Abstract
Background. Tako-tsubo syndrome (TTS) refers to the apical ballooning of the left ventricle observed when angiographic ventriculography is performed in patients presenting with electrocardiographic changes suggestive of acute coronary syndrome (new transient ST-segment deviation (>0.05 mV) or T-wave inversion (>0.2 mV)), mild elevation of cardiac markers, but normal coronary arteries at the angiogram. Case report. A 54-year-old woman developed the characteristic features of TTS 44 hours following nortriptyline overdose. The admission ECG showed increased QRS duration rapidly reversible after sodium bicarbonate infusion. There was a minimal increase in troponin I level. The ECG performed at the time of chest pain revealed deeply negative T waves in leads I, II, III, aVF, V1 to V6 and remained abnormal at 5 weeks follow-up. In contrast, a complete recovery of left ventricular function was observed within one week. Discussion. The pathophysiology of TTS, a variant of myocardial stunning, is still incompletely understood but could be related to sympathetic overstimulation. The possibility of TTS following toxic exposure is discussed.
Keywords: :
Introduction
“Taku-tsubo” is the Japanese name for a fishing pot with a round bottom and narrow neck that is used for trapping octopus. However, it refers also to the left ventricular apical ballooning observed in patients with ECG changes suggestive of acute coronary syndrome (ST segment elevation or T wave inversion), mildly elevated cardiac markers, left ventricular wall motion abnormalities in the apical region, but normal coronary arteries at the angiogram [Citation1]. To our best knowledge, apical ballooning syndrome or “tako-tsubo” syndrome (TTS) was never clearly identified following drug overdose.
Case report
A 54-year-old Caucasian woman arrived by ambulance in the emergency department following tricyclic antidepressants (TCA) overdose. This patient had a medical history of tobacco abuse (2 packs/day for 30 years). She ingested probably 1000 mg nortriptyline 8 hours prior admission.
Her vital signs were the following: temperature, 36˚C; respiratory rate, 20/min; heart rate, 61/min; and arterial blood pressure, 159/110 mm Hg. The admission Glasgow Coma Score was 8/15.
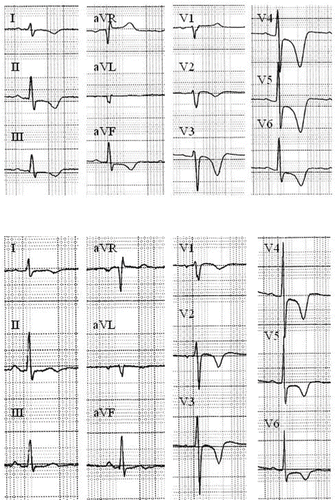
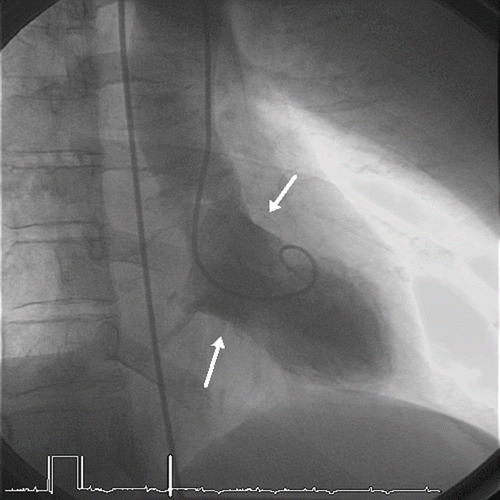
She was intubated 15 minutes after admission when her GCS decreased to 6, and in the absence of adequate upper airways protection. The admission electrocardiogram exhibited a sinus rhythm of 114/min, a wide QRS complex (146 msec) and a QTc interval of 472 msec, without significant changes in ST segment or T wave. The QRS widening reverted after the intravenous administration of 200 mmoles of sodium bicarbonate over 30 minutes (QRS 112 msec on the second ECG obtained 30 minutes after the end of infusion). Laboratory data showed creatine kinase of 68 IU/l (<200), troponin I of 0.23 ng/ml (<0.06). The hemodynamic condition remained perfectly stable during ICU stay. The serum concentration of nortriptyline on admission was measured at 858 ng/ml. Twelve hours after ICU admission, the patient was extubated but remained drowsy. Thirty-six hours after hospital admission (44 hrs after overdose), after having transferred to the general ward, the patient complained of typical chest pain. The ECG showed deeply negative T waves in leads I, II, III, aVF, and V1 to V6; the QRS duration was 106 msec (). Echocardiography showed apical akinesia with mild decreased systolic function. Cardiac catheterization confirmed the apical ballooning characteristic of “tako-tsubo” syndrome () and showed normal coronary arteries. No increase either in CK-MB or troponin I was measured in the blood samples. The patient was treated daily by 160 mg acetylsalicylic acid and 5 mg bisoprolol. Cardiac magnetic resonance imaging on day 8 demonstrated normal regional wall motion with no evidence of necrosis. The patient was discharged by the same day. On the ECG obtained 5 weeks later, T waves were still negative from V1-V6 but in a lesser extent. The prescription of bisoprolol was discontinued.
