Abstract
Identification and quantification of carotenoids in the epidermis of nine patients of chronic arsenic poisoning were done using isocratic reverse phase high performance liquid chromatography (HPLC). The major carotenoids in all the skin biopsies were all-E lutein and 3′-epilutein. Small amount of 2′,3′-anhydrolutein, all-E zeaxanthin, and 13-Z zeaxanthin were also present in some of the biopsy samples. Alpha-carotene, beta-carotene, and lycopene were not detected in any sample. The mean (± SD) concentration of all-E lutein in the epidermis of healthy volunteers was 1.09 ± 0.26 microgram/g of wet tissue, whereas it was only 0.29 ± 0.10 microgram/g in the diffuse dark brown spots of chronic arsenic poisoning. In raindrop-shaped discoloration spots of skin the mean concentration of all-E lutein was 0.86 ± 0.29 microgram/g of wet tissue. The difference between the concentrations of all-E lutein in the epidermis of healthy volunteers versus patients was for the diffuse dark brown spots statistically significantly (p < 0.05) lower, while this was not significant for the raindrop-shaped discoloration spots. This study suggests that arsenic exposure reduces the number, as well as concentrations of, carotenoids in skin.
Introduction
About half of the total population (about 57 million) of Bangladesh, at present, are consuming arsenic through drinking and cooking (Citation1,Citation2). Already more than 40,000 people developed signs and symptoms of chronic arsenic poisoning. Early diagnosis of poisoning is based on skin manifestations and history of arsenic exposure. Skin manifestations include melanosis and keratosis (Citation3). There are raindrop-shaped discoloration spots, diffuse dark brown spots, or diffuse darkening of the skin on the limbs and trunk. Diffuse keratosis is identified as bilateral thickening of the palms and soles, and nodular keratosis is identified as small protrusions appearing on the palms, soles, and occasionally on the dorsum of the hands, feet, or on the legs (Citation4). The reason that the skin becomes discolored spots, diffuse dark brown spots or diffuse darkening, is not known. The normal color of the skin is due to the presence of melanin, hemoglobin, and carotenoids (Citation5). Any change in the amount or the structure of any one of these may alter the color of skin. Among the three major cell types of skin cancer, squamous cell carcinoma and basal cell carcinoma appear to be associated with the ingestion of arsenic. Such association was not observed for malignant melanoma (Citation6). In a study conducted by Momin et al. (2002), it was shown that squamous cell carcinoma was found more often in chronic arsenic poisoning (Citation7). Epidemiological studies have suggested an association between the high intake of carotenoid-rich fruits and vegetables and a reduced risk of cancer (Citation8,Citation9). However, arsenic-induced skin lesions occur among Atacameno people in Northern Chile, despite a good nutritional status and daily consumption of fruits and vegetables including carrots, the richest source of beta-carotene (Citation10). In the present study, we estimated the amount of carotenoids in the raindrop-shaped discolored spots and diffuse dark brown spots of the skin of chronic arsenic poisoning to find out the role of carotenoids in the pathogenesis of skin manifestations.
Method
Twenty cases of chronic arsenic poisoning were randomly selected (using a random number table) from an Arsenic Treatment Center at Chatkhil Upazilla of the Noakhali District (170 km northwest of Dhaka). Each patient presented with diffuse dark brown spots or diffuse darkening and hypopigmentation (raindrop-shaped discoloration spots) throughout the whole body surface except the face, palms, and soles; palms and soles had discrete hyperkeratosis. To confirm the clinical diagnosis, drinking water (source: shallow tubewell, 100 ml of water), midstream urine (10 ml), and nails (both fingers and toes, about 100 mg) were collected from each patient to estimate the concentration of total arsenic. After confirming the diagnosis, only nine patients (age 21∼35 years; four males and five females) cooperated to perform skin biopsies from areas other than the face. The remaining 11 cases were dropped. We also took the skin biopsies of nine healthy volunteers (age 24∼34 years; four males and five females) from the same area. These volunteers had normal skin and no history of arsenic exposure. This study was approved by the Bangladesh Medical Research Council and informed written consent was taken from both the patients and the control group.
