Abstract
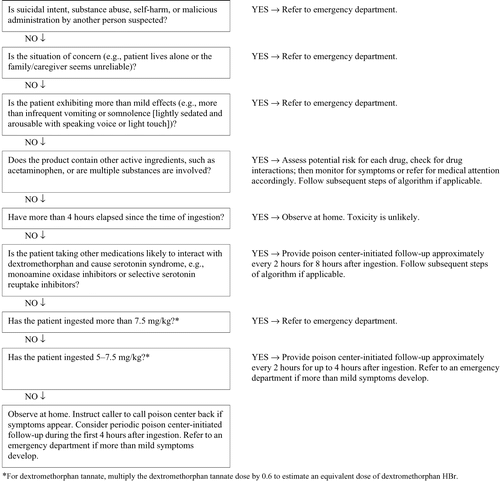
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial out-of-hospital management of patients with a suspected ingestion of dextromethorphan by 1) describing the process by which an ingestion of dextromethorphan might be managed, 2) identifying the key decision elements in managing cases of dextromethorphan ingestion, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to the ingestion of dextromethorphan alone. Co-ingestion of additional substances could require different referral and management recommendations depending on the combined toxicities of the substances. This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions might be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment. The grade of recommendation is in parentheses. 1) All patients with suicidal intent, intentional abuse, or in cases in which a malicious intent is suspected (e.g., child abuse or neglect) should be referred to an emergency department (Grade D). 2) Patients who exhibit more than mild effects (e.g., infrequent vomiting or somnolence [lightly sedated and arousable with speaking voice or light touch]) after an acute dextromethorphan ingestion should be referred to an emergency department (Grade C). 3) Patients who have ingested 5–7.5 mg/kg should receive poison center-initiated follow-up approximately every 2 hours for up to 4 hours after ingestion. Refer to an emergency department if more than mild symptoms develop (Grade D). 4) Patients who have ingested more than 7.5 mg/kg should be referred to an emergency department for evaluation (Grade C). 5) If the patient is taking other medications likely to interact with dextromethorphan and cause serotonin syndrome, such as monoamine oxidase inhibitors or selective serotonin reuptake inhibitors, poison center-initiated follow-up every 2 hours for 8 hours is recommended (Grade D). 6) Patients who are asymptomatic and more than 4 hours have elapsed since the time of ingestion can be observed at home (Grade C). 7) Do not induce emesis (Grade D). 8) Do not use activated charcoal at home. Activated charcoal can be administered to asymptomatic patients who have ingested overdoses of dextromethorphan within the preceding hour. Its administration, if available, should only be carried out by health professionals and only if no contraindications are present. Do not delay transportation in order to administer activated charcoal (Grade D). 9) For patients who have ingested dextromethorphan and are sedated or comatose, naloxone, in the usual doses for treatment of opioid overdose, can be considered for prehospital administration, particularly if the patient has respiratory depression (Grade C). 10) Use intravenous benzodiazepines for seizures and benzodiazepines and external cooling measures for hyperthermia (>104°F, >40°C) for serotonin syndrome. This should be done in consultation with and authorized by EMS medical direction, by a written treatment protocol or policy, or with direct medical oversight (Grade C). 11) Carefully ascertain by history whether other drugs, such as acetaminophen, were involved in the incident and assess the risk for toxicity or for a drug interaction.
Introduction
Scope of the problem and importance of the guideline
Dextromethorphan was approved by the US Food and Drug Administration in 1958 as a nonprescription drug to suppress cough and is now marketed throughout the world. Poisoning with dextromethorphan can follow the unintentional ingestion of a single large dose or it can follow chronic use of supratherapeutic doses. Dextromethorphan is also used as a substance of abuse or a means to attempt suicide. Based on a report from the Drug Abuse Warning Network of US emergency departments, 12,584 people sought emergency treatment in 2004 for dextromethorphan-related problems, representing 0.7% of all drug-related visits. The reasons for the dextromethorphan-related emergency department visits involved non-medical use (abuse) in 5,581 patients (44.3%), adverse effects from therapeutic use in 3,810 (30.3%), attempted suicide in 1,770 (14.1%), and unintentional ingestion in 1,423 (11.3%). Patients aged 12–20 years accounted for nearly one-half (48%) of all the visits resulting from the abuse of dextromethorphan. For unintentional ingestions, 94% of patients were under 12 years of age (Citation1).
The problem of dextromethorphan abuse and poisoning has been sporadic since its introduction on the market, but it appears to be increasing (Citation2,Citation3). According to the Toxic Exposure Surveillance System (TESS) of the American Association of Poison Control Centers, the number of cases involving dextromethorphan abuse or misuse by teenagers reported to poison centers in the US tripled between 2000 and 2003 (Citation4). A national survey of 50,000 high school students in 2006 found that 4, 5, and 7% of 8th, 10th, and 12th graders, respectively, claimed dextromethorphan abuse during the previous year (Citation5). Websites developed by organizations such as the Partnership for a Drug-Free America (www.drugfree.org) strive to raise awareness of the risks of dextromethorphan abuse. Alternatively, websites that promote abuse of dextromethorphan have also proliferated (Citation6).
During 1999–2004, US poison control centers managed 15,543 cases of dextromethorphan abuse as a single substance. In a subset analysis of 1,382 of these cases by the California Poison Control System, the annual frequency of cases increased 10-fold over this 6-year period. Adolescents (9–17 years of age) were involved in 74.5% of cases. The outcomes were mild in 46%, moderate in 41.8% and severe in 0.5% of the cases. There were no deaths reported, and the outcomes were reported as asymptomatic or unknown in 11.6%. A co-ingestant was involved in five of the seven cases with major effects. In this series, 17 patients claimed concurrent acetaminophen ingestion, but 26 patients had confirmed acetaminophen serum concentrations and 16 received acetylcysteine as therapy for acetaminophen poisoning (Citation7). During 2000–2005, US poison control centers managed 28,227 ingestions of dextromethorphan as a single substance in children under 6 years of age. Of these, 2564 (9.1%) children were evaluated at healthcare facilities and 25,663 (90.9%) were managed elsewhere, typically at home. Since follow-up after initial contact with the poison center was not conducted in 18,631 cases (65%), the outcomes of these cases can not be characterized. The increasing rate of abuse and misuse of dextromethorphan and the frequent unintentional ingestion of dextromethorphan by children makes this a substance of concern for poison center personnel.
Background on dextromethorphan
Dextromethorphan is the d-isomer of levorphanol, an opioid related to codeine. Its antitussive activity is based on its action on σ-opioid receptors without significant affinity for the μ and δ receptors, which are responsible for analgesic and CNS depressant effects. Dextromethorphan is metabolized in part by CYP2D6; 85% of Americans are rapid metabolizers due to genetic polymorphism. Dextrorphan, an active metabolite of dextromethorphan, antagonizes the actions of excitatory amino acids on N-methyl-d-aspartate (NMDA) receptors as do phencyclidine and ketamine. This action could account for the dissociative effects (e.g., dysphoria, hallucinations, agitation, sedation) experienced by those who abuse large doses of dextromethorphan. Rapid metabolizers might be more likely to achieve the dissociative effects at lower doses. Antagonism of NDMA receptors by dextromethorphan and metabolites might also be responsible for the adrenergic effects (e.g., hypertension, tachycardia, diaphoresis) sometimes observed with large doses of dextromethorphan as a result of inhibition of catecholamine reuptake. Dextromethorphan also binds to serotonergic receptors, which could contribute to its abuse potential and risk for serotonin syndrome. (Citation4,Citation6,Citation8,Citation9).
There are approximately 140 prescription and nonprescription drug products that contain dextromethorphan in combination with other active ingredients such as acetaminophen, antihistamines, decongestants, topical anesthetics, guaifenesin, promethazine, and ethanol. Several products contain only dextromethorphan as the active ingredient (). Typical amounts of dextromethorphan hydrobromide (HBr) in various products include: liquids, 3.3–15 mg/5 mL; tablets and capsules, 10–30 mg; extended-release tablets, 30–60 mg; lozenges, 2.5–7.5 mg; and powders, 20–30 mg. Dextromethorphan-containing sprays, rapidly dissolving oral strips, and solids intended to dissolve in beverages are also available. Most formulations contain dextromethorphan HBr, but some combination products contain dextromethorphan tannate, which is equivalent to 58% of dextromethorphan HBr (multiply the tannate dose by 0.6 for an approximate equivalent dose of dextromethorphan HBr) (Citation10). The concentration and dosage for the extended-release oral polistirex suspension are expressed as dextromethorphan HBr. Dextromethorphan powder is available through the Internet and by home laboratory extraction from pharmaceutical products (Citation3,Citation6,Citation11). Five deaths in the US have been attributed to dextromethorphan powder sold over the Internet (Citation12).
