Abstract
Background
The mushroom Amanita exitialis is reported to cause acute liver injury. It is found in Southern China, and has been previously associated with a high incidence of mortality.
Methods
We described a series of 10 patients with Amanita exitialis poisoning admitted to The Second Affiliated Hospital of the Chinese University of Hong Kong (Shenzhen) in April 2022. Patient demographics, clinical features, laboratory results, therapeutic interventions, and outcome data were collected.
Results
Among the 10 patients, 9 survived, while 1 died. Gastrointestinal symptoms were the first to appear (average latency period, 11 ± 4.2 h). Diarrhea was the most common clinical symptom (average duration, 4.4 days). Abdominal distention was an important sign, especially in severely-ill patients. Thrombocytopenia occurred on day 2 after mushroom ingestion and persisted for 3–4 days. Alanine aminotransferase and total bilirubin peaked on days 2–3.
Conclusion
Amanita exitialis poisoning is characterized by gastrointestinal symptoms and liver injury. In the patient who died, acute hepatic failure led to hepatic encephalopathy and cerebral edema. Abdominal distension accompanied by thrombocytopenia was common in critically ill patients in this outbreak.
Introduction
The genus Amanita contains approximately 600 known species of agarics worldwide [Citation1]; among these, 37 cyclopeptide-containing Amanita species [Citation2] are responsible for >90% of all mushroom poisoning-related fatalities [Citation3]. In southern China, a recent report on mushroom poisoning included 852 patients over an 18-year period and reported with a mortality rate of 21.5%. Of those who died 70.5% were caused by species in the genus Amanita [Citation4]. Notably, the mushroom Amanita exitialis (A. exitialis) had a mortality of 60.6% in this series. There is a greater incidence of poisonings with this mushroom in rainy seasons, specifically March and April [Citation5–7].
Amatoxins have a stable physical structure; they are heat-, acid-, and alkali-resistant, and water-soluble [Citation8]. The lethal dose of α-Amanitin is 0.1 mg/kg for adults [Citation9]. The genus Amanita exhibits gene polymorphism and degeneracy due to the need for environmental adaptation [Citation10]. Therefore, the clinical manifestations may vary in different regions. Classic clinical characteristics and course include a “latency phase,” a “gastroenteritis phase,” a “quiescent phase,” and an “organ impairment phase”. The liver is the most commonly affected, and fulminant hepatic failure is the most common cause of death [Citation11].
In this case series, the clinical presentation of an outbreak with A. exitialis poisoning differed from the aforementioned classic course.
Herein we analyzed the clinical and laboratory features of patients with A. exitialis poisoning, to further the clinicians’ knowledge of the characteristics and subsequent treatment of A. exitialis poisoning.
Methods
Study population
In April 2022 a cluster of 10 patients who all consumed A. exitialis presented to the intensive care unit (ICU) of The Second Affiliated Hospital of the Chinese University of Hong Kong (Shenzhen). We reviewed their medical records and abstracted their clinical symptoms, laboratory results, and clinical course.
Diagnosis
The diagnosis of A. exitialis poisoning was based on the region’s epidemiological history, the patient’s history of mushroom ingestion, their clinical manifestations, and the morphological identification of ingested mushrooms performed by at least two expert toxicologists. Mushroom samples were collected from the incident location and confirmed by the patients ().
Assessment of poisoning severity
The severity of the poisoning was classified according to the Poisoning Severity Score (PSS) established by International Programme on Chemical Safety/European Community/European Association of Poisons Centres and Clinical Toxicologist (IPCS/EC/EAPCCT) [Citation12] as follows: None (0), no symptoms or signs related to poisoning; Minor (1), mild, transient, and spontaneously resolving symptoms; Moderate (2), pronounced or prolonged symptoms; Severe (3), severe or life-threatening symptoms; and Fatal (4), death.
