Abstract
Background. Copper is an essential element. Poisoning with elemental copper is infrequent and manifestations rarely include the ones that our case presented. Case report. A previously healthy 2-year-old female patient unintentionally inhaled copper dust, developed respiratory failure a few hours later, and required mechanical ventilation. On hospital day three, the patient developed acute respiratory distress syndrome and was treated with high-frequency oscillatory ventilation for six days. She also developed hemolytic anemia, liver failure, oliguric renal failure, and evidence of acute tubular injury. During her stay in the intensive care unit she received inotropic support, packed red cells transfusion, and diuretics. A sample of bronchoalveolar lavage showed macrophages that stained positive for copper. Serum and urine copper concentrations were within the normal range after several days. Extubation was successfully achieved after two weeks and the patient was discharged on day 30 without sequelae. This is the first report of acute respiratory distress syndrome secondary to copper aspiration in a pediatric patient. Conclusion. To our knowledge, this is the first case reported of acute respiratory distress syndrome secondary to elemental copper aspiration. It is important to the clinician to be aware of acute respiratory distress syndrome as a differential diagnosis to copper aspiration by treating the patient aggressively in an adequate clinical setting.
Introduction
Copper is the third most abundant essential element in humans. This element catalyzes several reactions and is essential to the function of many enzymes and to the synthesis of the heme group and iron absorption. Copper poisoning is infrequent and manifestations rarely include the ones that our case presented with, such as vomiting, central nervous system impairment, hemolytic anemia, elevated hepatic enzymes, and acute lung injury that evolved into acute respiratory distress syndrome (Citation1).
Case report
A previously healthy 2-year-old child unintentionally spilled a bottle containing a powdered golden pigment on her face, inhaling part of the contents. She immediately developed dry cough, perioral cyanosis, and severe dyspnea. Fifteen minutes later, her parents took her to the emergency department (ED) where she demonstrated dyspnea, sensory impairment, and hypoxemia (pulse oximetry saturation 80% in room air). Her vital signs were temperature 36°C, heart rate 143 beats/minute, respiratory rate 38 breaths/minute, and blood pressure 110/80 mm Hg. During her stay on the ED she vomited brownish material. The parents said that the pigment was purpurin (1,2,4-trihydroxyanthraquinone) available at their home for school homework.
The venous blood gas analysis values were pH 7.19, pCO2 60 mmHg, and BE −6.3. A chest radiograph showed bilateral hyperinflation and mild interstitial infiltrates. Activated charcoal was administrated (1 g/kg) through a nasogastric tube. Intravenous fluids were started as well as oxygen and nebulized albuterol.
The patient was transferred to the pediatric intensive care unit because of progressively increasing oxygen demand associated with worsening radiological findings and increasing respiratory distress. The arterial blood gas analysis showed a pH 7.26 and mechanical ventilation was instituted (peak inspiratory pressure 29 cm H2O, positive end-expiratory pressure 8 cm H2O, inspiratory time 0.95 seconds, respiratory rate 18 breaths/minute, and fraction of inspired oxygen 0.80). Midazolam (0.2 mg/kg/h) was administered to induce sedation and empiric antibiotic therapy was started with penicillin and cefotaxime.
On day two, she was hemodynamically unstable, requiring fluid administration (30 ml/kg saline plus 50 ml/kg albumin 10% in 24 hours) and dopamine infusion (8 mcg/kg/min). She suffered repeated episodes of hemolytic anemia with hemoglobin of 8.2 g/dl and indirect bilirubin of 2 mg/dl, requiring a total of three packed red cell transfusions. A fiber bronchoscopy was performed showing normal airways with no signs of discoloration; a bronchoalveolar lavage sample was taken and sent for cytologic analysis and bacterial culture. The blood tests revealed elevated aspartate aminotransaminase (105 U/I) and ammonium (120 umol/l, normal 11.2–48.2 umol/l), and a prothrombinemia of 20%. Her arterial blood gas values were pH 7.1, HCO3 13.7 and BE −15.1) and glycosuria of 150–500 mg/dl without hyperglycemia.
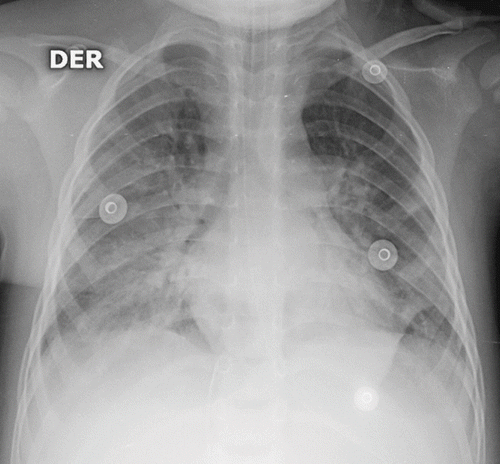
On day 3 she developed oliguric renal failure (urea 84 mg/dl and plasma creatinine 1.9 mg/dl). Furosemide infusion was initiated (0.4 mg/kg/h). At this time the patient still showed respiratory failure and acute respiratory distress syndrome (ARDS) was diagnosed according to chest radiograph () and a PaO2/FiO2 ratio of 180. She was switched to high-frequency oscillatory ventilation (HFOV) (mean airway pressure of 21, frequency of 10 Hz, and amplitude of 55 cm H2O). HFOV was used for six days and the patient gradually improved; conventional mechanical ventilation was then instituted.
Fig. 1. Chest radiograph on third day. Diffuse alveolar infiltrates compatible with acute respiratory distress syndrome.
On day 7 laboratory results of the aspirated powder revealed it to be copper dust. The serum copper level on day 11 was 68 mcg/dl.