The amount of total arsenic was estimated using the atomic absorption spectrophotometer with continuous flow hydride generator (Citation11). The limit of detection (LOD) of total arsenic in water was 0.88 microgram/l. The mean (± SD) concentration of total arsenic in drinking water was 193.6 ± 23.2 microgram/l in the case of patients, and 35.1 ± 9.3 microgram/l in the case of the control group. Patients excreted a high concentration of total arsenic in urine (64.8 ± 11.6 microgram/l) as compared to the control group (7.88 ± 4.16 microgram/l). The mean concentrations of total arsenic in the nail of patients and the control group were 5.83 ± 2.16 and 0.62 ± 0.11 microgram/g, respectively. The mean duration of suffering from symptoms by patients was 5.9 ± 2.7 years. All had no history of taking any antioxidant or drug(s) used for the treatment of chronic arsenic poisoning. In every patient of chronic arsenic poisoning, two punch biopsies (2–3 mm in diameter and mainly from epidermis) were taken - one from the diffuse dark brown spot and the other from the raindrop-shaped discolored spot. A single biopsy was taken from the skin of healthy volunteers. All the biopsies were taken from the abdomen. The weight of the biopsies were 1.6∼3.7 mg. Each biopsy material was kept in a separate container containing 1 ml of formaldehyde fully covered with aluminum foil and transported to the laboratory at 0–4°C on the same day of collection. Biopsy materials were then stored at −70°C until analysis (i.e., within three days of collection).
The concentration of carotenoids in the skin biopsies was measured by high performance liquid chromatography (HPLC). Before introduction into HPLC, carotenoids were extracted twice from each tissue separately; at first, in 1 ml of a mixture of n-hexane:acetone:methanol (2:1:1) for 60 minutes and then only n-hexane (1 ml) for another 30 minutes. The pooled extracts were then evaporated to dryness under nitrogen. The residue was dissolved in 100 μl of mobile phase and filtered through a 0.2 μm membrane filter. Twenty microliters was injected into the HPLC system.
Identification, separation, and quantification of carotenoids (with geometrical isomers) and their metabolites in skin were done using isocratic reverse phase HPLC with a UV-VIS detector set (ESA, USA) at 450 nm (Citation12). The separation was performed on a C18 column (250 × 4.6 mm, 5 μm particle size; ProntoSIL, Bischoff Chromatography, Germany) at room temperature. The mobile phase containing HPLC grade acetonitrile:methanol:chloroform (47:47:6) was used to achieve maximum separation and sensitivity. It was prepared daily, filtered, and delivered at a flow rate of 0.5 ml/min. After injecting each sample, the HPLC system ran for 70 min in order to get all of the carotenoids. The retention time for beta-carotene (last peak) was 65 min. Butylated hydroxytoluene (0.05%) was used both in the mobile phase and in the solvent used during extraction. All analyses were carried out in duplicate with the mean repeated; results should not vary by more than 10%. The chromatographic data were analyzed with the software EZCHROM elite (Scientific Software Inc, USA). All extractions and analyses were performed under dimmed light to prevent photo-isomerization and degradation of carotenoids. The LOD and limit of quantification (LOQ) were calculated using standard deviation (Citation13). Using 1.8 mg epidermis, the LOD for all-E lutein was 4.35 ng/g and LOQ was 13.05 ng/g.
The standards of all-E lutein, beta-carotene, beta-carotene, and all-E lycopene were purchased from Sigma Chemical Co. (USA). The standard of all-E zeaxanthin was a gift from Square Pharmaceuticals Ltd. (Bangladesh). 3′-epilutein, 2′,3′-anhydrolutein, 9-Z lutein, 9′-Z lutein, 13-Z zeaxanthin, and beta-cryptoxanthin were purified (at least 2 mg of each individual compound) from patients' serum using column chromatography and HPLC, and finally identified by 1H- and 13C-NMR (400 MHz) spectroscopy. The 1H-NMR data of individual isomer was confirmed using the data published by Khachik et al. (Citation14). All the standards were stored at −70°C in solvent free condition aerated with argon gas.
The significance of differences in lutein concentrations of epidermis between normal and chronic arsenic poisoning was determined using a multiple comparison test (Dunnett's test). The level of statistically significance was p < 0.05.
