Abstract
Introduction
Mushrooms containing amatoxin are found worldwide and represent a challenging poisoning for the clinician and consulting poison center. This study evaluates the experience of a large poison system with possible amatoxin-containing mushroom ingestion calls.
Methods
A 10-year retrospective review of the California Poison Control System database was performed for amatoxin mushroom ingestion calls resulting in hospitalization. Cases found were abstracted and data statistically analyzed for association with a composite endpoint of death, liver transplant, and/or the need for dialysis.
Results
Amatoxin-containing mushroom calls are infrequent with the vast majority (98.4 percent) coming from Northern California during the rainier first and fourth quarters (October through March) of the year. Elevated initial aminotransferase activities and international normalized ratios were predictive of the composite negative outcome. The mortality plus liver transplant and hemodialysis composite rate was 8.2 percent, consistent with current literature.
Conclusion
The California Poison Control System has relatively few amatoxin-containing mushroom ingestion calls that result in hospitalization but those that are reported mostly occur in Northern California. Treatment bias towards the sickest patients may explain the association of intravenous fluid use or treatment with acetylcysteine or silibinin with meeting the composite outcome. The initial presence of elevated hepatic aminotransferase activity and international normalized ratios are poor prognostic indicators and are likely reflective of late presentation, an advanced toxic phase of amatoxin poisoning, and/or delays in time to obtain poison center consultation.
Keywords:
Introduction
Mushroom ingestion calls represent a unique challenge for poison centers. The primary concern in cases is whether the mushroom ingested will result in serious acute or delayed toxicity, with particular concern for hepatotoxic amatoxins. Amatoxin-containing mushrooms contain a group of toxic cyclopeptides. These toxins are found in many species including Amanita phalloides, Amanita virosa, Amanita biosporigera, Amanita ocreata, Amanita verna, Galerina marginata and some Lepiota spp. and Conocybe spp. [Citation1]. In California, Amanita phalloides poisonings represent the most toxic and severe ingestions. Two important toxic cyclopeptides (bicyclic octapeptides) in amatoxin-containing mushrooms include α-amanitin and toxophallin, both isolated from Amanita phalloides [Citation2,Citation3].
The toxin α-amanitin is a cyclopeptide that is thought to cause liver and kidney damage by binding with high affinity to the largest subunit of ribonucleic acid (RNA) polymerase II known as RNA pb1. This results in a dose-effect inhibition of RNA polymerase II that causes undetectable RNA pb1 in animal studies and results in cell death [Citation3]. Toxic mechanisms of α-amanitin include an increase in cellular oxidative stress that contributes to cell injury and death [Citation3]. Toxophallin has also been isolated from Amanita phalloides [Citation2]. It is thought to be an L-amino acid oxidase that further contributes to oxidative stress and cell injury/death. Although significant work has been done on the toxic mechanisms of amatoxin-containing mushrooms, a complete understanding of the complex potential toxic mechanisms has not yet been completely elucidated.
The clinical presentation after ingestion of amatoxin-containing mushrooms is phasic with the first phase being asymptomatic [Citation4]. After about 6 to 8 h, the second phase begins with gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea). If these symptoms are severe, then dehydration will often occur. Between 36 to 48 h after ingestion, a third phase of toxicity can begin. Evidence of liver damage is noted with elevation in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) activities, and total bilirubin concentration. As the hepatic cellular death/damage worsens, the international normalized ratio (INR) increases from disruption of the production of hepatically generated clotting factors [Citation4]. Volume depletion and direct toxin-induced nephrotoxicity result in elevations in blood urea nitrogen (BUN) and creatinine concentrations.
In a series of 144 amatoxin poisoned patients in Turkey, patients who demonstrated low mean arterial pressures, encephalopathy, mucosal hemorrhage, oliguria/anuria, hypoglycemia, thrombocytopenia, low serum sodium concentrations, and high BUN concentrations, AST activities, ALT activities, total bilirubin concentration, and INR were found to have higher mortality rates [Citation5]. The mortality rate was 9.7% which was consistent with the fatality rate of 8.8% reported after suspected cyclopeptide mushroom poisoning in the United States (US) using the National Poison Data System [Citation6]. These mortality rates are less than the historic reported rates of 20-50% after amatoxin-containing mushroom ingestions [Citation6]. This improved mortality is thought to be due to improved supportive care now available for liver and kidney failure patients.