Table 1. Recommended therapeutic doses and maximum daily doses of dextromethorphan (Citation86)
Pharmacokinetic studies indicate that the time to achieve peak serum concentrations (2–3 hours) and serum elimination half-lives (approximately 3 hours) with a liquid, tablet, or extended-release suspension of dextromethorphan are similar. In a study of eight adults given dextromethorphan HBr 60 mg as syrup and tablets, the median (range) times to achieve peak serum concentrations were 2 (Citation1–2) and 3 (1.5–4) hours, respectively. The serum half-lives (mean ± SD) for the syrup and tablet formulations were 3.3 ± 0.63 and 2.7 ± 0.77 hours, respectively (Citation13). An extended-release oral polistirex suspension (Delsym) delivers dextromethorphan from an ion-exchange complex over a period of 9–12 hours. As reported in a study of six adults given 60 mg dextromethorphan HBr equivalent doses of the polistirex suspension, the time to achieve peak plasma concentrations (3.7 ± 0.82 hours) and elimination half-life (3.02 ± 0.83 hours) were not clinically different from those reported for immediate-release tablets or syrup (Citation14). The serum half-life could vary if the person is a fast or slow metabolizer, irrespective of the dosage form.
Following an acute ingestion of a large amount of dextromethorphan, central nervous system effects are prevalent and include stupor, ataxia, nystagmus, hyperexcitability, dystonia, coma, and toxic psychosis. Some cases have exhibited respiratory depression, tachycardia, hypertension, and diaphoresis (Citation15). The ingestion of large amounts of liquid preparations of dextromethorphan often causes nausea and vomiting due to the syrup base or ethanol content (up to 25% ethanol in some products). Some of the effects attributed to dextromethorphan might be caused or exacerbated by concomitant ingestion of sympathomimetics (e.g., pseudoephedrine, phenylephrine), antihistamines (e.g., diphenhydramine, brompheniramine), or ethanol found in combination products. Cases of long-term dextromethorphan abuse have exhibited tolerance, dependence, and physical withdrawal symptoms (e.g., vomiting, night sweats, myalgia, diarrhea, feeling cold, restlessness) beginning approximately 3 days after dextromethorphan discontinuation (Citation16).
Dextromethorphan has also been associated with the development of the serotonin syndrome, which manifests as autonomic instability, altered mental status, seizures, extrapyramidal syndrome including muscle rigidity, hyperthermia, and, rarely, death. Serotonin syndrome appears to be due, in large part, to excessive stimulation of the 5-HT2A subtype of central nervous system serotonin receptors. Typically, these cases involve patients who use multiple serotonergic agents or who take large amounts of a single serotonergic agent. Drug interactions are also commonly implicated in serotonin syndrome. Drugs that might interact with dextromethorphan to cause serotonin syndrome include selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, meperidine, lithium, clonazepam, methylenedioxy-methamphetamine (MDMA, Ecstasy), and the dietary supplements tryptophan and St. John's wort (Citation17).
Dextromethorphan is in FDA pregnancy category C. It is generally considered to be safe to use during pregnancy in recommended doses and is commonly used by pregnant women (Citation18,Citation19).
The effectiveness of dextromethorphan for relief of cough or cold symptoms has been questioned to the extent that its use has been discouraged by some organizations (Citation20–23). A 2007 report by the Centers for Disease Control and Prevention described three infants aged 1–6 months whose deaths were attributed to the use of cough and cold products. Two of the infants had measurable concentrations of dextromethorphan in their sera, but its role in their deaths was not stated. Based on these cases and other literature, the authors of the CDC report concluded that “the dosages at which cough and cold medications can cause illness or death in children aged under 2 years are not known.” They went on to advise that “because of the risks for toxicity, absence of dosing recommendations, and limited published evidence of effectiveness of these medications in children aged under 2 years, parents and other caregivers should not administer cough and cold medications to children in this age group without first consulting a health-care provider and should follow the provider's instructions precisely.” (Citation24)
Definition of terms
For the purpose of this guideline, two age groups were defined as either children less than 6 years of age or older children and adults. The older age group is more likely to attempt self-harm, engage in dextromethorphan abuse, and to conceal an ingestion. To be consistent with TESS definitions, acute ingestions are defined as those occurring over a period of up to 8 hours and chronic ingestions are those that occur over a period of more than 8 hours. Acute-on-chronic ingestion is an acute ingestion by a patient who has already been exposed to dextromethorphan for more than 8 hours (Citation25).
The following definitions for clinical effects are used throughout the guideline and are adapted from those used by TESS. Mild adverse or toxic effects are defined as those not requiring specific treatment or hospital admission and are generally limited to vomiting and somnolence (lightly sedated and arousable with speaking voice or light touch). Moderate effects are defined as those requiring treatment or admission to a hospital for observation and include vital sign abnormalities (particularly mild hyperthermia) agitation, lethargy (sedated but arousable with more than speaking voice or with irritating stimuli), movement disorders, hallucinations and those classified by the reporting article as “moderate” by TESS criteria, or serious enough to warrant discontinuation of medication in clinical trials. Severe effects are defined as those that appear to have been life-threatening (e.g., severe hyperthermia or rigidity, coma [requiring painful stimuli to arouse or unarousable] or sedation requiring intubation, seizures, respiratory depression, hypotension), or which were classified in the article as “severe” by TESS criteria. The severity of serotonin syndrome can be similarly categorized (Citation17). Assessments of severity required the medical judgment of the reviewer in some cases.
Intended users of this guideline
The intended users of this guideline are personnel in US poison control centers. This guideline has been developed for the conditions prevalent in the US. While the toxicity of dextromethorphan is not expected to vary in a clinically significant manner in other nations, the out-of-hospital conditions could be much different. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of this Guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and out-of‐hospital management of patients with suspected ingestions of dextromethorphan by 1) describing the process by which an ingestion of dextromethorphan might be managed, 2) identifying the key decision elements in managing cases of dextromethorphan ingestion, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research.
This guideline applies to the ingestion of dextromethorphan alone. Co-ingestion of additional substances could require different referral and management recommendations depending on the combined toxicities of the substances. This review focuses on the ingestion of more than a single therapeutic dose and the effects of overdoses. Although therapeutic doses of dextromethorphan can cause adverse effects in adults and children, some idiosyncratic and some dose-dependent, these cases are not considered here.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions might be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment.
Methodology
The methodology used for the preparation of this guideline was developed after reviewing the key elements of practice guidelines (Citation26,Citation27). An expert consensus panel was established to develop the guideline (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant US poison control center experience, and be an opinion leader with broad esteem. Two specialists in poison information were included as full panel members to provide the viewpoint of the end-users of the guideline.
Search strategy
Literature searches for relevant articles were performed by a single investigator. The National Library of Medicine's PubMed database was searched through March 2006 using dextromethorphan as a MeSH term with the subheadings poisoning or toxicity, limited to humans. The PubMed database was further searched using dextromethorphan as a textword (title, abstract, MeSH term, CAS registry) plus either poison* or overdos* or intox* or toxic*, limited to humans. This process was repeated in International Pharmaceutical Abstracts (1970–March 2006, excluding abstracts of meeting presentations), Science Citation Index (1977–March 2006), Database of Abstracts of Reviews of Effects (accessed March 2006), Cochrane Database of Systematic Reviews (accessed March 2006), and Cochrane Central Register of Controlled Trials (accessed March 2006). Reactions (1980–March 2006), the dextromethorphan poisoning management in Poisindex, and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, abstracts from the North American Congress of Clinical Toxicology published in the Journal of Toxicology Clinical Toxicology (1995–2004) and Clinical Toxicology (2005) were reviewed for original human data.
