinskis , Jonas Surkus, Wesley van den Busken, Irma van den Hengel-Koot, Federico Vigorita, Wojciech Waldman, William Stephen Waring, Sergej Zacharov (Affiliations detailed in Table S2).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- European Monitoring Centre for Drugs and Drug Addiction. European drug report 2020: trends and developments. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2020. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf
- Tracy DK, Wood DM, Baumeister D. Novel psychoactive substances: types, mechanisms of action, and effects. BMJ. 2017;356:i6848.
- Gibbons S. Legal highs’–novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clin Toxicol (Phila). 2012;50(1):15–24.
- European Monitoring Centre for Drugs and Drug Addiction. European drug report 2018: trends and developments. Lisbon: Office for Official Publications of the European Communities; 2018. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/8585/20181816_TDAT18001ENN_PDF.pdf
- United Nations Office on Drugs and Crime. World drug report 2018. 2018. [cited 2022 Feb 1]. https://www.unodc.org/wdr2018/en/exsum.html
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol. 2011;49(8):705–719.
- Logan BK, Mohr ALA, Friscia M, et al. Reports of adverse events associated with use of novel psychoactive substances, 2013–2016: a review. J Anal Toxicol. 2017;41(7):573–610.
- Luethi D, Liechti ME. Designer drugs: mechanism of action and adverse effects. Arch Toxicol. 2020;94(4):1085–1133.
- Wood DM, Ceronie B, Dargan PI. Healthcare professionals are less confident in managing acute toxicity related to the use of new psychoactive substances (NPS) compared with classical recreational drugs. QJM. 2016;109(8):527–529.
- Wood DM, De La Rue L, Hosin AA, et al. Poor identification of emergency department acute recreational drug toxicity presentations using routine hospital coding systems: the experience in Denmark, Switzerland and the UK. J Med Toxicol. 2019;15(2):112–120.
- Wood DM, Conran P, Dargan PI. ICD-10 coding: poor identification of recreational drug presentations to a large emergency department. Emerg Med J. 2011;28(5):387–389.
- Heyerdahl F, Hovda KE, Giraudon I, et al. Current European data collection on emergency department presentations with acute recreational drug toxicity: gaps and national variations. Clin Toxicol (Phila). 2014;52(10):1005–1012.
- Wood DM, Heyerdahl F, Yates CB, et al. The European drug emergencies network (Euro-DEN). Clin Toxicol (Phila). 2014;52(4):239–241.
- European Monitoring Centre for Drugs and Drug Addiction. Drug-related hospital emergency presentations in Europe: update from the Euro-DEN Plus expert network. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2020. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/12725/TD02AY20001ENN.pdf
- Grafinger KE, Liechti ME, Liakoni E. Clinical value of analytical testing in patients presenting with new psychoactive substances intoxication. Br J Clin Pharmacol. 2020;86(3):429–436.
- Potts AJ, Cano C, Thomas SHL, et al. Synthetic cannabinoid receptor agonists: classification and nomenclature. Clin Toxicol (Phila). 2020;58(2):82–98.
- Hill SL, Najafi J, Dunn M, et al. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB-CHMICA. A report from the identification of novel psychoActive substances (IONA) study. Clin Toxicol. 2016;54(8):638–643.
- White JC, Wood DM, Hill SL, et al. Acute toxicity following analytically confirmed use of the novel psychoactive substance (NPS) methiopropamine. A report from the identification of novel psychoActive substances (IONA) study. Clin Toxicol. 2019;57(7):663–667.
- Blanco G, Vidler D, Roper C, et al. Acute toxicity from the synthetic cathinone N-ethylpentylone (ephylone) in the United Kingdom. Clin Toxicol (Phila). 2021;59:1270–1273.
- Helander A, Backberg M, Beck O. Drug trends and harm related to new psychoactive substances (NPS) in Sweden from 2010 to 2016: experiences from the STRIDA project. PLoS One. 2020;15(4):e0232038.
- Helander A, Backberg M, Hulten P, et al. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci Int. 2014;243:23–29.
- Monte AA, Hopkinson A, Saben J, et al. The psychoactive surveillance consortium and analysis network (PSCAN): the first year. Addiction. 2020;115(2):270–278.
- Partridge E, Alfred S, Camilleri A, et al. Establishing the protocols for the South Australian emergency department admission blood psychoactive testing (EDABPT) programme for drug surveillance. Emerg Med Australas. 2021;33:883–887.
- United Nations Office on Drugs and Crime. World drug report 2020. San Francisco (CA): United Nations; 2020. [cited 2022 Feb 1]. https://wdr.unodc.org/wdr2020/en/index2020.html
- Smyth BP, O'Farrell A, Cullen W. Drug-related medical hospital admissions during and after a period of head shop expansion. Eur J Public Health. 2021;31(2):285–291.
- Webb NE, Wood DM, Greene SL, et al. Change in the new psychoactive substances associated with emergency department acute toxicity presentations associated with the introduction of the UK 2016 psychoactive substances act. Clin Toxicol (Phila). 2019;57(1):36–41.
- Dines AM, Wood DM, Yates C, et al. Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European drug emergencies network (Euro-DEN). Clin Toxicol (Phila). 2015;53(9):893–900.