Expert treatment guidelines based on quality randomized clinical trials are lacking. This study examines the experience of the California Poison Control System over a recent ten-year period with amatoxin-containing mushroom ingestions. The California Poison Control System handles about 240,000 calls/year and services a state of about 40 million people using four divisions (San Francisco, San Diego, Fresno, and Sacramento) utilizing one set of treatment guidelines and a single electronic medical record. Specialists in Poison Information are encouraged to seek backup toxicologist consultation on all suspected cases of amatoxin mushroom poisoning.
Methods
The investigation is a retrospective observational study from the California Poison Control System database queried from January 2013 to February 2023 using the terms mushroom poisoning/Amatoxin/Amanita phalloides with ingestion as the route of exposure and healthcare facility as the management site. The shared database used by the California Poison Control System is VLDE Software (Visual Dotlab Enterprise version 5.5.5p, Fresno, CA. USA). Abstraction of the deidentified data on each case was performed by one of the California Poison Control System division medical directors (RFC, TEA, RBV, and CGS). Seventy-one cases were identified by the computer search. Of these, 10 cases were eliminated because of a lack of information, wrong diagnostic codes entered, or ingestion/poisoning/exposure clearly unrelated to Amanita phalloides. Predetermined data points were entered into a Microsoft Excel Spreadsheet (Microsoft Corp, Seattle, Washington).
All cases were seen in the emergency department and/or hospitalized. They had age, sex, date of call, date of ingestion, mushroom exposure (raw or cooked exposure), alone or in a group, co-ingestions, initial symptoms, organ systems affected, location in California where mushroom obtained, abnormal initial available laboratory findings (AST, ALT, total bilirubin, BUN, creatinine, glucose, INR, pH, lactate and hemoglobin/hematocrit), complications/abnormalities, and a combined composite outcome that included death and/or liver transplant and/or need for acute hemodialysis recorded. Treatment modalities were also recorded. Recording of time points occurred if explicitly mentioned in the history field and if not explicitly mentioned then the time that the progress notes first addressed the variable of interest was used. Recorded time points were rounded to the nearest 0.5 h. Some of the cases through December 2018 were included in the study by De Olano, et al. [Citation6] (Supplementary Table 1).
Table 1. Patient characteristics stratified by those that develop the composite outcome of organ failure requiring transplant, hemodialysis, and/or death compared to patients who did not develop the composite endpoint.
For continuous descriptive variables, the mean and standard deviation are reported. For categorical variables, the total number and percentage are reported. Fisher’s Exact test and Wilcoxon rank sum test were used to compare the composite endpoint of death, liver transplant, and/or the need for hemodialysis to non-composite endpoint patients for categorical and continuous variables, respectively. Significance was set a priori at P < 0.05. Data were analyzed using Stata MP® version 18.
A univariate logistic regression was performed to access potential risk factors associated with the composite endpoint. Unadjusted odds ratios and 95% confidence intervals were calculated. All predictors supported by prior studies and predictors with P < 0.20 on both univariate logistic regression analysis and Fisher’s Exact test or Wilcoxon rank sum test hypothesis testing were assessed for collinearity; variables with a variance inflation factor >2.5 were excluded from consideration for multivariable analysis [Citation7]. Multivariable logistic regression models were developed using purposeful variable selection [Citation8] and a 10% change-in-estimate procedure [Citation9] to determine if the potential for confounding was present and warranted adjustment. All interaction terms were analyzed and noted to have no effect. For the final model selection, Akaike’s Information Criteria and Bayesian Information Criteria were used to identify the best model fit [Citation10]. Through this multivariable logistic regression model fitting, final adjusted odds ratios and 95% confidence intervals were calculated. Model performance was evaluated using the area under the receiver operating characteristic curve (AUC).