Six major toxicology textbooks were reviewed for recommendations on the management of dextromethorphan poisonings and for citations of additional articles with original human data in the chapter bibliographies. All US poison control centers were surveyed in 2006 to ascertain their out-of-hospital management and triage practices for dextromethorphan poisonings.
Criteria used to identify applicable studies
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of these articles were reviewed, searching specifically for those that dealt with estimations of doses with or without subsequent signs or symptoms of toxicity and management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles that did not meet either of the preceding criteria, did not add new data (e.g., reviews, editorials), or that exclusively described inpatient-only procedures (e.g., dialysis) were excluded.
Data extraction process
All articles that were retrieved from the original search were reviewed by a trained physician abstractor (second author). The articles were reviewed for original human data regarding the toxic effects of dextromethorphan or data directly relevant to the out-of-hospital management of patients with dextromethorphan toxicity or overdose. Relevant data (e.g., dose, effects, time of onset of effects, therapeutic interventions or decontamination measures provided, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief description of each article was written. This evidence table is available at http://www.aapcc.org/DiscGuidelines/DM%20evidence%20table%202006-8-8.pdf. The table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Attempts were made to locate foreign language articles and have their crucial information extracted, translated, and tabulated. A written summary of the data was created and distributed by the abstractor. Copies of all of the abstracted articles were made available for reading by the panel members on a secure AAPCC website.
Criteria used to assign levels of evidence
The articles were assigned level-of-evidence scores based on the Grades of Recommendation table developed by the Centre for Evidence-Based Medicine at Oxford University (Appendix 2). Single case reports and case series were classified as level 4.
Guideline writing and review
A guideline draft was prepared by the lead author (listed first). The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the panel members were collected, anonymously copied into a table of comments, and submitted to the lead author for response. The lead author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the lead author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, and ACMT members and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through e-mail communication to AAPCC staff. All submitted comments were rendered anonymous, copied into a table of comments, and reviewed by the expert consensus panel and the lead author. The lead author responded to each comment in the table and his responses and subsequent changes in the guideline were reviewed and accepted by the panel.
Estimation of dose
The estimation of dose is based largely on the patient's history and the type of product. If precise data for the ingestion are unknown or unclear (package size, unit size, number of units ingested), poison centers often utilize a method in which the maximum potential dose is calculated. For example, if the actual dose ingested cannot be ascertained, the amount of the drug product that is missing from the container is multiplied by the concentration of the formulation.
When the mg/kg dose or a patient's weight was not included in an article, the mg/kg dose was estimated by the use of growth charts up to 20 years of age (Citation28). The 95th percentile weight was used for a particular age and sex. When the sex of the patient was not stated, the weight for males was used. This approach errs on the side of estimating a lower mg/kg dose. Estimated mg/kg doses are italicized throughout the guideline whenever they are presented.
Time since ingestion
Ascertaining the time since ingestion is useful in evaluating the potential for toxicity and determining the need for healthcare facility referral and the duration of observation there. Once the time of peak effect has passed, an asymptomatic patient with an unintentional ingestion might not require referral to a healthcare facility solely based on a dose that exceeded a threshold dose.
Evaluation of evidence
Poison control centers
The expert consensus panel solicited referral and management guidelines for dextromethorphan from all US poison centers in 2006 and received documents from 12 poison centers. Five other centers indicated that they did not have written guidelines for dextromethorphan poisoning. The remaining centers did not respond to the request. Of the 11 centers reporting doses for emergency department referral, nine used 10 mg/kg or more, another used 15 mg/kg or more, and one used more than 180 mg. All nine centers that reported doses at which a patient could remain at home listed less than 10 mg/kg. Three centers that submitted guidelines limited the application of the dose thresholds to patients less than 6 years of age. None listed references for the source of the referral dose. Since all 12 centers submitting guidelines did not state their practices for all elements examined, the totals vary.
Review of textbooks
The review of dextromethorphan poisoning in toxicology textbooks revealed that five provided no information on referral dose, toxic dose, time of onset, or prehospital care (Citation29–33). One indicated that symptoms usually occur with ingestions in excess of 10 mg/kg and that toxicity is highly variable (Citation34). Naloxone was mentioned by two textbooks as potentially useful for dextromethorphan toxicity (Citation33,Citation34). Three books did not address dextromethorphan toxicity (Citation29,Citation30,Citation31).
Review of poisindex
Poisindex, a computerized toxicology reference used by poison control centers, did not provide specific recommendations regarding doses at which emergency department referral is recommended for adolescents or adults. According to Poisindex, children who ingest less than 10 mg/kg of regular dextromethorphan can be managed at home, but a reference for this dose was not cited. It also stated “it has been recommended that all children who are symptomatic and have ingested a long-acting dextromethorphan preparation be referred to a health care facility for evaluation.” For adults, it stated that 960 mg per day has been tolerated with minor adverse effects and the ingestion of 720 mg over 36 hours led to coma in one case. It went on to state “acute dextromethorphan overdosage probably will not result in severe signs and symptoms of intoxication unless massive amounts have been ingested” and “long-acting preparations and combination products may have greater potential for toxicity in children.” (Citation15) These statements were neither referenced nor qualified by age or dose.
Review of the literature
Overall, there were few articles specifically addressing either out-of-hospital or in-hospital management of dextromethorphan ingestions. Evidence regarding dose, toxicity, and time of onset is primarily limited to case reports and case series (level 4). The literature search did not identify any level 1 article specifically investigating a toxic threshold dose or time to onset of effects of dextromethorphan.
Dose resulting in toxicity
Acute ingestions in patients less than 6 years of age
There was one article (level 2b) in which single doses of dextromethorphan were prospectively given to patients under 6 years of age and associated with subsequent mild toxicity. Doses of 7.5–30 mg dextromethorphan syrup (0.35–0.94 mg/kg), given to 33 children aged 2–17 years with upper respiratory illnesses resulted in a few reports of mild adverse effects (6 hyperactivity, 2 nausea or stomachache, 3 insomnia, 1 dizziness, 1 nervousness) (Citation35).
One abstract (level 6) described three children, aged 1 month to 4 years, in whom doses of 5–21 mg/kg (combination or extended-release products) resulted in one or more symptoms (lethargy, coma, nystagmus, mydriasis, ataxia, and dystonia) in all patients ranging from moderate to severe (Citation36).
Another abstract (level 6) described five children aged 2–4 years who ingested unstated doses of extended-release dextromethorphan polistirex suspension. They exhibited one or more symptoms of urticaria, restlessness, lethargy, nystagmus, ataxia, tachycardia, and blood pressure elevation. Two patients were admitted to an intensive care unit for unstated reasons. One patient received naloxone 3.6 mg, which resulted in “incomplete clearing of lethargy.” Severity of the five cases ranged from mild to severe in the authors' estimation (Citation37).
There were also seven level 4 articles () with individual dose and effect information (Citation38–44). In one case, a 3‐month-old infant died soon after receiving 0.6–1.2 mL of Children's Tylenol Plus Cough and Cold (in 0.8 mL: acetaminophen 80 mg, dextromethorphan HBr 2.5 mg, pseudoephedrine HCl 7.5 mg); the duration of dosing was unclear and the exact cause of death was uncertain (Citation41). In another instance, a 10-month-old was found unresponsive and eventually died after being given 10 mL of an unspecified dextromethorphan-containing cough and cold product for an unknown duration (Citation38). A 20-month-old boy developed moderate toxicity (mydriasis, blank stare, hypertonia, hypotonia) after the ingestion of 150 mg (11.5 mg/kg) of an unspecified dextromethorphan product (Citation39).