Discussion
Apical ballooning syndrome or “tako-tsubo” syndrome (TTS) often mimics acute coronary syndromes [Citation2–4]. The mechanism of this reversible cardiomyopathy is still poorly understood and various hypotheses have been proposed. The most commonly debated etiology is a catecholamine-induced myocardial stunning [Citation5,Citation6]. In a series of 19 patients (18 women), who presented with left ventricular dysfunction after sudden emotional stress, Wittstein et al. compared in 13 patients plasma catecholamine levels with those in 7 patients with Killip class III myocardial infarction [Citation6]. On hospital day 1 or 2, plasma levels of catecholamines among patients with stress cardiomyopathy were 2 to 3 times the values of among patients with Killip class III myocardial infarction and 7 to 34 times published normal values. By hospital day 7, 8, or 9, plasma catecholamine levels remained substantially higher in the stress cardiomyopathy group. The mechanism underlying the association between sympathetic stimulation and myocardial stunning is unknown. A direct myocyte injury has been debated, with contraction-band necrosis that can be demonstrated histologically in certain conditions including pheochromocytoma, subarachnoid hemorrhage, or fatal asthma. Catecholamines have well known vasoconstrictive effects. Epicardial coronary arterial spasm could result in transient ischemia. But the patients usually have contractile abnormalities in multiple vascular territories and multivessel epicardial spasm was never demonstrated at angiogram. Microvascular spasm with sympathetically mediated microcirculatory dysfunction is another possibility. The absence of coronary artery stenosis at coronary angiography does not exclude the presence of coronary artery disease with transient thrombus formation. Ibanez et al. suggested that a transient obstruction of the left anterior descending (LAD) artery could be caused by a ruptured atherosclerotic plaque that can only be seen by intravascular ultrasound examination, but these patients have usually abnormal LAD anatomy [Citation7]. Finally, in order to explain the angiographic pattern, a transient left ventricular obstruction could lead to an apical ballooning because of restricted endomyocardial blood flow [Citation8]. This phenomenon is typically observed in hypertrophic cardiomyopathy. The left ventricular obstruction could result from the catecholamine-induced basal segments hyperkinesis or from an abnormal myocardial functional architecture such as a localised mid ventricular septal thickening, which in presence of raised catecholamines levels, leads to a transient mid-cavity obstruction.
TTS has been described predominantly in women and frequently occurs after emotional or physical stress [Citation9]. Originally described in the Japanese population, it was also demonstrated in the European and North-American populations [Citation10,Citation11]. The patients developing TTS have usually cardiovascular risk factors, including arterial hypertension, hyperlipidemia, diabetes mellitus, or smoking [Citation12].
The diagnosis of TTS requires the exclusion of other conditions such as pheochromocytoma, acute myocarditis, hypertrophic cardiomyopathy, or acute neurological disease (particularly subarachnoid hemorrhage). A large number of other underlying disorders have been associated with TTS [Citation1]. However, to our best knowledge, a recent episode of drug overdose has never been mentioned among the precipitating factors.