Results
Among the carotenoids, only lutein (geometrical isomers: all-E lutein, 9-Z lutein, 9′-Z lutein), zeaxanthin (geometrical isomers: all-E zeaxanthin, 13-Z zeaxanthin), cryptoxanthin, and metabolites of lutein (3′-epilutein, 2′,3′-anhydrolutein) were found in the epidermis of healthy volunteers. The major carotinoids in all the biopsy samples were identified as all-E lutein and 3′-epilutein. Chromatogram shows that the retention time for all-E lutein and 3′-epilutein were 10.9 and 15.5 min, respectively. The minor carotinoids were beta-cryptoxanthin (n=8), all-E zeaxanthin (n=6), 13-Z zeaxanthin (n=6), 9-Z lutein (n=1) and 9′-Z lutein (n=4). Only one biopsy showed all-E lycopene; alpha-carotene and beta-carotene were not detected in any sample. In the epidermis of chronic arsenic poisoning, all-E lutein and 3′-epilutein were mainly present (). 9-Z Lutein and 9′-Z lutein were totally absent; beta-Cryptoxanthin was present in one biopsy. all-E Zeaxanthin, 13-Z zeaxanthin, and 2′,3′-anhydrolutein were present in two biopsies. None of the biopsies showed detectable concentration of alpha-carotene, beta-carotene, and lycopene.
Fig. 1. HPLC profile of the carotenoids in skin biopsies of arsenic non-exposed and raindrop-shaped discolored spots of chronic arsenic poisoning. Peak 1: all-E lutein (retention time- 10.9 min); peak 2: 13-Z zeaxanthin (13.3 min); peak 3: all-E zeaxanthin (13.9 min); peak 4: 3′-epilutein (15.5 min); peak 5: 9-Z lutein (18.0 min); peak 6: 9′-Z lutein (18.7 min); peak 7: 2′,3′-anhydrolutein (19.5 min); and peak 8: cryptoxanthin (22.4 min).
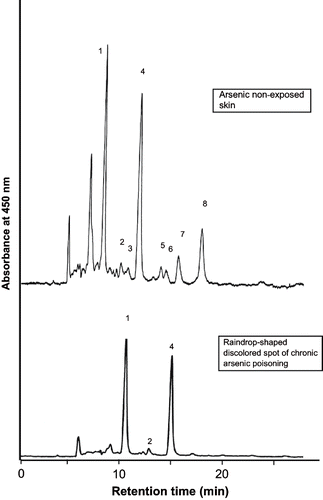
The mean concentration of all-E lutein in the epidermis of healthy volunteers was 1.09 ± 0.26 microgram/g of wet tissue (). The concentration of all-E lutein in the epidermis of chronic arsenic poisoning varies. In the diffuse dark brown spots of skin, the mean concentration was 0.29 ± 0.10 microgram/g of wet tissue. This difference between the normal epidermis and diffuse dark brown spots was statistically significant (p < 0.05). But in the raindrop-shaped discoloration spots, all-E lutein concentration increased toward the normal level to 0.86 ± 0.29 microgram/g of wet tissue. The difference of concentrations between the normal epidermis and raindrop-shaped discoloration spots was not statistically significant (p > 0.05). The mean concentrations of 3′-epilutein in the epidermis of healthy volunteers, diffuse dark brown spots and raindrop-shaped discoloration spots of chronic arsenic poisoning were 0.71 ± 0.27, 0.20 ± 0.11, and 0.43 ± 0.25 microgram/g of wet tissue, respectively. The difference of 3′-epilutein concentrations between healthy volunteers and diffuse dark brown spots was statistically significant (p < 0.05), whereas the difference between healthy volunteers and raindrop-shaped discoloration spots was not statistically significant (p > 0.05).
Table 1. Concentration of all-E lutein and 3′-epilutein in the epidermis of normal volunteers and patients of chronic arsenic poisoning
Discussion
More than 600 carotenoids have been identified of which only 25 dietary carotenoids and nine of their metabolites have been detected in human tissues, although the specific profile is dependent on an individual diet (Citation15). Among the dietary carotenoids, 13 are all-E isomers and the rest, 12, are Z isomers. In well-nourished humans, carotenoids are present primarily in the adipose tissue (80∼85%), liver (8–12%), and muscle (2–3%) with small amounts in other tissues, including skin (Citation16). The major carotenoids in the skin are lutein, zeaxanthin, cryptoxanthin, lycopene, alpha-carotene, and beta-carotene (Citation17,Citation18). The present study shows that lutein, zeaxanthin, and cryptoxanthin are present in the skin of healthy volunteers; among them lutein is dominant. Lutein is more polar than beta-carotene and has been suggested to be absorbed five times more efficiently from the gut (Citation19). Absence of alpha-carotene, beta-carotene, and lycopene is related with dietary intake. There are a few studies where the concentration of carotenoids either in whole skin or epidermis was found. The concentration of carotenoids in epidermis has physiological importance and is 3–6-fold higher than the dermis (Citation20,Citation21). Our data shows that the mean total concentration of carotenoids (as lutein + epilutein) in normal epidermis is 1.80 microgram/g, which is lower than the concentration of beta-carotene reported by Vahlquist et al. (Citation21). Vahlquist et al. estimated the concentration of carotenoids using methods other than HPLC. Several-fold lower concentration of carotenoids was reported by Peng et al. using HPLC but they used whole skin instead of epidermis (Citation17). Higher concentration of lutein in the epidermis of our normal individuals is due to a high intake of green leafy vegetables (Citation22). The site of skin and higher exposure to sunlight due to geographical location may influence the concentration of carotenoids. Our data also shows high concentration of 3′-epilutein which may be explained by 1) its transfer from the plasma and 2) extensive metabolism of lutein within the epidermis. The allylic oxidation of lutein can result in the formation of (3R, 6′R)-3-hydroxy-beta,beta-carotene-3′one(3′-oxolutein), which may undergo reduction either to revert back to lutein or epimerize at C-3′ to form 3′-epilutein (Citation23). Arsenic non-exposed normal epidermis also contains small amount of 9-Z lutein, 9′-Z lutein, all-E zeaxanthin, 13-Z zeaxanthin, and 2′,3′-anhydrolutein. Similar isomers are detected in plasma (Citation14).