Treatment
All patients were managed with a bundle of treatments, which included the followings: (i) resuscitation fluids; (ii) oral administration of activated charcoal 5 g, 6 times per day for the first 3 days; (iii) non-specific antidotes (intravenous acetylcysteine at an initial dose of 130 mg/kg given over 60 min, followed additional dose of 60 mg/kg given over 4 h, and then by 120 mg/kg given over 16 h, and ultimately followed by 60 mg/kg given twice daily for 5–7 day; intravenous penicillin G 300,000 IU/kg/day for 5 days; oral silymarin 1400 mg thrice daily for 5–7 days, because intravenous silymarin is unavailable in China).
Meanwhile, the following therapeutic options were used based on the patients’ conditions. Hemoperfusion was performed once for one patient and twice for eight. Plasma exchange was performed twice for one patient and thrice for four. Three patients received a single treatment of hemodialysis on the first hospital day, and an additional patient had two treatments on days 1 and 2. The most seriously ill patient (patient 10) received daily hemodialysis for ten hospital days, and a double plasma molecular adsorption system treatment for 6 days, in addition to three platelet transfusions. Besides patient 10, one other patient (patient 9) required mechanical ventilation.
Results
The patients’ demographic and outcome information
summarizes demographic characteristics and clinical outcomes. All patients concurrently presented to the emergency department of the hospital because of severe diarrhea, nausea, and vomiting (which generally occurred 2–15 h after mushroom ingestion). Their average age was 50 years, and their mean latency period was 11 ± 4.2 h. The median Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 5 (at 24 h post-admission). The mean length of hospital stay was 12 days; mean length of stay in the ICU was 8 days. One patient died of fulminant hepatic failure.
Table 1. Patients’ demographic characteristics and outcome.
Clinical characteristics
Diarrhea was severe and persistent, with an average duration of 4.4 days (range, 1–9 days). All patients in the outbreak had watery stools that resulted in fluid and electrolyte imbalances.
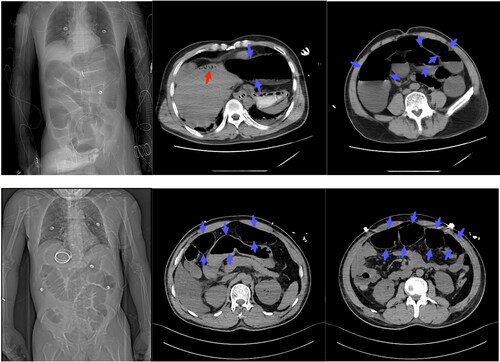
Abdominal pain occurred in four patients. Abdominal distension occurred in 5 patients and lasted an average of 3.8 days (range 2–7). Imaging from two patients are shown in . The treatment for these two patients included decompressive suction via colonoscopy. The incidence of jaundice was 40%, at the peak incidence of the disease, while its duration was short-lived (average, 2.75 days) except in Patient 10.
Figure 2. Represented radiographic images of abdomen. The first row of images shows patient 9, the second row shows patient 10. The images illustrate hepatic portal venous gas (red arrow) and dilated bowel (blue arrow).
Fever occurred only in Patient 10 during the early stage, with a peak of 38 °C and a short duration (1 day). No significant abnormality in inflammatory markers were identified before or after the fever.
Patient 10 was treated with the medical supportive care and antidotes as described in the methods section from hospital day 1 through hospital day 5. Abdominal distention gradually improved after endoscopic suction for colonic decompression on hospital day 5. He had progressive hepatic failure and received daily hemodialysis after hospital day 4, and a double plasma molecular adsorption system treatment on hospital day 5 and daily for 6 days, in addition to three platelet transfusions. The patient became comatose on hospital day 3. His bilirubin increased while transaminase and platelet decreased. Over the next 15 days the patient developed liver failure accompanied by hepatic encephalopathy and multiple organ dysfunction syndrome. He died on hospital day 20 secondary to cerebral edema with herniation, despite aggressive supportive therapy.