Results
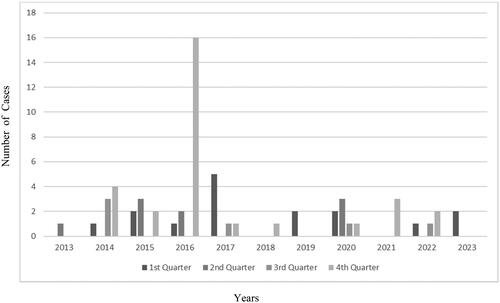
There were 61 calls to the California Poison Control System of possible amatoxin mushroom exposure evaluated at a hospital over a 10-year period; only five cases were previously reported as part of the De Olano Study (Supplementary 1). Late fall to the end of winter were the most common times for amatoxin mushroom related calls (see ). All but one of these calls were from Northern California and appear to be seasonally clustered as exampled by the large number of calls in December 2016. The overall composite endpoint was met in five out of 61 cases (8.2%) in this study. Three of these patients died including 60- and 31-year-old males and a 35-year-old female treated with hemodialysis. Two underwent liver transplantation (1.5- and 38- year-old females) and were discharged. The actual survival rate was 95.1% (58/61).
Figure 1. Cases of amatoxin‐containing mushroom case as a function of year and quarter of the year. A total of 46 out of 61 occurred during quarters 4 and 1. Only nine out of 61 were reported during second quarters and six out of 61 in third quarters. A large outbreak was noted in the fourth quarter of 2016.
The overall diagnostic confirmation of amatoxin containing mushrooms was limited to 23% of the patients. Forty percent of those who reached the composite outcome had diagnostic mushroom confirmation, while 21.4% of the patients who did not reach the composite outcome had diagnostic confirmation. All confirmations were by mycologists. Of the 61 patient calls with possible amatoxin-mushroom exposures, 10 were 4 years of age or younger. Eight spontaneously ingested mushrooms while outside and remained asymptomatic. Two patients were fed the mushrooms, and both were symptomatic. One of these children developed severe symptoms that led to a liver transplant.
summarizes the characteristics of the patients with presumed amatoxin mushroom poisoning. Initial signs, symptoms and available laboratory results of these patients are found in with elevated initial liver function tests defined as twice the upper limit of normal (n = 5) and INR (n = 4) being statistically significant (P < 0.05) for the patients who reached the composite endpoint compared to the 56 patients that did not reach the composite endpoint. Treatment data are summarized in . All patients who met the composite endpoint of were treated with acetylcysteine, compared to 27% who did not reach the composite endpoint (P = 0.003). Treatment with intravenous fluids (100% v. 52%) and silibinin (60% v. 18%) were more prevalent in patients who reached the composite endpoint, but this difference fell short of statistical significance (P = 0.06). Unadjusted odds ratios (OR) from univariate analysis are presented in . Initial abnormal INR (OR = 20.89 [95% CI 2.09–209.27], P = 0.01) and the use of silibinin (OR = 6.9, [95% CI 1.01–46.85], P = 0.048) were significantly associated with the composite outcome.
Table 2. Symptom and laboratory characteristics: Patients who ingested mushrooms and developed the composite outcome of organ failure requiring liver transplant, hemodialysis, and/or death compared to patients who did not reach the composite endpoint.
Table 3. Treatment characteristics: Patients who ingested mushrooms and developed organ failure, requiring liver transplant, died and/or required hemodialysis compared to patients who did not reach the composite endpoint.
Table 4. Unadjusted odds ratios: patients who ingested mushrooms and developed organ failure requiring liver transplant, hemodialysis and/or died compared to patients who did not develop the composite endpoint.
On multivariable logistic regression, initial elevated INR (adjusted OR = 19.13, [95% CI 1.26–291.25], P = 0.034) was statistically significantly associated with the composite outcome, with sex (female) (adjusted OR = 4.40, [95% CI 0.47–41.02]), multiple organ signs/symptoms (adjusted OR = 0.44, [95% CI 0.2–9.02]), and multiple organ symptoms noted (adjusted OR = 2.91, [95% CI 0.15–56.38]) present as confounders (). These variables define the best model. provides the receiver operating curve (ROC) data from the model with an area under the ROC of 0.8964, indicating excellent discrimination.