Table 2. Acute dextromethorphan poisonings with dose information in patients less than 6 years of age
Acute ingestions in patients 6 years of age and older
There was one article reviewed (level 2b) in which single doses of dextromethorphan were prospectively given to patients older than 6 years of age (range 2–17 years) and associated with subsequent mild toxicity. This article was described above (Citation35). There were also three case series (level 4) and one abstract (level 6) of a retrospective case review (Citation45–48). Since all of these articles reported dextromethorphan doses and durations, patient ages, and subsequent effects as ranges or percentages, it was impossible to determine which doses were associated with a particular effect. In one review of Coricidin HBP Cough and Cold (per tablet: chlorpheniramine maleate 4 mg, dextromethorphan HBr 30 mg) use by 92 patients, 11–65 years of age, the ingestion of 2–60 tablets (dextromethorphan 60–1800 mg) was associated with mild to moderate effects (50 tachycardia, 29 hypertension, 40 lethargy, 20 mydriasis, 5 agitation, 20 ataxia or dizziness, 9 vomiting, 16 confusion, 8 elevated temperature, 6 dry mouth, 5 slurred speech, 3 hallucinations) (Citation46). A retrospective review of 78 patients at least 8 years of age (77% between 13 and 17 years of age) with abuse of 4–63 tablets of several types of Coricidin (dextromethorphan in combination) reported mild to moderate effects as documented by one poison center (Citation45). A self-reporting survey of 53 adults with histories of dextromethorphan abuse in doses of 70–2700 mg (products not specified) taken in 1–200 episodes reported that 51 associated their use with mild-moderate adverse effects, but nearly all claimed abuse of other substances (Citation48). An abstract of a review of poison center records of 44 patients aged 13–21 years who acutely abused 6–23 tablets of dextromethorphan (various products of dextromethorphan alone or in combination) reported mild to moderate toxicity (Citation47).
There were several level 4 or 6 articles () with detailed individual case information on acute dextromethorphan toxicity but all cases involved a co-ingestant or intentional dextromethorphan abuse. Specifically, there were 12 separate cases reported in eight articles (Citation4,Citation49–55). Among them, the lowest dextromethorphan dose reported to result in adverse effects was 80 mg in an 8-year-old boy (2.2 mg/kg) as a liquid combination product that also contained carbinoxamine maleate 40 mg and pseudoephedrine hydrochloride 500 mg. The product was ingested over 4 hours, which resulted in moderate toxicity (hallucinations, mydriasis, tachycardia, elevated temperature, decreased bowel sounds, warm dry skin, tongue and jaw dyskinesia). The authors suggested that any one or all of the three drugs could have been responsible his signs and symptoms. His symptoms responded to a benzodiazepine (Citation53). The lowest dose of a solid dosage form associated with toxicity in a case report was 480 mg (7.7 mg/kg) ingested by a 12-year-old girl who exhibited moderate toxicity (Citation4). The lowest dose of dextromethorphan resulting in severe toxicity (seizures, mydriasis, nystagmus, cyanosis, apnea) was 600 mg (300 mg in the evening and 300 mg in the morning, total = 9.7 mg/kg) that was ingested by a 15-year-old boy with a history of regular dextromethorphan abuse (Citation50). The reported doses of dextromethorphan HBr associated with moderate toxicity ranged from 80 mg (2.2 mg/kg) to 600 mg (7.7 mg/kg) and those with severe or fatal toxicity ranged from 600 mg (9.7 mg/kg) to 3000 mg (37 mg/kg) as described in .
Table 3. Acute dextromethorphan poisonings with dose information in patients 6 years of age and older
Acute-on-chronic ingestions
There were no papers that expressly examined the issue of acute-on-chronic poisonings with dextromethorphan. In many of the adult case reports of chronic ingestion, patients ingested repetitive acute doses daily over periods of days to years, but these cases were generally categorized as chronic exposures.
Chronic ingestions in patients less than 6 years of age
There were no articles reviewed in which multiple doses of dextromethorphan were prospectively given to patients under 6 years of age and associated with subsequent toxicity.
There were seven level 4 or 6 reports with detailed individual case information on chronic dextromethorphan toxicity (Citation36,Citation38,Citation41,Citation56–59). A 20-month-old boy was found dead 6 hours after allegedly receiving dextromethorphan 6 mg in an unspecified liquid formulation over a period of 13 hours (Citation56). The fact that the boy's dextromethorphan serum concentration was nearly 100 times greater than that associated with therapeutic doses raises questions about the dose ingested. This case was not included in subsequent considerations of toxic doses. A 5-month-old boy developed moderate effects (irritability, ashen appearance, dehydration, fever, tachycardia, tachypnea, bizarre behavior, muscle movement disorder, mydriasis) after being given a total dose of dextromethorphan of 67.5 mg (13.4 mg/kg) in a combination liquid product during 3 days with other drugs for the treatment of otitis media (Citation58).
Chronic ingestions in patients 6 years of age and older
There were two level 1b articles in which multiple doses of dextromethorphan were prospectively given to patients 6 years of age and older and associated with subsequent toxicity (Citation60,Citation61). In a prospective controlled trial in 121 adult volunteers, dextromethorphan 60 mg/day in two divided doses for 7 days (formulation not specified) was associated with mild adverse effects in 74% of subjects (nausea, vomiting and abdominal pain in one person caused withdrawal from study; others reported headache, loose stools, light-headedness, dizziness, nausea) (Citation60). In the second trial, 15 adult patients on methadone maintenance were given dextromethorphan 120–480 mg daily for 12 days and some patients reported mild effects (Citation61).
A retrospective self-reporting survey of 53 adults with a history of dextromethorphan abuse (level 4) was described above (Citation48). There were 16 level 4 articles with detailed individual case information on chronic ingestions of dextromethorphan (e.g., case reports, case series) (Citation16,Citation50,Citation52,Citation62–74). All but one article (Citation67) described cases that involved co‐ingestants or intentional dextromethorphan abuse. In one case, an 8-year-old boy developed moderate toxicity (head deviation, generalized muscle stiffness) after receiving a combination syrup containing dextromethorphan 10 mg per dose every 6 hours for 3 days and after discontinuing dextroamphetamine therapy shortly before (Citation65). A 29-year-old man with suspected underlying bipolar disorder developed mania after ingesting 60 mg dextromethorphan in a combination product (Citation69). In another case, a 54-year-old man developed symptoms of moderate toxicity (psychosis, paranoia, hallucinations) after ingesting dextromethorphan 240 mg in a combination product over 24 hours (Citation72). A 15-year-old boy developed hepatotoxicity and moderate effects 1 day after ingesting 360 mL of NyQuil Liquid (per 15 mL: acetaminophen 500 mg, dextromethorphan HBr 15 mg, doxylamine succinate 6.25 mg, and 25% ethanol) over 12 hours (Citation63). He was treated with acetylcysteine and recovered. A 30-year-old man snorted approximately 250 mg of dextromethorphan HBr as a powder 2–3 times daily for 2–3 months thinking it was amphetamine. After snorting the powder, he felt “high” and developed restlessness, followed by feelings of depression, fatigue, dizziness, and nausea (Citation64). A 41-year-old woman with a history of asthma developed moderate effects (dyspnea, cough, lethargy, hypertension, tachycardia, tachypnea, shallow respirations, wheezing, miosis, atelectasis) after ingesting dextromethorphan 720 mg as a liquid over 36 hours (Citation71).
Since most products contain dextromethorphan HBr, concern has been raised about whether they could produce bromide toxicity with overdosage or chronic use and whether bromide might account for some symptoms attributed to dextromethorphan. No cases of documented bromide poisoning following acute or chronic dextromethorphan HBr ingestion were located in the literature search for this guideline. In one case, the serum chloride concentration was falsely elevated and the anion gap decreased due to bromide interfering with the assay following chronic ingestion of dextromethorphan HBr, but bromide toxicity was not evident (Citation75). In another case, similar lab interference occurred following the chronic use of cough and cold medications containing dextromethorphan HBr and a nonprescription drug adulterated with bromvalerylurea, which contains bromine. This patient exhibited lethargy, fever and acneiform eruptions on her face (Citation76).
Serotonin syndrome
Serotonin syndrome has been associated with drug interactions of dextromethorphan and agents that affect serotonin. Nonselective monoamine oxidase inhibitors, such as phenelzine (Citation77), isocarboxazid (Citation78), or drugs with such activity, such as the antibiotic linezolid (Citation79), have produced serotonin syndrome when dextromethorphan was taken in therapeutic doses for suppression of cough. The selective serotonin reuptake inhibitors fluoxetine (Citation80) and paroxetine (Citation81) have been associated with serotonin syndrome following interactions with dextromethorphan. These case reports have led to the generalized warning about drug interactions with dextromethorphan and any drugs in the classes of nonselective monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, and drugs known to produce serotonin syndrome (Citation17).