Our patient met the criteria for TTS diagnosis. However, we acknowledge that a provocative test for coronary spasm was not performed during angiography and that we did not exclude atherosclerotic plaque rupture by ultrasound examination. The patient had diffuse symmetric T-wave inversion. As she started to complain of chest pain only when her consciousness was improving, we can not exclude that other ECG abnormalities could have been present earlier.
Is there a possible realtionship between this cardiac event and TCA overdose?
Transient ECG changes mimicking acute coronary syndrome are common during the course of TCA overdose [Citation13,Citation14]. The ECG changes are usually not associated with abnormal regional wall motion at echocardiography. Rarely, elevated cardiac biomarkers consistent with an acute myocardial infarction (troponin or CK-MB) have also been observed as stated below, suggesting a cardiac toxicity. Kiyan et al. reported after the ingestion of 300 mg amitriptyline an “acute myocardial infarction” in a 33-year-old woman without cardiovascular risk factors [Citation15]. Initially, the ECG showed sinus tachycardia and mild QRS widening (120 msec). Forty hours after the ingestion, she complained of chest pain and the ECG was modified with ST segment depression and T-wave inversion in V2 to V5 leads. Creatine kinase and troponin I were elevated. In this case, the echocardiography did not reveal abnormalities such as global or segmental hypokinesia or pericardial effusion. No coronary angiography was obtained because of normal myocardial perfusion at the single photon emission tomography. Similarly, Arya et al. described a case of dothiepin overdose complicated by a typical electrocardiographic feature of acute myocardial infraction [Citation16]. No data were available regarding cardiac markers. While coronary angiography was not performed, echocardiography revealed hypokinesia in the apical and anteroseptal regions. Other authors reported a 22-year-old woman who had ST-segment elevation in the antero-septal leads 26 hours after the ingestion of 300 mg amitriptyline. Creatine kinase MB was increased and the echocardiography revealed hypokinesia of the septum [Citation17]. No coronary angiography was performed and the patient was lost for follow-up. Guthrie et al. described in 1986 a 41-year-old woman who had been found comatose after an amitriptyline overdose [Citation18]. A mild elevation of serum CK and MB fraction was observed without any cardiac complications, ECG changes, regional function abnormalities at the echo, or coronary lesions at the angiography.
So far, no clinical observation meeting all the criteria of TTS has been published after TCA overdose.
It remains difficult to speculate about the exact role of nortriptyline in the genesis of TTS. The delay between drug overdose and the onset of chest pain remains imprecise and relatively long. We were also not able to obtain plasma catecholamine levels. When plasma catecholamine levels could be determined in patients with TCA overdose, there was some correlation between QRS duration and plasma norepinephrine levels [Citation19]. However, commensurate physiologic changes were not found in the presence of elevated catecholamine levels. Older publications also suggest that nortriptyline could reduce coronary flow in experiments on the isolated heart muscle and could raise the tone of isolated coronary blood vessels [Citation20].
Other substances could have similar effects and cocaine use has been associated with TTS in a recent observation [Citation21]. Systemic effects of cocaine are mediated by the inhibition of norepinephrine, epinephrine, dopamine, and serotonine amine uptake in the presynaptic neurons. The resulting increase in catecholamines can cause a variety of cardiovascular dysfunctions, including a typical pattern of acute myocardial infarction. The reason why cocaine could cause in some patients a preferential contractile dysfunction of the apex is unknown; the enhanced responsiveness of the apical myocardium to sympathetic stimulation remains a possible mechanism [Citation22].
The prognosis of TTS is usually excellent, with improvements in left ventricular function within 2–4 weeks of symptom onset. The patient should be treated according to standard care for acute coronary syndrome. If the catecholamine hypothesis is plausible, and as the global left ventricular function is usually preserved, it appears logical not to use some specific catecholamines, like dobutamine, to increase contractility [Citation6]. Obviously, if the patient is presenting with hypotension related to vasoplegia after TCA overdose, the use of norepinephrine is not contraindicated. Concerning bêta-blockers, there is no direct evidence for a protective effect. They could be indicated when basal hypercontractility is associated with the echocardiographic evidence of an intraventricular pressure gradient [Citation23]. The decision to perform conorary angiography should be guided by the interpretation of patient's complaints, cardiac markers, ECG, and echocardiographic findings.