Figure 2. Area under the receiver operating characteristic (ROC) curve. The receiver operating characteristic curve for fitted logistic regression predicting organ failure or death is shown. This model achieved an observed area under the receiver operating curve (AUC) of 0.8964.
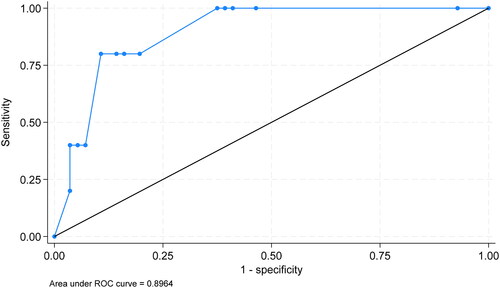
Table 5. Adjusted odds ratios of variables used in best fit model. Patients who ingested mushrooms and developed organ failure or died compared to patients who did not develop organ failure or die.
Time from ingestion to onset of gastrointestinal symptoms was specifically documented in 44/69 (72%) of cases. Symptom onset that occurred 6 h or greater from mushroom ingestion occurred in 35 (57% of total and 79.5% of those with times documented) and ranged from 6–48 h with a median of 12 h. A total of 15 cases had already developed elevations in hepatic aminotransferase activities by the time they arrived at the hospital with a range of 3–96 h (median time 19 h) taken to seek medical care. Of the 27 total patients that developed elevated hepatic aminotransferase activities, 22 developed impairments in hepatic synthetic ability as measured by an elevated INR or total bilirubin concentration over a period of 24-96 h post ingestion. Treatment with other drug therapies (acetylcysteine, silibinin or benzylpenicillin) occurred in 27 patients with the time from ingestion to treatment ranging 9-100 h (median 35 h). Gastrointestinal symptoms that developed less than 6 h post-ingestion occurred in 9 of 61 patients (15% of total and 20.5% of those with times documented). Six of the nine cases had hepatic aminotransferase activities recorded and none developed peak AST activities > 500 U/L or met the composite endpoint.
Discussion
This study found that the majority (98.4%) of calls to California Poison Control System about possible amatoxin-containing mushroom ingestions that resulted in hospitalization occurred in Northern California. The overall incidence was low but did cluster in the rainier first (January through March) and fourth (October through December) quarters of the calendar year. Consistent with the known toxicity of amatoxin-containing mushrooms, most patients who died presented with nausea/vomiting/diarrhea/abdominal pain and developed signs and symptoms of organ failure, requiring hemodialysis or liver transplantation. On multivariable logistic regression, an initial elevated INR was significantly associated with the composite outcome. The use of acetylcysteine, intravenous fluids, and silibinin was more frequent in patients with the composite outcome. The patients with poor outcomes tended to be the sickest patients and were likely to be treated most aggressively, including these modalities.
The presence of an initially elevated INR in many patients at the time the poison center was contacted is likely reflective of late presentation to the hospital post ingestion in which cases are in advanced toxic phase of amatoxin poisoning had already developed. Lack of rigorous randomized controlled trials on this topic makes it unclear how soon after ingestion medical intervention is needed to affect clinical outcome, though early intervention is likely best. Education directed at the public and mushroom foraging groups should encourage seeking medical care or poison control consultation as soon as mushroom toxicity is suspected.
Silibinin is not routinely available in California, but an open-labeled clinical trial was available in California for several years during this study period and it is now available through a US Food and Drug Administration compassionate use protocol. However, this often delays the time to treatment.
Routine diagnostic confirmation by toxin assay or by a mycologist was not done in all cases in this series. Expert mycologist identification can be useful in confirming amatoxin mushroom exposure if available and mushrooms or parts remain for examination. The lack of mycologists has encouraged the use of three popular mushroom identification software applications, but their clinical usefulness has been questioned. The lack of confirmation that the toxicity is related to the ingestion of amatoxin-containing mushrooms adds to the difficulty in confirming a diagnosis and contributes to the variability in the data.