Onset of effects
The expert consensus panel members considered the time to onset of toxicity after acute dextromethorphan ingestion valuable in making decisions about out-of-hospital management. All articles with toxicity information were searched for estimates of times of onset; however, the majority of articles reported times of hospital presentation rather than times of symptom development. In such cases, it was only possible to establish an upper limit for the time to the onset of effect. Care should be taken to distinguish time to onset of initial effects from time to onset of serious or major effects, the time to onset of peak effects, or the time to onset of subsequent deterioration or complications.
Only a few of the reviewed articles reported time intervals specific for the onset of effect after ingestion. In these cases, onsets ranged from 30 to 120 minutes (Citation4,Citation43,Citation54). A few others reported times to arrival at a hospital (2½–18 hours) or time to when the patient was found symptomatic (6½–12 hours). In such cases, it was only possible to establish a potential upper limit for time-to-effect onset rather than an actual time of onset (Citation11,Citation41,Citation44,Citation56). In the only case involving the extended-release suspension, a 2½-year-old child ingested 540 mg (38 mg/kg) of dextromethorphan, and developed moderate toxicity (dizziness, ataxia, nystagmus, opisthotonus, mydriasis) that began approximately 1 hour after ingestion (Citation44). There were no cases of delayed onset of effects reported in the reviewed literature. Pharmacokinetic studies indicate that the times to achieve peak serum concentrations for dextromethorphan liquid, tablet, or extended-release suspension are essentially the same with the peaks observed at 2–3 hours after ingestion (Citation13,Citation14).
The onset of serotonin syndrome is generally within hours of ingestion with an escalating severity of clinical abnormalities. The onset and progression of clinical toxicity is generally gradual and typically 60% of patients present within 6 hours after starting a medication, overdosing or changing a dose. Its onset can be abrupt after a large overdose or with certain drug interactions (Citation17).
Decontamination measures
There were no controlled studies examining the efficacy of decontamination measures at reducing dextromethorphan absorption. There were no cases in which the use of ipecac syrup was reported. The potentially quick onset of sedation and movement disorders following dextromethorphan overdose, the risk of aspirating ipecac-induced vomitus, and lack of proven benefit argue against its use (Citation82). A number of uncontrolled case series and case reports reported the use of activated charcoal in individual patients, but it was impossible to determine its efficacy given the lack of controls, the concurrent use of other therapies, and the fact that decontamination procedures do not generally produce immediate clinical improvement. Although the literature search did not reveal any evidence that activated charcoal adsorbs dextromethorphan, given the non-specific adsorption of most drugs by activated charcoal (Citation83), single-dose activated charcoal would be expected to be efficacious for dextromethorphan. Activated charcoal was used in several cases of dextromethorphan ingestion without apparent adverse effects, but its efficacy could not be evaluated in these case reports (Citation40,Citation44,Citation50,Citation52,Citation74,Citation84).
Treatment measures
There were no controlled studies examining the use of specific treatments or antidotes for dextromethorphan. A number of uncontrolled case series and case reports reported the use of various treatment measures in individual patients, but it was difficult in most cases to determine their potential efficacy given the lack of controls, the concurrent use of other therapies, and the potential for clinical improvement with the passage of time.
Naloxone
There were several reports of patients improving significantly after naloxone administration (Citation36,Citation40,Citation42,Citation43,Citation71). Although uncontrolled, some of these cases appeared convincing given the temporal relationship to naloxone administration and the rapidity and degree of clinical improvement in coma (Citation36), lethargy (Citation40,Citation71), hyperexcitability (Citation42), and ataxia (Citation43). Naloxone produced no significant temporally-related clinical improvement in: a case with opisthotonus, nystagmus, and blank stare (Citation44); a case with agitation, hallucinations, lethargy, hypertension, hypertonia, diaphoresis, and nystagmus (Citation74); a case with disorientation, ocular deviation, hypertonia-hypotonia (Citation39); and a case of lethargy (Citation37). The effect of naloxone on NMDA receptor antagonism by dextromethorphan is unclear and its use might not affect symptoms related to NMDA antagonism such as dysphoria and hallucinations.
Other
There were two case reports of children with dystonia who improved after diphenhydramine administration (Citation44,Citation65). The use of antipsychotics or benzodiazepines was reported in cases of dextromethorphan toxicity with subsequent clinical improvement in some (Citation46,Citation49,Citation51,Citation53,Citation58,Citation72,Citation84,Citation85).
The use of various general supportive measures was reported by a number of authors, and many of these might have contributed to successful outcomes (e.g., airway protective measures, IV fluids, thiamine). However it was difficult to assess their efficacy from the information provided.
A number of more specific treatment measures were also reportedly used in cases of dextromethorphan toxicity (e.g., anticonvulsants, antidepressants, barbiturates, diuretics, steroids, theophylline). However, in such instances, the measures were either ineffective, a clear treatment-related temporal improvement could not be established from the information provided, or they would not be expected to be available in an out-of-hospital setting.
Limitations of the literature
The literature on dextromethorphan poisoning generally exhibited a number of limitations: 1) much of the data was determined by retrospective studies and case reports and based on estimates of dose provided by patients or family members, which raise questions about the accuracy of the dose estimate; 2) the dose-effect information was confounded, in most cases, by the presence of co-ingestants, differences in treatment measures provided, the effects of drug withdrawal, chronic substance abuse, and concurrent medical conditions (e.g., cold symptoms, upper respiratory infection) that could have altered the clinical presentation or outcome; 3) product formulations of dextromethorphan alone and in combination might have changed over time and many authors failed to report all ingredients and strengths that were involved in their reported cases; 4) in case series, many of the patients remained asymptomatic and product formulations, ingestion doses, and frequency and severity of effects were typically reported as ranges of values, percentages, or means, so individual doses resulting in specific effects could not be determined; and 5) among the few prospective trials available, dextromethorphan was administered in therapeutic doses, which would be expected to be much smaller than doses likely to be seen in an overdose or poisoning.
The level of clinical detail presented in the case reports and abstracts varied widely. In most, the dextromethorphan ingestion was not independently verified or confirmed by laboratory testing nor could the influence of co-ingestants be adequately evaluated. There were no reports of adults without a co-ingestant or not associated with intentional abuse. The unclear time interval from ingestion to onset of toxicity is confounded by a lack of a definition for consequential toxicity. For example, after a dextromethorphan overdose the development of drowsiness in a child could indicate the onset of toxicity or could represent the approach of nap time.
Conclusions
Key decision points for triage
The expert consensus panel chose to emphasize the importance of information that would be needed in order to make a sound triage decision for a patient with a known dextromethorphan poisoning. These variables include the patient's intent, dose and formulation of the product, the presence of symptoms, and the time since ingestion. The expert consensus panel agreed that in each case, the judgment of the specialist in poison information, the poison center medical director, or other poison center-affiliated clinicians might override any specific recommendation from this guideline.
Patient intent
The panel concluded that all patients with suicidal intent, substance abuse, or in whom a malicious intent (e.g., child abuse or neglect) was suspected should be expeditiously transported to an emergency department, regardless of the dose ingested. Patients without these characteristics (e.g., adults with definite unintentional ingestion or children less than 6 years of age in whom abuse is not suspected) are candidates for consideration of out-of-hospital management of their ingestion.