In conclusion, we report a case of TTS following nortriptyline overdose. A recent drug overdose (and perhaps particularly with substances accompanied by an intense sympathetic stimulation) should be added to the list of the precipitating factors.
References
- Stöllberger C, Finsterer J, Schneider B. Transient left ventricular dysfunction (tako-tsubo phenomenon): Findings and potential pathophysiological mechanisms. Can J Cardiol 2006; 22: 1063–1068
- Abe Y, Kondo M. Apical ballooning of the left ventricle: A distinct entity?. Heart 2003; 89: 974–976
- Ogura R, Hiasa Y, Takahashi H, Yamaguchi K, Fujiwara K, Ohara Y, Nada T, Ogata T, Kusunoki K, Yuba K, Hosokawa S, Kishi K, Ohtani R. Specific findings of the 12-lead ECG in patients with “Takotsubo” cardiomyopathy: Comparison with the findings of the acute anterior myocardial infarction. Circ J 2003; 67: 687–690
- Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakamura S, Yoshida M, Mitsuba N, Hata T, Sato H. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: Comparison with acute myocardial infarction with minimal enzymatic release. Circ J 2004; 68: 77–81
- Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K. 123I-MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004; 45: 1121–1127
- Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539–548
- Ibanez B, Navarro F, Cordoba M, M-Alberca P, Farre J. Tako-tsubo transient left ventricular ballooning: is intravascular ultrasound the key to resolve the enigma?. Heart 2005; 91: 102–104
- Barriales Villa R, Bilbao Quesada R, Iglesias Rio E, Bayon Meleiro N, Mantilla Gonzalez R, Penas Lado M. Transient left ventricular apical ballooning without coronary stenosis syndrome: Importance of the intraventricular gradient. Rev Esp Cardiol 2004; 57: 85–88
- Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: Transient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141: 858–865
- Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005; 11: 472–479
- Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: First series in white patients. Heart 2003; 89: 1027–1031
- Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Koike H, Sasaka K. The clinical features of takotsubo cardiomyopathy. QJM 2003; 96: 563–573
- Smith DB, Tyznik JW. Desimipramine-induced conduction disorder mimicking myocardial infarction. Postgrad Med 1987; 82: 86–88
- Steeds RP, Muthusamy R. Abnormal ventricular conduction following dothiepin overdose simulating acute myocardial infarction. Heart 2000; 83: 289
- Kiyan S, Aksay E, Yanturali S, Atilla R, Ersel M. Acute myocardial infarction associated with amitriptyline overdose. Basic Clin Pharmacol Toxicol 2006; 98: 462–466
- Arya B, Hirudayaraj P, Wilmer K. Myocardial infarction: a rare complication of dothiepin overdose. Int J Cardiol 2004; 96: 493–494
- Chamsi-Pasha H, Barnes PC. Myocardial infarction: a complication of amitriptyline overdose. Postgrad Med J 1988; 64: 968–970
- Guthrie RM, Lott JA. Abnormal serum creatine kinase and MB fraction following an amitriptyline overdose. J Fam Pract 1986; 22: 550–555
- Merigian KS, Hedges JR, Kaplan LA, Roberts JR, Stuebing RC, Pesce A, Rashkin MC. Plasma catecholamine levels in cyclic antidepressant overdose. J Toxocol Clin Toxicol 191; 29: 177–190
- Banzdkiewicz W, Mrozikiewicz A. The influence of some antidepressant drugs on cardiac function. III. Influence on the isolated heart muscle and isolated coronary blood vessels. Arch Immunol Ther Exp (Warsz) 1976; 24: 471–475
- Arora S, Alfayoumi F, Srinivasan V. Transient left ventricular apical ballooning after cocaine use: Is catecholamine cardiotoxicity the pathologic link?. Mayo Clin Proc 2006; 81: 829–832
- Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman JI, Okino H. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 1993; 27: 192–198
- Kyuma M, Tsuchihashi K, Shinshi Y, Hase M, Nakata T, Ooiwa H, Abiru M, Hikita N, Adachi T, Shoji T, Fujise Y, Shimamoto K. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circ J 2002; 66: 1181–1184