A recent systematic review of 40 years of α-amanitin or amatoxin-containing mushroom poisonings that ended in July 2020 found 131 publications describing 877 unique patient cases [Citation4]. The overall mortality rate in that review was 15.7% (138/877), a survival rate of 84.3%. Treatment options for amatoxin-containing mushroom poisonings are supportive, absorption or enterohepatic circulation disruption/prevention, increasing the elimination, blocking hepatic cell uptake of α-amanitin, blocking toxin metabolism to an active form, and providing increasing glutathione as an antioxidant and free radical scavenger. Like in our calls, many of the cases would have been classified as possible amatoxin-containing mushroom ingestions not being confirmed by toxin assay or by mycologist identification.
Supportive care includes aggressive fluid and electrolyte replacement to maintain appropriate intravascular volume, vasopressor support, airway protection, and renal support in severely poisoned patients. Reducing absorption and recirculation and increasing elimination of amatoxins includes the use of activated charcoal, multiple dose activated charcoal, and perhaps hemodialysis, which were used in patients in this study [Citation2]. None of these approaches have been studied with rigorous clinical trials to date.
Drug therapies used to treat these patients included benzylpenicillin thought to inhibit the organic anion transporting polypeptide 1B3 (OATP1B3) transporter located in hepatic cell walls blocking α-amanitin uptake into hepatocytes. Another drug therapy used in treating amatoxin-containing mushroom poisoning that was used in this series was acetylcysteine. Acetylcysteine is a free radical scavenger and reducing agent that is a precursor to hepatic glutathione when endogenous hepatic cellular stores are depleted [Citation2]. It is commonly used in the treatment of paracetamol overdoses. Silibinin was also used in our patients. Milk thistle (Silybum marianum) seed extract silymarin contains several flavonoid compounds such as taxifolin, silychristin, silydanin and silibinin [Citation11]. These agents may provide regulation to cell membrane permeability, leukotriene inhibition, act as a reactive oxygen species scavenger and suppress deoxyribonucleic acid (DNA) expression in amatoxin-containing mushroom poisoning [Citation12]. In addition to providing an antioxidant effect, the use of silibinin or silymarin may also block the OATP1B3 entry transporter used for α-amanitin hepatocyte entry [Citation13]. A lack of rigorous randomized-controlled trials has prevented confirmation of the efficacy of these treatments.
As in our series, liver failure associated with amatoxin poisoning can be treated with liver transplantation [Citation14]. The first orthotopic liver transplant after amatoxin poisoning was in a 3-year-old in 1983 [Citation15]. By 1989, a report suggested that liver transplantation had become accepted as a treatment in patients with fulminant hepatic failure from amatoxin poisoning [Citation16]. A decision model for liver transplantation following amatoxin poisoning that relies on the prothrombin index based on the relative activity of clotting factors II, V, VII, X, and fibrinogen (compared with normal controls or serum controls) and serum creatinine concentration from day 3 to 10 after ingestion exists [Citation17]. The model predicted amatoxin-induced mortality with high sensitivity and specificity and therefore was useful in committing a patient to liver transplantation. Other standard criteria such as Escydie’s, King’s College, Clichy’s and Ganzert’s criteria have an accuracy in predicting mortality after amatoxin poisoning of 100, 90, 80 and 70%, respectively [Citation18]. The use of liver transplantation is well established as a rescue option in severe fulminant liver failure from amatoxin ingestions.