All of the extracts of epidermis of chronic arsenic poisoning contain mainly all-E lutein and 3′-epilutein. Small amount of 2′,3′-anhydrolutein is present in some of the cases. Cryptoxanthin is detected in one case, whereas it is present in eight biopsy samples of normal epidermis; all-E zeaxanthin and 13-Z zeaxanthin are present in two biopsies. That is, arsenic accumulation in the epidermis may stimulate the utilization of cryptoxanthin and some isomers of lutein and zeaxanthin. Arsenic-induced skin cancer cases had a significantly lower serum level of beta-carotene (Citation24), but there is yet no information about the carotenoid concentration in the skin of cancer patients.
The mean concentration of all-E lutein in the diffuse dark brown spots is several-fold lower than the mean concentration in normal epidermis. Reduced concentration of lutein in the diffuse dark brown spots may be due to oxidative stress caused by accumulation of arsenic in epidermis. Arsenic is usually accumulated in different organs of the body including skin, which is mostly in inorganic form (about 98%) (Citation25). The accumulated arsenic causes oxidative stress (Citation26). Carotenoids are sensitive to free radical attack (Citation27). The effect of arsenic-induced free radical on carotenoid concentrations is not known. However, UV radiation decreases the carotenoid concentration in skin (Citation28).
We have estimated the amount of carotenoids in the discolored spots and the neighboring diffuse dark brown spots. The mean concentration of lutein in the discolored spots is 0.86 microgram/g of wet tissue, which is about three times higher than concentration in diffuse dark brown spots (0.29 microgram/g of wet tissue). This higher lutein concentration may be responsible to alter the color of the skin. All of the family members in arsenicosis-hyperendemic areas are drinking high concentration of arsenic but usually one or two members show signs of hyperpigmentation and hyperkeratosis. Our present findings raises the question as to whether the appearance of discoloration spot is a good sign because there is a higher concentration of lutein (an antioxidant) than in the diffuse dark brown spots. The limitation of this study is that we could not compare the data of concentration of carotenoids in the uninvolved skin (from the face) of arsenic-poisoned patient. In addition, the distribution of melanin in discolored spots is not known.
In conclusion, this study suggests that chronic arsenic exposure reduces the number and concentrations of carotenoids in the epidermis of skin, and that higher accumulation of lutein and 3′-epilutein in some of the areas of skin may be responsible for raindrop-shaped discoloration spots. Further studies are required to understand the pathophysiology of accumulation of these xanthophylls in order to find out the best drug for the treatment of chronic arsenic poisoning.
Acknowledgement
This work was supported by a grant from the World Health Organization, Bangladesh (Project no. PHE BAN 003). The authors gratefully acknowledge Mr. Shahidul Islam from the Bangladesh Council for Scientific and Industrial Research, Dhaka for doing 1H- and 13C-NMR. We thank Prof. Md. Shahidullah for help in statistical analysis.
