Abstract
Introduction. In Hong Kong, Chinese medicine is popular and coexists with orthodox Western medicine. Despite a long history of use, many herbs have not been submitted to rigorous scientific testing and there are reports of hepatotoxicity. We describe a woman who developed acute hepatitis after drinking an herbal remedy containing Teucrium viscidum. Case report. A previously healthy 51-year-old woman was admitted to a regional hospital because of jaundice, with complaints of nausea, vomiting, and tea-colored urine for three days prior to admission. She denied any recent ingestion of known hepatotoxins, but she had consumed an herbal remedy for low back pain for three days before the onset of symptoms. She was icteric and had a serum total bilirubin level of 11.4 mg/dL, alanine aminotransferase of 2620 U/L, aspartate aminotransferase of 1876 U/L, and alkaline phosphatase level of 186 U/L. Discontinuation of the herbal remedy resulted in normalization of the liver enzymes two months later. Discussion. This is the first report of hepatitis probably related to use of Teucrium viscidum. The herb is infrequently used in Chinese medicine for treatment of rheumatic and bleeding disorders. T. viscidum contains teucvin, similar to other Teucrium species and is related to T. chamaedrys, commonly known as germander, which is a well documented cause of hepatotoxicity. Conclusions. Our findings suggest that Teucrium viscidum can cause hepatotoxicity similar to that of germander.
Keywords:
Introduction
In Hong Kong, Chinese medicine is popular and coexists with orthodox Western medicine. Eighteen percent of the population consults herbalists first for treatment of an illness. Despite a long history of use, many herbs have not been submitted to rigorous scientific testing and there are reports of hepatotoxicity. It has been shown that clinically relevant liver enzyme elevations occur in 1% of users of Traditional Chinese Medicine. Paradoxically, some herbal remedies used to treat liver disease may be hepatotoxic themselves. For instance, the Chinese medicine Syo-saiko-to, touted for its properties against hepatitis, has been shown to be associated with liver injury. The potential toxic effects of many Chinese medicinal herbs are either unknown or cannot be found in the literature.
Herbs associated hepatotoxicity are regularly reported in the literature and new agents are constantly added to the list.
We describe a woman who developed hepatitis after drinking herbal remedy containing Teucrium viscidum. It is a related species of T. chamaedrys, commonly known as germander, which is a well documented cause of hepatotoxicity. This is the first report of acute hepatitis associated with the use of T. viscidum.
Case report
A previously healthy 51-year-old woman was admitted to a regional hospital because of jaundice. She complained of nausea, vomiting and tea-colored urine for three days prior to admission. The patient denied any recent ingestion of pharmaceuticals including paracetamol, mushrooms, and other known hepatotoxins, but she had consumed an herbal remedy three days before the onset of symptoms. This was the only remedy used. According to the prescribed instruction, about 60 grams of a medicinal herb was crushed and suspended in rice wine for drinking once a day. The herbal remedy was indicated for treatment of low back pain. She had taken the remedy in the same way three months ago with no ill effects. Upon admission this time, the patient was icteric but there was no stigma of chronic liver disease or flapping tremor. Abdominal examination showed neither hepatosplenomegaly nor ascites.
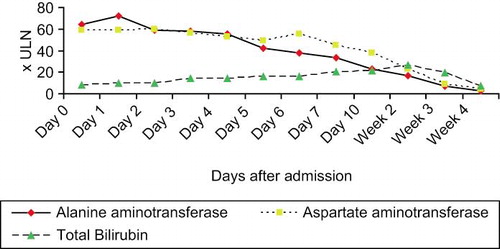
The initial laboratory work-up revealed a serum total bilirubin level of 11.4 mg/dL (reference 0.1 to 1.0) [195 umol/L, reference 2 to 23]; alanine aminotransferase level of 2620 U/L (reference <41 U/L); aspartate aminotransferase level of 1876 U/L (reference <32 U/L) and alkaline phosphatase level of 186 U/L (reference 42 to 98 U/L). Her International Normalized Ratio was 2.0. Hepatitis serology including anti-hepatitis A immunoglobulin M, hepatitis B surface antigen, anti-hepatitis B core immunoglobulin M, anti-hepatitis C antibodies, anti-hepatitis E immunoglobulin M, and Epstein-Barr virus immunoglobulin M were negative. Anti-nuclear factor was negative but anti-smooth muscle antibodies were weakly positive. Immunoglobulin pattern showed a mildly increase in immunoglobulin G of 16.4 g/L (reference 7.0 to 16.0 g/L). The C-reactive protein was 8.5 mg/L (reference <5.0 mg/L) and the erythrocyte sedimentation rate was 1 mm/hour. Complete blood picture showed a platelet count of 102 × 109/L. The white blood cell and absolute eosinophil count were 6.1 × 109/L (reference 4 to 11 × 109/L) and 0.1 × 109/L (reference <0.5 × 109/L), respectively. A comprehensive urine drug screen was negative. Paracetamol and salicylate levels were not detectable. Abdominal ultrasonography showed normal liver and biliary tract.