Dose and formulation
The expert consensus panel concluded that home observation is suitable for both adult and children who are asymptomatic, have acute unintentional ingestions of up to 7.5 mg/kg dextromethorphan HBr, and have not ingested any other potentially toxic substances. This is based on very limited case report data and consideration that currently most poison centers likely observe the 10 mg/kg threshold as published in Poisindex (Citation15). The panel selected this referral dose based on cases in the literature reporting moderate symptoms beginning at amounts of 2.2 mg/kg (Citation53) ranging from 2.2 to 7.7 mg/kg (Citation4,Citation49,Citation52–54); whereas, severe symptoms were associated with doses as low as 7.8 mg/kg (Citation54). Most of these cases involved other drugs, so the sole contribution to symptoms from dextromethorphan can not be determined. Since 65% of poison center cases of dextromethorphan ingestion do not receive follow-up after the initial call, the panel recommends that patients who have ingested 5–7.5 mg/kg of dextromethorphan should receive poison center-initiated follow-up approximately every 2 hours for up to 4 hours after ingestion. Patients should be referred to an emergency department if more than mild symptoms develop. This recommended follow-up procedure is intended to identify potential symptomatic cases whether the symptoms are due to dextromethorphan or other ingredients found in many cough and cold products. The 7.5 mg/kg threshold for dextromethorphan proposed in this guideline is more conservative than the 10 mg/kg dose reported by several poison centers, but the basis for the 10 mg/kg dose is not stated in any of the poison centers' guidelines, textbooks (Citation34), or Poisindex (Citation15). Until more information is available, the 7.5 mg/kg dose for emergency department referral accounts for the severe poisoning experiences reported in the literature as summarized in this guideline. There appears to be no difference in the toxicities of different formulations of dextromethorphan (immediate- vs. extended-release and solid vs. liquid) based on the sparse literature available.
Presence of symptoms
A number of articles reported adverse effects occurring with therapeutic dextromethorphan doses, mostly mild in nature. The expert consensus panel concluded that patients with mild complaints, such as infrequent vomiting or somnolence (lightly sedated and arousable with speaking voice or light touch), could remain at home with poison center follow-up. Symptoms beyond these mild complaints would warrant medical evaluation in an emergency department.
There is no adequate support from the literature on which to base triage decisions for the development of the serotonin syndrome. Since many consider the serotonin syndrome to be more likely to develop in patients on multiple serotonergic drugs, it seems prudent to closely observe for symptoms any patient who ingests dextromethorphan while taking another serotonergic agent (e.g., tricyclic antidepressant, selective serotonin reuptake inhibitor, monoamine oxidase inhibitor, lithium).
Time of onset of toxicity after overdose
In the majority of reported cases, patients were symptomatic within 4 hours of ingestion. The time to achieve peak serum concentrations with therapeutic doses of dextromethorphan is approximately 2–3 hours. Therefore, the panel concluded that an asymptomatic patient who unintentionally ingested a dextromethorphan product is unlikely to develop symptoms if the interval between the ingestion and contact with the poison center is longer than 4 hours. It is unlikely that these patients will become symptomatic after this time unless toxicity develops from co-ingestants like acetaminophen. Consideration should be given to the time of day that home observation will take place, since observation during normal sleep hours might not be practical or reliable. Serotonin syndrome from a drug interaction with dextromethorphan typically becomes evident within 6 hours. Periodic poison center-initiated follow-up every 2 hours for 8 hours is recommended by the consensus panel when a patient is taking other medications likely to interact with dextromethorphan and cause serotonin syndrome, such as monoamine oxidase inhibitors or selective serotonin reuptake inhibitors.
Potential out-of-hospital management
The expert consensus panel concluded that there is no role for ipecac syrup or any means to induce emesis in the treatment of patients who have ingested dextromethorphan due to the potential for rapid onset of sedation that could lead to pulmonary aspiration of vomitus. Use of ipecac syrup is not supported by sufficient evidence of benefit to warrant its use.
The panel concluded that activated charcoal could be administered as part of the management of a dextromethorphan-poisoned patient despite the lack of specific data to support its use. Since dextromethorphan can produce sedation and emesis and there are no data to suggest a specific clinical benefit of activated charcoal, the routine out-of-hospital use of activated charcoal in patients with unintentional dextromethorphan ingestion cannot be recommended at this time. It might be considered for out-of-hospital use in some regions in which prehospital activated charcoal is commonly administered by emergency medical personnel and when a long transportation time to an emergency department is anticipated. Transportation to an emergency department should not be delayed in order to attempt activated charcoal administration.
The benefit of naloxone in the treatment of dextromethorphan poisoning is unclear. There are case reports in which it appeared that the patients responded to the drug and also case reports in which no response was apparent. Since naloxone has a low risk of adverse effects, the expert consensus panel concluded that the use of naloxone could be considered in patients who are comatose, particularly those with respiratory depression. The doses used in the case reports are the same doses commonly used to reverse opiate effects (adults and children >20 kg: 0.4–2 mg IV, repeated every 2–3 minutes until response is achieved; children <20 kg: 0.01 mg/kg repeated every 2–3 minutes until response is achieved). As with any overdose situation in which naloxone is used, consideration must be given to the potential for precipitation of withdrawal symptoms in patients addicted to opiates. In these cases, the starting dose should be lower (e.g., 0.05 mg for adults and children).
The expert consensus panel concluded that close monitoring of vital signs as well as respiratory, cardiovascular, and neurological status of patients with possible severe dextromethorphan poisoning is of critical importance. Supportive and symptomatic care should be provided as required. This is particularly important when dextromethorphan is one of several drugs in a combination cough and cold pharmaceutical product or is contained in an illicit drug product.
Combination products
Many of the cases reviewed for this guideline involved combination cough and cold products in which dextromethorphan was included with other drugs such as decongestants, antihistamines, and acetaminophen. The panel concluded that it is of critical importance to carefully ascertain whether other drugs, besides dextromethorphan, were involved in the ingestion and assess the risk for toxicity.
Recommendations
All patients with suicidal intent, intentional abuse, or in cases in which a malicious intent is suspected (e.g., child abuse or neglect) should be referred to an emergency department (Grade D).
Patients who exhibit more than mild effects (e.g., infrequent vomiting or somnolence [lightly sedated and arousable with speaking voice or light touch]) after an acute dextromethorphan ingestion should be referred to an emergency department (Grade C).
Patients who have ingested 5–7.5 mg/kg should receive poison center-initiated follow-up approximately every 2 hours for up to 4 hours after ingestion. Refer to an emergency department if more than mild symptoms develop (Grade D).
Patients who have ingested more than 7.5 mg/kg should be referred to an emergency department for evaluation (Grade C).
If the patient is taking other medications likely to interact with dextromethorphan and cause serotonin syndrome, such as monoamine oxidase inhibitors or selective serotonin reuptake inhibitors, poison center-initiated follow-up every 2 hours for 8 hours is recommended (Grade D).
Patients who are asymptomatic and more than 4 hours have elapsed since the time of ingestion, can be observed at home (Grade C).
Do not induce emesis (Grade D).
Do not use activated charcoal at home. Activated charcoal can be administered to asymptomatic patients who have ingested overdoses of dextromethorphan within the preceding hour. Its administration, if available, should only be carried out by health professionals and only if no contraindications are present. Do not delay transportation in order to administer activated charcoal (Grade D).
For patients who have ingested dextromethorphan and are sedated or comatose, naloxone, in the usual doses for treatment of opioid overdose, can be considered for prehospital administration, particularly if the patient has respiratory depression (Grade C).
Use intravenous benzodiazepines for seizures and benzodiazepines and external cooling measures for hyperthermia (>104°F, >40°C) from serotonin syndrome. This should be done in consultation with and authorized by EMS medical direction, by a written treatment protocol or policy, or with direct medical oversight (Grade C).
Carefully ascertain by history whether other drugs, such as acetaminophen, were involved in the incident and assess the risk for toxicity or for a drug interaction (Grade D).
These recommendations are summarized in Appendix 4.
Implications for research
The panel identified the following topics where additional research is needed or analysis of existing databases might be useful.
Additional detailed reports are needed to more precisely establish the threshold dose for emergency department referral for the unintentional ingestion of dextromethorphan.
The influence of other co-ingestants typically found in dextromethorphan-containing combination products on the development of toxicity needs to be determined.
The safety and efficacy of activated charcoal in the management of dextromethorphan poisoning need evaluation.
The risks and benefits of naloxone in the management of dextromethorphan poisoning need clarification.
The predisposing causes and risk groups for the development of the serotonin syndrome need further study.
The risk of bromism from acute or chronic use of dextromethorphan HBr needs assessment.
Disclosures
At the time of his work on this guideline, Dr. Erdman was employed by AstraZeneca. Dr. Booze's husband is employed by AstraZeneca. There are no other potential conflicts of interest reported by the expert consensus panel or project staff regarding this guideline.
Notes
*Guidelines for the Management of Poisoning supported in full by Cooperative Agreement 8 U4BHS00084 between the American Association of Poison Control Centers and the Health Resources and Services Administration, Department of Health and Human Services.