She was extubated after 10 days of conventional mechanical ventilation but experienced post-extubation laryngitis (subglotic edema on a new fiber bronchoscopy). Despite dexamethasone (0.6 mg/kg/day) therapy she needed re-intubation and ventilation for another four days.
Analysis of the bronchoalveolar lavage sample revealed phagocytic intracellular copper in macrophages using the Shikata technique (). Blood, urine, and bronchoalveolar fluid cultures were negative.
Fig. 2. Cytologic evaluation of broncheo-alveolar lavage fluid sample. Copper-laden alveolar macrophages with Shikata Positive Stain.

During the second week, her hepatic and renal functions progressively improved. One month after the hospital admission she was asymptomatic and without supplementary oxygen. A complete blood count (CBC), brain computed tomography, renal, and hepatic functions were all normal. The urinary copper level was less than 10 mcg/dl.
Health authorities were informed about the illegal sale of copper as a pigment for school use.
Discussion
Copper is readily absorbed after ingestion, inhalation, and dermal exposure (Citation1,Citation3,Citation4,Citation7). Since several mechanisms regulate the intestinal absorption of copper, systemic poisoning after ingestion is infrequent (Citation4). After absorption, copper is transported through the bloodstream (Citation1). Nearly all absorbed copper is deposited in the liver (Citation2). The liver not only stores copper but also excretes the element to the bile (Citation6).
Copper that is not absorbed through the intestinal tract is bound within intestinal mucosal cells and excreted when these cells are desquamated. Excess copper excreted in the bile is tightly bound to amino acids, thus preventing its reabsorption (Citation1). Copper is found extensively in red blood cells (Citation6) and is incorporated into ceruloplasmin in the liver (Citation1). Copper concentration in the lungs of a patient after a suicidal copper ingestion was 24 times higher than the normal range, and the concentration in the kidneys was 15 times higher (Citation5).
Approximately 2 to 4% of ingested copper is excreted in the urine (Citation1,Citation2), and around 18% of the ingested copper that is absorbed passes through the intestinal wall into the bowel for excretion (Citation1). Fecal and biliary excretion accounts for 80% of excretion of the ingested copper that is absorbed (Citation1,Citation6). The main route of elimination is reported to be biliary (Citation8). Small amounts of copper are eliminated in the skin, hair, and sweat (Footnote9). The dietary copper biological half-life is reported to be 13 to 33 days, with biliary excretion being the main route of elimination (Citation8). Copper produces hemolysis by increasing oxidation of hemoglobin sulfhydryl groups, leading to an increase in red blood cell permeability (Citation10). Copper inhibits sulfhydryl group enzymes such as glucose-6-phosphate dehydrogenase and glutathione reductase, which protect the cell from free oxygen radicals (Citation1,Citation3).
The assessment of acute copper poisoning should include whole blood copper levels, CBC, and liver and renal function in all patients with clinical toxicity. The literature about the use of chelators in an acute copper poisoning is limited. Data on efficacy are derived from patients with chronic copper intoxication and from animal studies. Dimercaprol, penicillamine, sodium 2,3-dimercaptopropanesulfonate (DMPS), and ethylene-diamino-tetraacetic acid (EDTA) have been used for this purpose (Citation11–14). Chelation therapy is generally recommended for symptomatic patients. If the patient is asymptomatic, laboratory confirmation is necessary before instituting chelation therapy (Citation1).
Conclusion
To our knowledge, this is the first case reported of acute respiratory distress syndrome secondary to elemental copper aspiration. It is important that the clinician be aware of ARDS as a differential diagnosis to copper aspiration by treating the patient aggressively in an adequate clinical setting.
Notes
9. Ruffing R. Copper: Normal metabolism, poisoning and a review of Palm Island mystery disease, unpublished, 1988.
References
- Editorial Staff. Copper (Management/Treatment Protocol). POISINDEX® System, RK Klasco. Thomson Micromedex, Greenwood Village, Colorado 6/2006
- Bingham E, Cohrssen B, Powell CH. Patty's Toxicology 5th. John Wiley & Sons, New York 2001; 2
- Nelson L. Copper. Goldfrank's Toxicologic Emergencies 7th, LR Goldfrank, NE Flomenbaum, NA Lewin, RS Weisman, MA Howland, RS Hoffman. Appleton & Lange, New York 2002; 1262–1271
- Barranco VP. Eczematous dermatitis caused by internal exposure to copper. Arch Dermatol 1972; 106: 386–387
- Kurisaki E, Kuroda Y, Sato M. Copper-binding protein in acute copper poisoning. Forens Sci Internat 1988; 38: 3–11
- Dowdy RP. Copper metabolism. Am J Clin Nutr 1969; 22: 887–892
- Bentur Y, Koren G, McGuigan M. An unusual skin exposure to copper: Clinical and pharmacokinetic evalution. Clin Toxicol 1988; 26: 371–380
- Barceloux DG. Copper. Clin Toxicol 1999; 37: 217–230
- Metz EN, Sagone AL. The effect of copper on the erythrocyte hexose monophosphate shunt pathway. J Lab Clin Med 1972; 80: 405–413
- Owen CA, Jr, Randall RV, Goldstein NP. Effect of dietary D-penicillamine on metabolism of copper in the rat. Am J Physiol 1975; 228(1)88–91
- Bhusnurmath SR, Walia BNS, Singh S. Sequential histopathologic alterations in Indian childhood cirrhosis treated with d-Penicillamine. Human Pathol 1991; 22: 653–658
- Rodeck B, Kardoff R, Melter M. Treatment of copper associated liver disease in childhood. Eur J Med Res 1999; 4(6)253–6
- Mitchell WM, Basinger MA, Jones MM. Antagonism of acute copper(II)-induced renal lesions by sodium 2,3-dimercaptopropanesulfonate. Johns Hopkins Med J 1982; 151(6)283–5
