Abstract
The plastic hardener methyl ethyl ketone peroxide (MEKP) is an unstable peroxide that releases free oxygen radicals. Ingestion of this compound induces widespread liver necrosis that is often fatal, extensive ulceration with subsequent scarring, and stenosis of the proximal digestive tract in survivors. Severe metabolic acidosis occurs due to the accumulation of formic acid and other organic acids inducing neurologic damage, such as optic nerve lesions. A 53-year-old man unintentionally ingested approximately 120 ml of a 33% solution of this compound in dimethylphtalate. The patient was treated with the free radical scavenger N-acetylcysteine to counteract free radical-mediated damage and with hemodialysis to remove accumulated organic acids. Although our patient demonstrated considerable edema and ulceration of the distal esophagus, stomach and duodenum in the acute phase, there was never any sign of liver damage or neurological damage, nor were there any demonstrable lesions in the proximal digestive tract three weeks after the event. Treatment with a combination of N-acetylcysteine and haemodialysis may be a promising therapy for this severe and potentially life-threatening intoxication.
Introduction
Methylethylketone peroxide (MEKP) belongs to the organic peroxides. Organic peroxides are non-volatile, very reactive oxidizing agents that readily form organic radicals. This property is utilized in polymerization reactions. Commercially, MEKP is used as a hardener. The ability to cause eye irritation in rabbits has been shown to correlate with chemical reactivity. Systemic toxicity studies have shown that MEKP is the most toxic organic peroxide, followed by tert-butyl-hydroperoxide or cumene-hydroperoxide, and di-tert-butyl-peroxide, in that order (Citation1–3). Commercially, the term MEKP usually designates the technical grade of this material, which is really a solution of a mixture of various methylethyl ketone peroxides and hydroperoxides in dimethylphtalate. MEKP is a colourless and odourless liquid that is sometimes inadvertently ingested.
Ingestion of MEKP has been reported in the literature and is associated with a high morbidity and mortality (Citation3–13, ). Little is known about the actual fate of MEKP once it is absorbed. However, MEKP has several physico-chemical properties that are likely to contribute to its toxic action. In contact with metal ions, MEKP readlily forms various various alkylperoxyl radicals. This involves a decomposition process in which various organic acids (e.g., formic acid, acetic acid, propionic acid) may be formed. The MEKP monomer is likely to split into hydrogen peroxide (H2O2) and Methyl Ethyl Ketone (Footnote14). In biological systems, the heme group has been identified as a strong accelerator of this radical formation process (Citation15).
Table 1. Review of literature
The presence of free radicals induces lipid peroxidation of target organs, particularly of the digestive tract and the liver. The digestive tract in this intoxication is exposed directly to the agent after ingestion, which explains why this agent is more likely to induce proximal than distal lesions. The liver is exposed to MEKP only after systemic absorption. This may induce acute liver failure, which is the major cause of death in this intoxication (). Survivors, on the other hand, often suffer gastrointestinal sequelae due to fibrotic and stenotic repair reactions following the acute phase insult. Additionally the formation of the organic acids may cause metabolic acidosis, local burns of skin and mucosa on contact, and induce neurologic lesions such as inflammation and ischemia of the optic nerve (Citation16–17).
There is no known effective treatment for this intoxication, neither on the basis of accumulated experience in human intoxications nor on the basis of experimental studies. Treatment recommendations are directed at limiting gastrointestinal toxicity by recommending the administration of milk and at limiting systemic abroption of MEKP by administering activated charcoal. To the best of our knowledge these recommendations are not evidence based. The only therapeutic option for established target organ damage and metabolic acidosis is supportive care.
Case report
A 53-year-old man unintentionally ingested an estimated 120 ml solution of 33% MEKP in dimethyl phtalate out of a plastic water bottle while working in his garage. The chemical identity of the liquid in the bottle was later confirmed by NMR-spectrometry by the manufacturer (results not shown).
The patient arrived at the emergency room within 30 minutes of ingestion with symptoms of nausea and sore throat. The patient had not vomited prior to presentation but did vomit shortly after arrival after being given milk to drink. The previous medical history included a partial colectomy after a trauma 18 years ago and a necrotising fasciitis of the head and neck region three years ago.