Laboratory results
Four patients had electrolyte disorders. The trough value of serum potassium was 2.8 mEq/L (patient 8, Day 2), serum calcium was 1.7 mmol/L (patient 3, Day 1; patient 9, Day 4), serum magnesium was 0.53 mmol/L (patient 4, Day 1), and serum phosphorus was 0.36 mmol/L (patient 9, Day 3).
illustrates the laboratory abnormalities in this case series. The aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were abnormal of initial tests and had higher peak value in patient 9 and 10. The average time to reach peak values for all patients was 55.3 h (42–90 h) and 61 h (42–100 h) for AST and ALT, respectively. The total bilirubin (TBIL) concentration showed a similar trend.
Table 2. Laboratory results of patients with mushroom poisoning.
The exception of the deceased patient (Patient 10), the prothrombin activity (PTA), activated partial thromboplastin time (aPTT) in the surviving patients gradually returned to normal range on hospital day 4 (PTA: 70.5%; aPTT: 41.6 s). The peak state of D-dimer in Patient 10 was maintained from hospital day 5 to day 20.
The platelet count was normal on admission in all patients, but decreased in the patients with a PSS > 2 and liver injury (Patients 4, 6, 7, 9, and 10) on hospital days 2, and gradually recovery on hospital days 6–7. The decline lymphocytes count showed a similar trend to that of the platelet count.
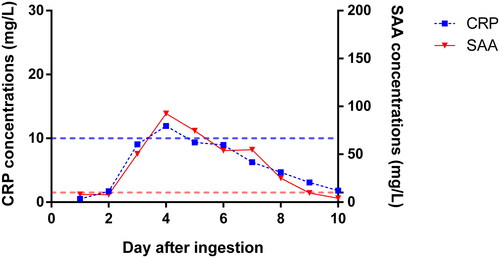
C-reactive protein (CRP) and serum amyloid-A (SAA) gradually increased on day 2 () while procalcitonin fluctuated in the normal range.
Figure 3. Course of inflammatory markers in all patients. The left Y-axis corresponds to the blue line plot to reflect the time-concentration trend of CRP, and the right Y-axis corresponds to the red line plot to reflect the time-concentration trend of SAA. The average values presented in figure. The blue-broken line represents the upper limit of normal value of CRP; the red-broken line represents the upper limit of normal value of SAA. CRP: C-reactive protein; SAA: serum amyloid-A.
The ammonia elevation in the surviving patients persisted for approximately 7 days (during hospital days 2–8), with the highest peak value of 149 μmol/L in patient 9, who like the fatal patient, also developed an ileus. Blood ammonia concentrations fluctuated in the first 3 days of illness.
Discussion
In China, ingestion of mushrooms of the genus Amanita is common, with A. exitialis having the highest proportion of reported mortality [Citation13]. Chen et al. [Citation4] analyzed 102 episodes of all types of mushroom poisoning in southern China which included 52 patients who consumed A. exitialis who had a mortality of 60.6%, while only one of ten patients who died in this limited cluster.
Although some studies [Citation14–16] have reported that a “gastroenteritis phase” usually progressed into a “quiescent phase” after 24–36 h, the average period of diarrhea in this patient group was longer, and some critically-ill patients experienced severe abdominal distention and secondary paralytic ileus.
The characteristics of early and severe diarrhea accompanied by abdominal distention were prominent in critically ill patients and may be a marker of severity. The clinical features that occurred in this outbreak were similar to those reported previously [Citation17–19]. In addition, prolonged periods of diarrhea increased fluid and electrolyte imbalances that required closer monitoring. Cathartics are not recommended for intestinal decontamination for such patients to avoid exacerbating dehydration [Citation20].
Thrombocytopenia is a common feature in amatoxin mushroom poisoning [Citation21–23]. We observed thrombocytopenia between days 3 and 7 post ingestion. Possible reasons for this abnormality may be higher expression of procoagulant microparticles that have been demonstrated in patients with acute liver injury (ALI) [Citation24,Citation25]. Increased platelet consumption results from inflammation-mediated [Citation26] and prothrombotic [Citation27] mechanisms to maintain a hypercoagulable state of microcirculation. Also, with the development of acute liver injury, there is a change of coagulation function from an early procoagulant state to a consumptive hypocoagulability [Citation28]. Finally, the impact of interventions, such as hemoperfusion is a known risk for thrombocytopenia.