References
- http://dawninfo.samhsa.gov/files/TNDR10DXMforHTML.pdf, Ball JK, Albright V. Emergency department visits involving dextromethorphan. The DAWN report, 2006, issue 32.
- Bem JL, Peck R. Dextromethorphan. An overview of safety issues. Drug Saf 1992; 7: 190–199
- http://www.usdoj.gov/ndic/pubs11/11563/11563p.pdf, US Department of Justice. Intelligence bulletin: DXM (dextromethorphan).
- Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care 2004; 20: 858–863
- www.monitoringthefuture.org, Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Teen drug use continues down in 2006, particularly among older teens; but use of prescription-type drugs remains high (December 21, 2006). University of Michigan News and Information Services: Ann Arbor, MI.
- Osterhoudt KC. A Cadillac ride to the emergency department. Pediatr Emerg Care 2005; 21: 877–879
- Bryner JK, Wang UK, Hui JW, Bedodo M, MacDougall C, Anderson IB. Dextromethorphan abuse in adolescence: an increasing trend: 1999–2004. Arch Pediatr Adolesc Med 2006; 160: 1217–1222
- Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Op Pediatr 2006; 18: 184–188
- Weinbroum AA, Rudick V, Paret G, Ben-Abraham R. The role of dextromethorphan in pain control. Can J Anaesth 2000; 47: 585–596
- The Merck Index 14th, MJ O'Neil, PE Heckelman. Merck, Whitehouse, NJ 2006
- Roll D, Tsipis G. “Agent Lemon”: a new twist on dextromethorphan toxicity [abstract]. J Toxicol Clin Toxicol 2002; 40: 655
- http://www.usdoj.gov/usao/ins/press_releases/Pressrelease06/20060110.DXM.pdf, US Department of Justice. Two Indianapolis men charged with selling dextromethorphan over the internet, five deaths of young men linked to shipment. Press release January 10, 2006.
- Aylward M, Maddock J, Davies DE, Protheroe DA, Leideman T. Dextromethorphan and codeine: Comparison of plasma kinetics and antitussive effects. Eur J Respir Dis 1984; 65: 283–2991
- Demirbas S, Reyderman L, Stavchansky S. Bioavailability of dextromethorphan (as dextrorphan) from sustained release formulations in the presence of guaifenesin in human volunteers. Biopharm Drug Dispos 1998; 19: 541–545
- Dextromethorphan management. Poisindex System, RK Klasco. Thomson Micromedex, Greenwood Village, CO June 2, 2006, Edition expires
- Miller SC. Dextromethorphan psychosis, dependence and physical withdrawal. Addict Biol 2005; 10: 325–327
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352: 1112–1120
- Einarson A, Lyszkiewicz D, Koren G. The safety of dextromethorphan in pregnancy: Results of a controlled study. Chest 2001; 119: 466–469
- Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. National Birth Defects Prevention Study. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005; 193: 771–777
- American Academy of Pediatrics. Committee on Drugs. Use of codeine- and dextromethorphan-containing cough remedies in children. Pediatrics 1997; 99: 918–920
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UB, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Smith Hammond C, Tarlo SM. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 1S–23S
- 22. Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2004, Issue 4. Art. No.: CD001831. DOI: 10.1002/14651858. CD001831.pub2.
- Smith MB, Feldman W. Over-the-counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA 1993; 269: 2258–2263
- 24. Pediatric Toxicology Committee and Data Committee, National Association of Medical Examiners. Srinivasan A, Budnitz D, Shehab N, Cohen A. Infant deaths associated with cough and cold medications - two states, 2005. MMWR Morb Mortal Wkly Rep 2007; 56:1–4.
- Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM. 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2005; 23: 589–666
- Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281: 1900–1905
- Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003; 139: 493–498
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts: United States. Advance Data from Vital and Health Statistics; No. 314. National Center for Health Statistics, Hyattsville, MD 2000
- Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient, J Brent, SD Phillips, KL Wallace, JW Donovan, K Burkhart. Mosby, Philadelphia 2005
- Medical Toxicology 3rd, RC Dart. Lippincott, Williams & Wilkins, Philadelphia 2004
- Clinical Management of Poisoning and Drug Overdose 3rd, LM Haddad, MW Shannon, JF Winchester. WB Saunders, Philadelphia 1998
- Kleinschmidt KC, Wainscott M, Ford MD. Opioids. Clinical Toxicology, MD Ford, KA Delaney, LJ Ling, T Erickson. WB Saunders, Philadelphia 2001; 627–639
- Nelson LS. Opioids. Goldfrank's Toxicologic Emergencies 8th, NE Flomenbaum, LR Goldfrank, RS Hoffman, MA Howland, NA Lewin, LS Nelson. McGraw-Hill, New York 2006; 590–613
- Albertson TE. Dextromethorphan. Poisoning & Drug Overdose 5th, KR Olson. McGraw-Hill, New York 2007; 181–183
- Paul IM, Shaffer ML, Yoder KE, Sturgis SA, Berlin CM, et al. Dose-response relationship with increasing doses of dextromethorphan for children with cough. Clin Ther 2004; 26: 1508–1514
- Henretig F, Cugini D, Durbin D, Kearney T, Vuignier BI, Torrey S, DeMarco J. Dextromethorphan (DM) overdose in children [abstract]. Vet Hum Toxicol 1988; 30: 364
- Devlin KM, Hall AH, Smolinske SC, Wruk KM, Kulig KW, Rumack BH. Toxicity from long-acting dextromethorphan preparations [abstract]. Vet Hum Toxicol 1985; 27: 296
- Hanzlick R. National Association of Medical Examiners Pediatric Toxicology (PedTox) Registry Report 3. Case submission summary and data for acetaminophen, benzene, carboxyhemoglobin, dextromethorphan, ethanol, phenobarbital, and pseudoephedrine. Am J Forensic Med Pathol 1995; 16: 270–277
- Iglesias Platas I, Fernandez Santervas Y, Luaces Cubells C, Garcia Garcia JJ, Pou Fernandez J. Intoxicación por dextrometorfano. An Esp Pediatr 2002; 57: 492–493
- Katona B, Wason S. Dextromethorphan danger. N Engl J Med 1986; 314: 993
- Marinetti L, Lehman L, Casto B, Harshbarger K, Kubiczek P, Davis J. Over-the-counter cold medications-postmortem findings in infants and the relationship to cause of death. J Anal Toxicol 2005; 29: 738–743
- Pender ES, Parks BR. Toxicity with dextromethorphan-containing preparations: A literature review and report of two additional cases. Pediatr Emerg Care 1991; 7: 163–165
- Shaul WL, Wandell M, Robertson WO. Dextromethorphan toxicity: Reversal by naloxone. Pediatrics 1977; 59: 117–118
- Warden CR, Diekema DS, Robertson WO. Dystonic reaction associated with dextromethorphan ingestion in a toddler. Pediatr Emerg Care 1997; 13: 214–215
- Baker SD, Borys DJ. A possible trend suggesting increased abuse from Coricidin exposures reported to the Texas Poison Network: Comparing 1998 to 1999. Vet Hum Toxicol 2002; 44: 169–171
- Banerji S, Anderson IB. Abuse of Coricidin HBP cough & cold tablets: Episodes recorded by a poison center. Am J Health Syst Pharm 2001; 58: 1811–1814
- Simone KE, Bottei EM, Siegel ES, Tsipis GB. Coricidin® abuse in Ohio teens and young adults [abstract]. J Toxicol Clin Toxicol 2000; 38: 532–533
- Ziaee V, Hamed EA, Hoshmand A, Amini H, Kebriaeizadeh A, Saman K. Side effects of dextromethorphan abuse, a case series. Addict Behav 2005; 30: 1607–1613
- Budai B, Iskandar H. Dextromethorphan can produce false positive phencyclidine testing with HPLC. Am J Emerg Med 2002; 20: 61–62
- Baumann P, Vlatkovic D, Macciardi F. Dextromethorphan poisoning in an adolescent with genetic cytochrome P450 CYP2D6 deficiency. Therapie 1997; 52: 607–608