On physical examination, the patient was somewhat anxious with a blood pressure of 130/70 mmHg, a pulse rate of 85 beats/min, a core temperature of 36.5°C, and a peripheral oxygen saturation of 100%. There was residual scarring of the skin of the neck and throat. Examination of the oropharynx demonstrated no burns or other abnormalities. Examination of the abdomen showed normal bowel movements and no tenderness. The rest of the physical examination was unremarkable.
Laboratory evaluation () revealed a slight leucocytosis, hyperglycemia, an elevated serum creatinine, and serum amylase but no elevations of liver enzymes. Liver function also remained normal during admission. Only the PTT was slightly increased on days two and three of admission ().
Table 2. Laboratory results
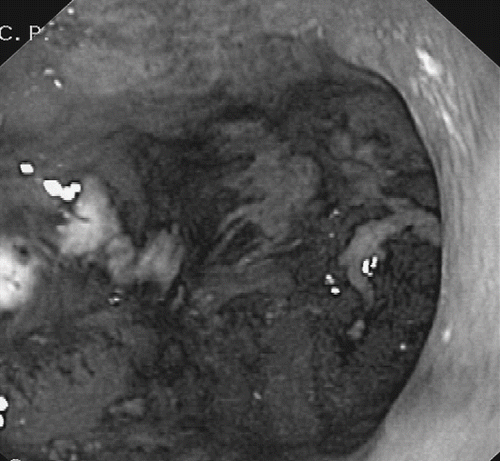
An esophagogastroduodenoscopy performed one hour after admission demonstrated considerable inflammation and necrotic lesions of the mucosal surfaces of the distal esophagus, the stomach and the duodenum with ulceration of the antral region of the stomach (). An ultrasound of the abdomen, a chest radiogram, and an electrocardiogram on the day of admission were all normal.
Fig. 1. Image during oesophagogastroduodenoscopy showing the antral region of the stomach with inflammation and necrotic lesions of the mucosal surfaces with ulceration.
After the diagnostic evaluation, the patient was admitted to the intensive care unit for monitoring of vital signs. The initial supportive care regimen consisted of the administration of one liter of cow's milk via a duodenal tube that had been inserted at endoscopy. He was also given metoclopramide and pantoprazol intravenously and lidocaine gel orally.
In an attempt to provide our patient with antidotal therapy, N-acetylcysteine was administered intravenously for 48 hours using the local dosing schedule for acetaminophen-poisoning (). Additionally, four hours of hemodialysis followed by continuous veno-venous haemofiltration (CVVH) were instituted after a metabolic acidosis with an elevated anion gap and osmolal gap developed (). The presence of methyl ethyl ketone and acetic acid in the patient's serum was later confirmed using gas chromatography (). Propionic acid and formic acid were not measurable at any time. CVVH was stopped once methyl ethyl ketone and acetic acid were no longer detectable, which was after three days.
Table 3. Schedule for acetaminophen-poisoning
During follow-up, the patient made an uneventful recovery. Initially there was macroscopic haematuria and secretory diarrhea in a frequency of about four times a day. These complaints subsided within two days. At no time was there any hemodynamic or respiratory instability, or any abnormal liver function test results. On day three of admission the patient was transferred to the ward from where he was discharged from hospital in good health on day eight with instructions to use only tube feeding until further notice. Three weeks after the event a second endoscopy did not show any residual abnormalities. The patient was then discharged from out-patient follow-up with instructions to resume normal oral feeding.
Discussion
To date, 23 other cases of MEKP ingestion have been reported in the literature (Citation4–13, ). Two articles seem to report on the same case, which is therefore counted only once (Citation10,Citation11).
The clinical spectrum of this intoxication in the acute phase can be subdivided into four categories. First, there are the gastrointestinal signs and symptoms due to inflammation and ulceration of exposed parts of the gastrointestinal tract, which can range from vomiting and acid burns to hemorrhage and perforation. The mechanism for this is not known. Second, there is the free radical mediated liver damage, which is the main cause of mortality (see ). Typically a patient who ingests MEKP develops liver function abnormalities within a few hours, progressing to acute liver failure due to generalized liver necrosis within the next two to three days leading to death within four days (Citation6,Citation8–9). On the basis of accumulated experience, the case fatality rate of this intoxication due to acute liver failure appears to be 26% (6/23). This figure could be an overestimation of the case fatality rate because of publication bias. Third, there are the complications due to the decomposition of MEKP that results in the generation of organic acids (Footnote14). Apart from metabolic acidosis, formic acid can induce a variety of neurological complications among which optic nerve toxicity is the most prevalent (Citation16–17). Finally, there are secondary acute complications such as acute renal failure due to rhabdomyolysis, ventilator-associated pneumonia, Adult Respiratory Distress Syndrome, and myocarditis ().