- Henshall DE, Innes CW, Morrison SR, et al. A prospective observational study of emergency department presentations following novel psychoactive substance use. Scott Med J. 2018;63(2):39–44.
- Tebo C, Mazer-Amirshahi M, Wax P, et al. Characterizing trends in synthetic cannabinoid receptor agonist use from patient clinical evaluations during medical toxicology consultation. J Psychoactive Drugs. 2020;23:1–8.
- Maxwell JC. The changing face of synthetic cannabinoids in Texas. J Psychoactive Drugs. 2018;50(4):281–286.
- Public Health England. Adult substance misuse treatment statistics 2019 to 2020. 2020. [cited 2022 Feb 1]. https://www.gov.uk/government/statistics/substance-misuse-treatment-for-adults-statistics-2019-to-2020/adult-substance-misuse-treatment-statistics-2019-to-2020-report
- Ralphs R, Gray P, Sutcliffe OB. The impact of the 2016 psychoactive substances act on synthetic cannabinoid use within the homeless population: markets, content and user harms. Int J Drug Policy. 2021;97:103305.
- European Monitoring Centre for Drugs and Drug Addiction. New psychoactive substances in prison. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2018. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/8869/nps-in-prison.pdf
- Public Health England. National poisons information service report 2018/19. 2019. [cited 2022 Feb 1]. https://www.npis.org/Download/NPISAnnualReport2018-19.pdf
- Giorgetti A, Busardo FP, Tittarelli R, et al. Post-Mortem toxicology: a systematic review of death cases involving synthetic cannabinoid receptor agonists. Front Psychiatry. 2020;11:464.
- Kraemer M, Boehmer A, Madea B, et al. Death cases involving certain new psychoactive substances: a review of the literature. Forensic Sci Int. 2019;298:186–267.
- Fockele C, Armenian P. A cross-sectional emergency department survey of novel psychoactive substance prevalence. Am J Emerg Med. 2017;35(10):1580.
- Peacock A, Bruno R, Gisev N, et al. New psychoactive substances: challenges for drug surveillance, control, and public health responses. Lancet. 2019;394(10209):1668–1684.
- Office for National Statistics. Drug misuse in England and Wales: year ending. 2020. [cited 2022 Feb 1]. https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/yearendingmarch2020
- ESPAD Group. ESPAD report 2019: results from the European school survey project on alcohol and other drugs. Luxembourg: ESPAD Group; 2020. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/13398/2020.3878_EN_04.pdf
- Barratt MJ, Ferris JA, Zahnow R, et al. Moving on from representativeness: testing the utility of the global drug survey. Subst Abuse. 2017;11:1178221817716391.
- Global Drug Survey. Global drug survey 2020: executive summary. 2020. [cited 2022 Feb 1]. https://www.globaldrugsurvey.com/wp-content/uploads/2021/01/GDS2020-Executive-Summary.pdf
- Marchei E, Ferri MA, Torrens M, et al. Ultra-high performance liquid chromatography-high resolution mass spectrometry and high-sensitivity gas chromatography-mass spectrometry screening of classic drugs and new psychoactive substances and metabolites in urine of consumers. Int J Mol Sci. 2021;22(8):4000.
- Bade R, White JM, Chen J, et al. International snapshot of new psychoactive substance use: case study of eight countries over the 2019/2020 New Year period. Water Res. 2021;193:116891.
- European Monitoring Centre for Drugs and Drug Addiction. Drugs in syringes from six European cities: results from the ESCAPE project 2017. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2019. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/system/files/publications/11287/20191061_TD0119176ENN_PDF.pdf
- European Monitoring Centre for Drugs and Drug Addiction. Final report of the European Drug Emergencies Network (Euro -DEN) March 2015. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2015. [cited 2022 Feb 1]. https://www.emcdda.europa.eu/drugs-library/final-report-european-drug-emergencies-network-euro-den-march-2015_no
- Ibrahim-Achi D, Miro O, Galicia M, et al. Spanish research network on drugs in hospital emergency departments - the REDUrHE registry: general analysis and comparisons between weekend and weekday poisonings. Emergencias. 2021;33(5):335–344.
- Liakoni E, Yates C, Dines AM, et al. Acute recreational drug toxicity: comparison of self-reports and results of immunoassay and additional analytical methods in a multicenter European case series. Medicine (Baltimore). 2018;97(5):e9784.
- Vallersnes OM, Persett PS, Oiestad EL, et al. Underestimated impact of novel psychoactive substances: laboratory confirmation of recreational drug toxicity in Oslo, Norway. Clin Toxicol (Phila). 2017;55(7):636–644.
- Davies S, Wood DM, Smith G, et al. Purchasing 'legal highs’ on the internet–is there consistency in what you get? QJM. 2010;103(7):489–493.
- Brandt SD, Sumnall HR, Measham F, et al. Second generation mephedrone. The confusing case of NRG-1. BMJ. 2010;341:c3564.
- Ramsey J, Dargan PI, Smyllie M, et al. Buying ‘legal’ recreational drugs does not mean that you are not breaking the law. QJM. 2010;103(10):777–783.
- Backberg M, Jonsson KH, Beck O, et al. Investigation of drug products received for analysis in the Swedish STRIDA project on new psychoactive substances. Drug Test Anal. 2018;10(2):340–349.