References
- Mudur G. Half of Bangladeshi population at risk of arsenic poisoning. Br Med J 2000; 359: 1127
- Misbahuddin M. Consumption of arsenic through cooked rice. Lancet 2003; 361: 435–436
- IPCS. Arsenic and arsenic compounds. Environmental health criteria2nd. World Health Organization, Geneva 2001
- Mitra SR, Guha Mazumder DN, Basu A, Block G, Haque R, Samanta S, Ghosh N, Smith MMH, von Ehrenstein OS, Smith AH. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ. Health Perspect 2004; 112: 1104–1109
- Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr 2002; 132: 399–403
- Guo HR, Yu HS, Hu H, Monson RR. Arsenic in drinking water and skin cancer: cell type specificity (Taiwan, ROC). Cancer Causes Control 2001; 12: 909–916
- Momin A, Haque M, Ali CM, Khan MR, Salam A, Khanam M, Khan AZ, Farah A, Bari A. Chronic arseniasm with skin cancer - 5 case reports and review. Bang J Dermatol Leprol Venereol 2002; 19: 30–33
- Van Poppel G, Goldbohm RA. Epidemiological evidence for beta-carotene and cancer prevention. Am J Clin Nutr 1995; 62(suppl)1393S–1402S
- Ziegler RG. Vegetables, fruits and carotenoids and the risk of cancer. Am J Clin Nutr 1991; 53(suppl)251S–259S
- Smith AH, Arroyo AP, Guha Mazumder DN, Kosnett MJ, Hernandez AL, Beeris M, Smith AH, Moore LE. Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect 2000; 108: 617–620
- Misbahuddin M, Maidul Islam AZM, Khandkar S, Ifthekar-Al-Mahmud Islam N, Anjumanara. Efficacy of spirulina extract plus zinc in patients of chronic arsenic poisoning: a randomized placebo-controlled study. Clin Toxicol 2006; 44: 135–141
- Thurnham DI, Smith E, Flora PS. Concurrent liquid-chromatography assay of retinol, α-tocopherol, β-carotene, lycopene and β-cryptoxanthin in plasma, with tocopherol acetate as internal standard. Clin Chem 1988; 34: 377–381
- Schmidt CK, Brouwer A, Nau H. Chromatographic analysis of endogenous retinoids in tissue and serum. Anal Biochem 2003; 315: 36–48
- Khachik F, Englert G, Daitch CE, Beecher GR, Tonucci LH, Lusby WR. Isolation and structural elucidation of the geometrical isomer of lutein and zeaxanthin in extracts from human plasma. J Chromatogr 1992; 582: 153–166
- Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomatoo carotenoids and their impact on human health. Exp Biol Med 2002a; 227: 845–851
- Bendich A, Oslon JA. Biological actions of carotenoids. FASEB J 1989; 3: 1927–1932
- Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biom 1993; Prev 2: 139–144
- Wingerath T, Sies H, Stahl W. Xanthophyll esters in human skin. Arch Biochem Biophys 1998; 355: 271–274
- van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RPM, van Dusseldorp M, Weststrate JA, Eskes TKAB, Hautvast JGAJ. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am J Clin Nutr 1999; 70: 261–268
- Lee R, Mathews-Roth MM, Pathak MA, Parrish JA. The detection of carotenoid pigments in human skin. J Inves Dermatol 1975; 64: 175–177
- Vahlquist A, Lee JB, Michaelsson G, Rollman O. Vitamin A in human skin: II Concentrations of carotene, retinol and dehydroretinol in various components of normal skin. J Inves Dermatol 1982; 79: 94–97
- Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V. Determination of major carotenoids in a few Indian leafy vegetables by High Performance Liquid Chromatography. J Agric Food Chem 2005; 53: 2838–2842
- Khachik F, Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformation of selected carotenoids in plasma, liver, and other tissues of humans and in nonprimate animal models. Inves Ophthamol Vis Sci 2002b; 43: 3383–3392
- Hsueh TM, Chiou HY, Huang TL, Wu WL, Huang CC, Yang MH, Lue LC, Chen GS, Chen CJ. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biom Prev 1997; 6: 589–596
- Yamauchi H, Yamamura Y. Concentration and chemical species of arsenic in human tissue. Bull Environ Contam Toxicol 1983; 31: 267–77
- Bernstam L, Nriagu J. Molecular aspects of arsenic stress. J Toxicol Environ Health 2000; 3: 293–322
- Chen G, Djuric Z. Carotenoids are degraded by free radicals but do not affect lipid peroxidation in unilamillar liposomes under different oxygen tensions. FEBS Lett 2001; 505: 151–154
- Biesalski HK, Hemmes C, Hopfenmuller W, Schmid C, Gollnick HPM. Effects of controlled exposure of sunlight on plasma and skin levels of beta-carotene. Free Radical Res 1996; 24: 215–224