The lack of high-quality data, particularly the lack of randomized-controlled trials for amatoxin-induced toxicity, has not dampened the support for various treatments for amatoxin containing mushrooms ingestions. Silibinin, silymarin, and acetylcysteine treatments for amatoxin-induced liver disease are advocated by reviews, clinical series and case reports [Citation12,Citation19–24]. At the same time, several authors have questioned whether the use of silibinin or silymarin actually changes outcomes after amatoxin-induced liver and kidney damage [Citation22,Citation25]. Survival rate after potential exposure to amatoxin-containing mushrooms have shown improvements over the years probably because of improved supportive care and have recently ranged between 84% and 98.2% consistent with this rate reported in this study [Citation4–6,Citation14,Citation24,Citation26–30]
Pediatric unintentional mushroom ingestions in the US are usually benign with toxic manifestations uncommon [Citation31,Citation32]. In an older review of the Toxic Exposure Surveillance System database, 4,235 of the total of 6,317 mushroom-related calls to California poison centers involved children less than 6 years of age. Of these 99.1% were asymptomatic or had minor effects from their exposure [Citation32]. Most of the ingestions occurred outside the home or school (95.3%) and only one (0.02%) had major effects after ingestion. No deaths were reported. Evaluating our patients 4 years of age or younger with suspected amatoxin ingestions, 80% were outside the house and all were asymptomatic. The one pediatric patient with major toxicities and met the composite endpoint in this study requiring a liver transplant was fed cooked mushrooms. These findings are consistent with the previous observations that pediatric unintentional or accidental mushroom exposures have limited risk for serious toxicity.
Several limitations exist for this study. Data from poison centers can be incomplete, not include all cases, be difficult to extract exact timings and may suffer from reporting and recall basis. In this study, selection bias may account for the association between acetylcysteine and silibinin use and the composite outcome and the increased odds of the composite outcome with intravenous fluids and silibinin use. Despite these limitations, this study reflects the experience of a large poison system with amatoxin-containing mushroom calls.
Conclusion
Amatoxin-containing mushroom calls to the California Poison Control System that result in hospitalization are rare and come from mostly Northern California. The overall composite outcome of 8.2% and survival rate of 95.1% is consistent with recent literature. Calls that initially report elevated hepatic aminotransferase activities and any increased INR above the upper range of the normal range of the laboratory identify a patient at risk for the composite outcome and imply a delay in seeking care or recognition of the association with mushroom ingestion.
Supplemental Material
Download MS Word (25.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kent R, Olson CGS. Poisoning & drug overdose. 8th ed. New York: McGraw Hill, LLC; 2022.
- Ye Y, Liu Z. Management of Amanita phalloides poisoning: a literature review and update. J Crit Care. 2018;46:17–22. doi:10.1016/j.jcrc.2018.03.028.
- Xue J, Lou X, Ning D, et al. Mechanism and treatment of alpha-amanitin poisoning. Arch Toxicol. 2023;97(1):121–131. doi:10.1007/s00204-022-03396-x.
- Tan JL, Stam J, van den Berg AP, et al. Amanitin intoxication: effects of therapies on clinical outcomes - a review of 40 years of reported cases. Clin Toxicol. 2022;60(11):1251–1265. doi:10.1080/15563650.2022.2098139.
- Trabulus S, Altiparmak MR. Clinical features and outcome of patients with amatoxin-containing mushroom poisoning. Clin Toxicol. 2011;49(4):303–310. doi:10.3109/15563650.2011.565772.
- De Olano J, Wang JJ, Villeneuve E, et al. Current fatality rate of suspected cyclopeptide mushroom poisoning in the United States. Clin Toxicol. 2021;59(1):24–27. doi:10.1080/15563650.2020.1747624.
- O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–690. doi:10.1007/s11135-006-9018-6.
- Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:10.1186/1751-0473-3-17.
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi:10.2105/ajph.79.3.340.
- Chakrabarti A, Ghosh JK. AIC, BIC and recent advances in model selection. In: Bandyopadhyay PS, Forster MR, editors. Handbook of the Philosophy of Science, Philosophy of Statistics. Vol. 7. North-Holland: Elsevier; 2011. p. 583–605.
- Diaz JH. Amatoxin-containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ Med. 2018;29(1):111–118. doi:10.1016/j.wem.2017.10.002.
- Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–2063. doi:10.2165/00003495-200161140-00003.
- Le Dare B, Ferron PJ, Gicquel T. Toxic effects of amanitins: repurposing toxicities toward new therapeutics. Toxins. 2021;13(6):417. doi:10.3390/toxins13060417.