Scarring following acute phase ulcerative lesions of the gastrointestinal tract is the predominant problem in survivors. However, the chronic complications of this intoxication have not been described extensively, since the largest case series of MEKP ingestion describing 13 patients does not report on the chronic sequelae of this ingestion (Citation7). The scarring process induces stenotic lesions of the gastrointestinal tract, which often requires therapy varying from esophageal dilatation to partial resections of the stomach and/or the proximal small bowel. To date, only two patients with this intoxication survived without any chronic sequelae. However, both of these patients ingested an unknown quantity of MEKP.
The present case has three features that distinguish it from the reported experience to date. First, our patient ingested about 120 ml of a clear fluid that was positively identified as MEKP by the manufacturer. The presence of methyl ethyl ketone in the blood was also documented. Second, our patient received causal therapy, whereas all previous patients only received supportive care. This therapy consisted of the administration of N-acetylcysteine and the elimination of accumulated organic acids using extracorporeal clearance techniques. Third, this patient is the only case of MEKP ingestion who suffered no significant acute or chronic complications after receiving a therapy that was directed at the hypothesized pathogenesis of this intoxication. The elevated serum creatinine at admission is very likely not due to the ingestion because creatinine would need more time to become elevated.
On the basis of experimental data, lipid peroxidation of target organs due to free radicals liberated from MEKP and toxicity due to the generation of organic acids such as acetic acid, formic acid, and propionic acid as a result of MEKP metabolism seem to be the dominant pathophysiological mechanisms in this intoxication (Citation2,Citation3,Citation9,Citation18). Treatment of rats with 40 mg/kg of MEKP quickly induced lipid peroxidation in vivo, and from one to four hours after treatment caused a depression in the plasma content of vitamin E and the liver content of glutathione, as well as elevations in serum activities of alanine and aspartate aminotransferases (Citation19). This free radical mediated damage of the liver has similarity to acetaminophen intoxication in which depletion of glutathione also occurs and which can be prevented by treatment with acetylcysteine (Citation20). N-Acetylcysteine has also been suggested as a therapeutic option for MEKP intoxication (Citation12,Citation13) but there are no published reports on its use in MEKP intoxications. Although it is impossible to come to any firm conclusions on the basis of the outcome in our case, the absence of any acute liver damage suggests that this therapeutic option should be pursued further in experimental studies.
Although there are currently no adequate studies, there is a good theoretical basis for the use of N-acetylcysteine and a risk-benefit ratio (Citation20) that would favor its use in such cases. We used the intravenous N-acetylcysteine protocol that we normally use for acetaminophen intoxications (). We continued N-acetylcysteine for 48 hours, but the duration of acetylcysteine administration could probably be considerably shorter if one considers the short half-life of peroxides.
The decomposition of MEKP results in the generation of organic acids, one of which is formic acid that is generated in excess in methanol intoxication. Hemodialysis is an established therapeutic option in severe methanol intoxication (Citation16,21). This was the basis for adding hemodialysis to the therapeutic regime after a metabolic acidosis with elevated anion and osmolal gaps developed. Although the presence of acetic acid in the serum of our patient was demonstrated, formic acid and proprionic acid were not found at any time. Dialysis was continued until acetic acid was no longer measurable in the blood. We feel that it is reasonable to add dialysis to the therapeutic regime when and for as long as toxic metabolites can be demonstrated. If such metabolites cannot be measured, dialysis could be continued for a fixed period of time (e.g., 48 hours).
In conclusion, the use of N-acetylcysteine and hemodialysis in a case of an ingestion of a significant quantity of MEKP was followed by the absence of hepatic injury and rapid resolution of clinical toxicity, with no permanent sequelae.