The inflammatory response after Amanita poisoning was described only in animal studies [Citation29,Citation30]. We found that this outbreak had a non-infectious inflammatory response with lymphocytopenia in the early period of mushroom poisoning. The role of immune response in organ injury caused by amanitin-containing mushroom poisoning needs further evaluation.
Several studies have reported that abnormal aminotransferase concentrations and coagulation dysfunction were associated with a poor prognosis, and may be useful as a valuable prognostic indicator [Citation7,Citation31–33]. Our results support these views, and in patients with higher grades of poisoning, ALT concentrations demonstrated an earlier rise and later peak, with similar findings for total bilirubin as well.
Gao et al. [Citation34] reported that an elevated ammonia concentration was associated with mortality and could predict the prognosis of patients with acute mushroom poisoning. In this outbreak, the ammonia concentration was higher in the one deceased patient than that in the surviving patients.
Currently, an early risk prediction scoring system for acute poisoning is lacking. The APACHE II score may predict outcomes in some other poisonings [Citation35,Citation36]. Otherwise, we observed that APACHE II score insufficiently reflects the severity of Amanita mushroom poisoning in the early stage. We considered that the APACHE II score has a higher range in chronic physiological or pathological injury, but because acute mushroom poisoning has specific characteristics, more research is needed for accurate evaluation of any prognostic score.
Limitations
This was a rare occurrence of a cluster of 10 simultaenous cases, and there was a high degree of consistency in the type of mushroom ingested, the time of admission, and the medical environment. However, some limitations are as follows: (a) it was a retrospective case series with a small amount of missing data; (b) it was a small cohort of only 10 patients; and (c) there was a lack of confirmation of the presence of amatoxin in either the mushrooms or patient samples.
Conclusions
This analysis of 10 cases of A. exitialis poisoning demonstrated that all cases had severe diarrhea, and abdominal distension and thrombocytopenia were prominent in critically ill patients. The fatal case developed hepatic failure with low prothrombin activity, rising aminotransferase concentrations, persistent hyperbilirubinemia, as well as an elevated D-dimer. The APACHE II score was a poor predictor of outcome for A. exitialis poisoning.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Diaz JH. Amatoxin-containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ Med. 2018;29(1):111–118.
- Cai Q, Tulloss RE, Tang LP, et al. Multi-locus phylogeny of lethal amanitas: implications for species diversity and historical biogeography. BMC Evol Biol. 2014;14:143.
- Tang S, Zhou Q, He Z, et al. Cyclopeptide toxins of lethal amanitas: compositions, distribution and phylogenetic implication. Toxicon. 2016;120:78–88.
- Chen ZH, Zhang P, Zhang GZ. Investigation and analysis of 102 mushroom poisoning cases in Southern China from 1994 to 2012. Fungal Diversity. 2014;64(1):123–131.
- Wu J, Gong X, Hu Z, et al. Acute liver failure caused by Amanita verna: a case series and review of the literature. BMC Surg. 2021;21(1):436.
- Geng J, Cao Z, Ma X, et al. Mushroom poisoning: an overlooked cause of acute liver injury in China. Liver Int. 2017;37(3):468–469.
- Sun J, Li HJ, Zhang HS, et al. Investigating and analyzing three cohorts of mushroom poisoning caused by Amanita exitialis in Yunnan, China. Hum Exp Toxicol. 2018;37(7):665–678.
- Le Daré B, Ferron PJ, Gicquel T. Toxic effects of amanitins: repurposing toxicities toward new therapeutics. Toxins. 2021;13(6):417.
- Tavassoli M, Afshari A, Arsene AL, et al. Toxicological profile of Amanita virosa – a narrative review. Toxicol Rep. 2019;6:143–150.
- Li P, Deng WQ, Li TH, et al. Illumina-based de novo transcriptome sequencing and analysis of Amanita exitialis basidiocarps. Gene. 2013;532(1):63–71.
- Hu J, Zhang P, Zeng J, et al. Determination of amatoxins in different tissues and development stages of Amanita exitialis. J Sci Food Agric. 2012;92(13):2664–2667.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213.