- Hendrickson RG, Hofrichter K. Crystal dex – purified DSM using and acid/base extraction [abstract]. J Toxicol Clin Toxicol 2004; 42: 757
- Kirages TJ, Sule HP, Mycyk MB. Severe manifestations of Coricidin intoxication. Am J Emerg Med 2003; 21: 473–475
- Nairn SJ, Díaz JE. Cold-syrup induced movement disorder. Pediatr Emerg Care 2001; 17: 191–192
- Olsen DG, Bronstein AC, Fisher JM, Bartalini M. Green Hornet: Teenage agony and ecstasy [abstract]. J Toxicol Clin Toxicol 2004; 42: 765
- Rammer L, Holmgren P, Sandler H. Fatal intoxication by dextromethorphan: A report on two cases. Forensic Sci Int 1988; 37: 233–236
- Bailey B, Daneman R, Daneman N, Mayer JM, Koren G. Discrepancy between CYP2D6 phenotype and genotype derived from post-mortem dextromethorphan blood level. Forensic Sci Int 2000; 110: 61–70
- Garcia Corcuera R, Cabero Perez MJ, Arteaga Manjon R, Herranz Fernandez JL. Intoxicación accidental por dextrometorfán. An Esp Pediatr 1993; 39: 364
- Myer P, Bryant B, Cartwright GW. Overdose with a combination decongestant, antihistamine and antitussive syrup. Indiana Med 1985; 78: 766–768
- Roberge RJ, Hirani KH, Rowland PL, 3rd, Berkeley R, Krenzelok EP. Dextromethorphan- and pseudoephedrine-induced agitated psychosis and ataxia: case report. J Emerg Med 1999; 17: 285–288
- Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol 2004; 44: 1132–1142
- Cornish JW, Herman BH, Ehrman RN, Robbins SJ, Childress AR, Bead V, Esmonde CA, Martz K, Poole S, Caruso FS, O'Brien CP. A randomized, double-blind, placebo-controlled safety study of high-dose dextromethorphan in methadone-maintained male inpatients. Drug Alcohol Depend 2002; 67: 177–183
- Craig DF. Psychosis with Vicks Formula 44-D abuse. CMAJ 1992; 146: 1199–1200
- Fleckenstein JL. Nyquil and acute hepatic necrosis. N Engl J Med 1985; 313: 48
- Fleming PM. Dependence on dextromethorphan hydrobromide. Br Med J (Clin Res Ed) 1986; 293: 597
- Graudins A, Fern RP. Acute dystonia in a child associated with therapeutic ingestion of a dextromethorphan containing cough and cold syrup. J Toxicol Clin Toxicol 1996; 34: 351–352
- Hinsberger A, Sharma V, Mazmanian D. Cognitive deterioration from long-term abuse of dextromethorphan: A case report. J Psychiatry Neurosci 1994; 19: 375–377
- Iaboni RP, Aronowitz JS. Dextromethorphan abuse in a dually diagnosed patient. J Nerv Ment Dis 1995; 183: 341–342
- Marsh LD, Key JD, Spratt E. Bulimia and dextromethorphan abuse. A case study. J Subst Abuse Treat 1997; 14: 373–376
- Mendez MF. Mania self-induced with cough syrup. J Clin Psychiatry 1992; 53: 173–174
- Price LH, Lebel J. Dextromethorphan-induced psychosis. Am J Psychiatry 2000; 157: 304
- Schneider SM, Michelson EA, Boucek CD, Ilkhanipour K. Dextromethorphan poisoning reversed by naloxone. Am J Emerg Med 1991; 9: 237–238
- Sharma A, Dewan V, Petty F. Acute psychosis with Coricidin cold medicine. Ann Pharmacother 2005; 39: 1577–1578
- Walker J, Yatham LN. Benylin (dextromethorphan) abuse and mania. BMJ 1993; 306: 896
- Wolfe TR, Caravati EM. Massive dextromethorphan ingestion and abuse. Am J Emerg Med 1995; 13: 174–176
- Ng YY, Lin WL, Chen TW. Spurious hyperchloremia and decreased anion gap in a patient with dextromethorphan bromide. Am J Nephrol 1992; 12(4)268–270
- Hung YM. Bromide intoxication by the combination of bromide-containing over-the-counter drug and dextromethorphan hydrobromide. Hum Exp Toxicol 2003; 22: 459–461
- Rivers N, Horner B. Possible lethal reaction between Nardil and dextromethorphan. Can Med Assoc J 1970; 103: 85
- Sovner R, Wolfe J. Interaction between dextromethorphan and monoamine oxidase inhibitor therapy with isocarboxazid. N Engl J Med 1988; 319: 1671
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: A review of postmarketing data. Clin Infect Dis. 2006; 42: 1578–1583
- Navarro A, Perry C, Bobo WV. A case of serotonin syndrome precipitated by abuse of the anticough remedy dextromethorphan in a bipolar patient treated with fluoxetine and lithium. Gen Hosp Psychiatry 2006; 28: 78–80
- Skop BP, Finkelstein JA, Mareth TR, Magoon MR, Brown TM. The serotonin syndrome associated with paroxetine, an over-the-counter cold remedy, and vascular disease. Am J Emerg Med 1994; 12: 642–644
- Krenzelok EP, McGuigan M, Lheur P. American Academy of Clinical Toxicology, European Association of Poison Centres and Clinical Toxicologists. Position statement: Ipecac syrup. J Toxicol Clin Toxicol 1997; 35: 699–709
- Chyka PA, Seger D, Krenzelok EP, Vale JA. American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 2005; 43: 61–87
- Nordt SP. “DXM”: a new drug of abuse?. Ann Emerg Med 1998; 31: 794–795
- Soutullo CA, Cottingham EM, Keck PE, Jr. Psychosis associated with pseudoephedrine and dextromethorphan. J Am Acad Child Adolesc Psychiatry 1999; 38: 1471–1472
- USP DI Drug Information for the Health Care Professional. Thomson Micromedex, Greenwood Village, CO, accessed 6/2/2006.
Appendix 1
Expert Consensus Panel Members
Lisa L. Booze, Pharm.D.
Certified Specialist in Poison Information
Maryland Poison Center
University of Maryland School of Pharmacy
Baltimore, Maryland
E. Martin Caravati, M.D., M.P.H., FACMT, FACEP
Professor of Surgery (Emergency Medicine)
University of Utah
Medical Director
Utah Poison Center
Salt Lake City, Utah
Gwenn Christianson, R.N., M.S.N.
Certified Specialist in Poison Information
Indiana Poison Center
Indianapolis, Indiana
Peter A. Chyka, Pharm.D., DABAT, FAACT
Professor, Department of Clinical Pharmacy
College of Pharmacy
University of Tennessee Health Science Center
Knoxville, Tennessee
Daniel J. Cobaugh, Pharm.D., FAACT, DABAT
Director of Research
ASHP Research and Education Foundation
Bethesda, Maryland
Former Associate Director, American Association of Poison Control Centers
Daniel C. Keyes, M.D., M.P.H.
Medical Director
Pine Bluff Chemical Demilitarization Facility
Associate Professor, Southwestern Toxicology Training Program
Dallas, Texas
Anthony S. Manoguerra, Pharm.D., DABAT, FAACT
Professor of Clinical Pharmacy and Associate Dean
School of Pharmacy and Pharmaceutical Sciences
University of California San Diego
Former Director, California Poison Control System, San Diego Division
San Diego, California
Lewis S. Nelson, M.D., FACEP, FACMT, FAACT
Associate Professor of Emergency Medicine
New York University School of Medicine
Associate Medical Director
New York City Poison Control Center
New York, New York
Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Director, West Virginia Poison Center
Professor, West Virginia University School of Pharmacy
Department of Clinical Pharmacy
Charleston, West Virginia
Paul M. Wax, M.D., FACMT
Attending Toxicologist
University of Texas Southwestern Medical Center
Dallas, Texas
Alan D. Woolf, M.D., M.P.H., FACMT
Director, Program in Environmental Medicine
Children's Hospital, Boston
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Appendix 2
Grades of recommendation and levels of evidenc
Appendix 3
Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Pharmacists Association
American Public Health Association
American Society of Health-System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control and Prevention – National Center for Injury Prevention and Control
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety
Appendix 4
Triage Algorithm for Acute Dextromethorphan HBr Poisoning