- Enjalbert F, Rapior S, Nouguier-Soule J, et al. Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol. 2002;40(6):715–757. doi:10.1081/clt-120014646.
- Woodle ES, Moody RR, Cox KL, et al. Orthotopic liver transplantation in a patient with amanita poisoning. JAMA. 1985;253(1):69–70. doi:10.1001/jama.1985.03350250077026.
- Klein AS, Hart J, Brems JJ, et al. Amanita poisoning: treatment and the role of liver transplantation. Am J Med. 1989;86(2):187–193. doi:10.1016/0002-9343(89)90267-2.
- Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol. 2005;42(2):202–209. doi:10.1016/j.jhep.2004.10.023.
- Ferreira R, Romaozinho JM, Amaro P, et al. Assessment of emergency liver transplantation criteria in acute liver failure due to Amanita phalloides. Eur J Gastroenterol Hepatol. 2011;23(12):1226–1232. doi:10.1097/MEG.0b013e32834c7b8f.
- Saller R, Brignoli R, Melzer J, et al. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15(1):9–20. doi:10.1159/000113648.
- Mitchell T, Spyker D. Is intravenous silibinin a failed antidote for the fulminant hepatic failure treatment of amatoxin mushroom poisoning? Interim results of the North American clinical trial. Clinical Toxicology. 2017;55(7):689–868.
- Mitchell ST, Olson KR. Intravenous silibinin for the management amatoxin poisoning: first usage in an American cohort. Clin Toxicology. 2008;46(5):351–421.
- Gores KM, Hamieh TS, Schmidt GA. Survival following investigational treatment of amanita mushroom poisoning: thistle or shamrock? Chest. 2014;146(4):e126–e9. doi:10.1378/chest.13-1573.
- Locatelli CD, Travaglia A, Manzo L. Prolonged high-dose n-acetylcysteine treatment of Amanita phalloides and chlorinated hydrocarbon poisoning. Pharmacol Res. 1992;26:201. doi:10.1016/1043-6618(92)91111-S.
- Liu J, Chen Y, Gao Y, et al. N-acetylcysteine as a treatment for amatoxin poisoning: a systematic review. Clin Toxicol. 2020;58(11):1015–1022. doi:10.1080/15563650.2020.1784428.
- Horowitz BZ. Silibinin: a toxicologist’s herbal medicine? Clin Toxicol. 2022;60(11):1194–1197.
- Trakulsrichai S, Sriapha C, Tongpoo A, et al. Clinical characteristics and outcome of toxicity from amanita mushroom poisoning. Int J Gen Med. 2017;10:395–400. doi:10.2147/IJGM.S141111.
- Lecot J, Cellier M, Courtois A, et al. Cyclopeptide mushroom poisoning: a retrospective series of 204 patients. Basic Clin Pharmacol Toxicol. 2023;132(6):533–542.
- Dluholucky S, Snitkova M, Knapkova M, et al. Results of diagnostics and treatment of amanita phalloides poisoning in Slovakia (2004-2020). Toxicon. 2022;219:106927. doi:10.1016/j.toxicon.2022.09.013.
- Karvellas CJ, Tillman H, Leung AA, et al. Acute liver injury and acute liver failure from mushroom poisoning in North America. Liver Int. 2016;36(7):1043–1050. doi:10.1111/liv.13080.
- Giannini L, Vannacci A, Missanelli A, et al. Amatoxin poisoning: a 15-year retrospective analysis and follow-up evaluation of 105 patients. Clin Toxicol. 2007;45(5):539–542. doi:10.1080/15563650701365834.
- Beuhler MC, Sasser HC, Watson WA. The outcome of North American pediatric unintentional mushroom ingestions with various decontamination treatments: an analysis of 14 years of TESS data. Toxicon. 2009;53(4):437–443. doi:10.1016/j.toxicon.2009.01.004.
- Nordt SP, Manoguerra A, Clark RF. 5-Year analysis of mushroom exposures in California. West J Med. 2000;173(5):314–317. doi:10.1136/ewjm.173.5.314.