- Deng WQ, Li TH, Zhang M, et al. Analysis of common poisonous species of amanita and their poisoning cases in South China. Mycosystema. 2020;39(9):1750–1758.
- Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning–from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96(11):3195–3198.
- Karlson-Stiber C, Persson H. Cytotoxic fungi–an overview. Toxicon. 2003;42(4):339–349.
- Diaz JH. Syndromic diagnosis and management of confirmed mushroom poisonings. Crit Care Med. 2005;33(2):427–436.
- Eyer F, Felgenhauer N, Zilker T. The development of a toxic megacolon due to Amanita phalloides poisoning. A rare complication. Dtsch Med Wochenschr. 2004;129(4):137–140.
- Huang L, Liu XL, Cao CS, et al. Outbreak of fatal mushroom poisoning with Amanita franchetii and Ramaria rufescens. BMJ Case Rep. 2009;2009:bcr06.2008.0327.
- Hilty MP, Halama M, Zimmermann AK, et al. Ulcerating ileocolitis in severe amatoxin poisoning. Case Rep Gastrointest Med. 2015;2015:632085.
- Barceloux D, McGuigan M, Hartigan-Go K. Position statement: cathartics. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35(7):743–752.
- Trabulus S, Altiparmak MR. Clinical features and outcome of patients with amatoxin-containing mushroom poisoning. Clin Toxicol. 2011;49(4):303–310.
- Wang Q, Sun M, Lv H, et al. Amanita fuliginea poisoning with thrombocytopenia: a case series. Toxicon. 2020;174:43–47.
- Lin LY, Tong YL, Lu YQ. The characteristics of liver injury induced by amanita and clinical value of α-amanitin detection. Hepatobiliary Pancreat Dis Int. 2022;21(3):257–266.
- Agarwal B, Wright G, Gatt A, et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol. 2012;57(4):780–786.
- Stravitz RT, Bowling R, Bradford RL, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58(1):304–313.
- Stravitz RT, Ellerbe C, Durkalski V, et al. Thrombocytopenia is associated with multi-organ system failure in patients with acute liver failure. Clin Gastroenterol Hepatol. 2016;14(4):613–620.e4.
- Mooberry MJ, Key NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. 2016;89(2):111–122.
- Lee KCL, Baker L, Mallett S, et al. Hypercoagulability progresses to hypocoagulability during evolution of acetaminophen-induced acute liver injury in pigs. Sci Rep. 2017;7(1):9347.
- Chen X, Shao B, Yu C, et al. Energy disorders caused by mitochondrial dysfunction contribute to α-amatoxin-induced liver function damage and liver failure. Toxicol Lett. 2021;336:68–79.
- Leist M, Gantner F, Naumann H, et al. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology. 1997;112(3):923–934.
- Eren SH, Demirel Y, Ugurlu S, et al. Mushroom poisoning: retrospective analysis of 294 cases. Clinics. 2010;65(5):491–496.
- Ward J, Kapadia K, Brush E, et al. Amatoxin poisoning: case reports and review of current therapies. J Emerg Med. 2013;44(1):116–121.
- Bonacini M, Shetler K, Yu I, et al. Features of patients with severe hepatitis due to mushroom poisoning and factors associated with outcome. Clin Gastroenterol Hepatol. 2017;15(5):776–779.
- Gao Y, Zhang H, Zhong H, et al. Lactate and blood ammonia on admission as biomarkers to predict the prognosis of patients with acute mushroom poisoning and liver failure: a retrospective study. Toxicol Res. 2021;10(4):850–855.
- Huang J, Xuan D, Li X, et al. The value of APACHE II in predicting mortality after paraquat poisoning in Chinese and Korean population: a systematic review and meta-analysis. Medicine. 2017;96(30):e6838.
- Wu X, Xie W, Cheng Y, et al. Severity and prognosis of acute organophosphorus pesticide poisoning are indicated by C-reactive protein and copeptin levels and APACHE II score. Exp Ther Med. 2016;11(3):806–810.