Acknowledgments
We wish to acknowledge C. Braun and K. Schutjes from Akzo Nobel Chemicals Company for the verification of the fluid as MEKP.
Notes
14. Akzo Nobel. Butanox M-50 product data sheet technical brochure, 2005.
References
- Anonymous. Industrial Toxicology and Dermatology in the Production and Processing of Plastics. Elsevier Publishing Company, Amsterdam 1964; 211–217, (10 references)
- Litov RE, Matthews LC, Tappel AL. Vitamin E protection against in vivo lipid peroxidation initiated in rats by methyl ethyl ketone peroxide as monitored by pentane. Toxicol Appl Pharmacol 1981; 9: 96–106
- Floyd EP, Stokinger HE. Toxicity studies of certain organic peroxides. Am Ind Assoc J 1958; 19: 205–212
- Deisher JB. Poisoning with liquid plastic catalyst: report of a case. Northwest Medicine 1958; 57: 46
- Dines DE, Shipman K. Toxic myocarditis: report of a case arising from ingestion of liquid plastic catalyst. Angiology 1962; 13: 297–302
- Burger LM, Chandor SB. Fatal ingestion of Plastic Resin Catalyst. Arch Environ Health 1971; 23: 402–404
- Wojdyla Z, Pach J, Kolodziej J. Fatal acute poisoning with ketonox. Arch Med sad i krym 1979; 29: 199–205
- Mittleman RE, Romig LA, Gressmann E. Suicide by ingestion of methyl ethyl ketone peroxide. J Forensic Sci 1986; 31(1)312–320
- Karhunen PJ, Ojanperä I, Lalu K, Vuori E. Peripheral zonal hepatic necrosis causes by accidental ingestion of methyl ethyl ketone peroxide. Hum and Exp Toxicol 1990; 9: 197–200
- Blazquez M, Laurent-Puig P, Chataigner D, Delchier J. Les lésions caustiques oesogastriques et hépatiques induites par l'ingestion d'un durcisseur de résine. Gastroenterol. Clin Biol 1991; 15: 554–564
- Chataigner D, Garnier R, Reygagne A, Blasquez M, Efthymiou M-L. Brulures chimiques du tractus digestif supérieur et hépatite cytolytique secondaires à l'ingestion d'un mélange de peroxyde de méthyléthylcétone, de diacétone-alcool et de phtalate de diisobutyle. J de Tox Clin et Exp 1992; 12: 205–206
- Prez-Martnez A, Gutirrez-Junquera C, Gonzlvez-Piera J, Marco-Macin A, Rubio-Guijarro JR, Moya-Marchante M. Oesophageal stenosis in a child caused by ingestion of methyl ethyl ketone peroxide. Eur J Pediatr 1997; 156: 967–977
- Bates N, Driver CP, Bianchi A. Methyl ethyl ketone peroxide ingestion: Toxicity and outcome in a 6-year-old child. Pediatrics 2001; 108(2)473–476
- Akaike T, Sato K, Ijiri S, Miyamoto Y, Kohno M, Ando M, Maeda H. Bactericidal activity of alkyl peroxyl radicals generated by heme-iron-catalyzed decomposition of organic peroxides. Archives of Biochemistry and Biophysics 1992; 294(1)55–63
- Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisening. J Toxicol Clin Toxicol 2002; 40(4)415–46
- Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG. Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology 2000; 21(3)321–30
- Ando M, Tappel AL. Methyl ethyl ketone peroxide damage to cytochrome P-450 peroxidase activities. Toxicol Appl Pharmacol 1985; 81: 517–24
- Warren DL, Reed DJ. Modification of hepatic vitamin E stores in vivo. I. Alterations in plasma and liver vitamin E content by methyl ethyl ketone peroxide. Arch Biochem Biophys 1991; 285(1)45–52
- Chyka PA, Butler AY, Holliman BJ, Herman MI. Utility of acetylcysteine in treating poisonings and adverse drug reactions. Drug Safety 2000; 22(2)123–148
- Sivilotti MLA. Ethanol, isopropanol and methanol. Medical Toxicology3rd, RC Dart. Lippincott Williams and Wilkins, Philadelphia 2004; 1211–1223