The herbal remedy was stopped after admission and the patient was treated conservatively. Her liver function tests and complete blood picture returned to normal 2 months later (). Anti-smooth muscle antibodies turned negative and immunoglobulin G level returned to normal. The provisional diagnosis was herb-induced hepatitis since there was clinical improvement after herb withdrawal and there was no other cause of icteric hepatitis.
Laboratory investigations
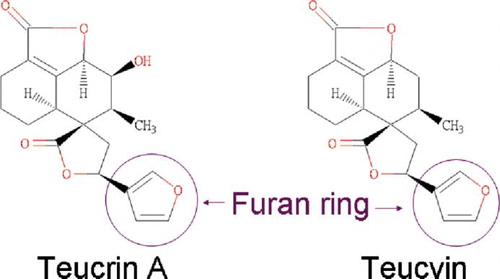
Unused herb was obtained from the patient and morphologically identified by botanist to be T. viscidum (). Gas chromatography mass spectrometry analysis of the herb detected teucvin, a known ingredient of Teucrium viscidum.
Discussion
This is the first report of hepatitis probably related to use of Teucrium viscidum. Teucrium viscidum is a medicinal plant infrequently used in Traditional Chinese Medicine for treatment of rheumatoid arthritis, hematemesis and dysmenorrhea. The herb bears the common name “Xue Jian Chou”, which means hemostasis. It is to be taken orally after decoction in water or crushed for topical application.
The safety of T. viscidum has not been documented. On the other hand, other Teucrium species have been cited to cause hepatotoxicity. T. chamaedrys, commonly known as germander, caused an epidemic of hepatitis that led to the prohibition of all the derived products Other related species that have been implicated for hepatotoxicity include T. capitatum and T. polium.
Germander's hepatotoxicity is mediated via its furano neoclerodane diterpenoids, mainly teucrin A. Activation of the furano ring by cytochrome P450 3A results in the formation of toxic reactive epoxides. It is likely that germander-induced hepatitis may be due to both direct toxicity and secondary immune reactions, with probably a varying contribution of these two mechanisms in different patients.
It is likely that Teucrium species that share similar chemical profile will share the same mechanism and will be hepatotoxic.
T. viscidum was analyzed and found to contain teucvin, which belongs to furano neoclerodane diterpenoid and shares great similarities to teucrin A (). In particular, the furan ring structure is present. Our findings suggest that intake of Teucrium viscidum could cause hepatotoxicity similar to other Teucrium species. Preparation in rice wine instead of water could potentially enhance the extraction of furano alkaloids from T. viscidum. A secondary immune mechanism might be involved as the symptoms occurred on re-exposure. Indeed the transient elevation of immunoglobulin G and weakly positive anti-smooth muscle antibodies also supported the hypothesis. Hepatitis was attributed to this herb since other causes were excluded and there was a temporal relationship between the administration of the herb and the onset of hepatitis.
To establish a correct diagnosis of herb related hepatotoxicity, it is crucial to obtain a detailed history, observe any specific pattern of liver injury and confirm the identity of the herb involved as well as the toxic ingredients wherever possible. However, such information is not always available. It has been shown that about half of patients who took herbs do not inform their physicians. Besides, the pattern of herb induced liver injury can be non-specific. With the exception of some uncommon liver injury patterns, the usual histologic appearances in most cases of herb related hepatotoxicity are indistinguishable from other forms of liver disease. As for hepatotoxic herbs, the toxic ingredients and mechanisms of liver injury are often not clearly known. Currently a wide variety of causality assessment scales exist to determine the extent of relationship between a drug and a suspected reaction. The Naranjo Probability Scale is one of the widely used methods. The probability of adverse drug reaction is assigned a score rating the reaction as definite, probable, possible or doubtful. The Naranjo Scale was used to assess the probability of T. viscidum associated acute hepatitis in this patient. A score of six was obtained, indicating a probable association.
Conclusions
We report the first case hepatotoxicity probably caused by T. viscidum. Our findings suggest that the use of T. viscidum could pose similar risks to that of germander and other related Teucrium species, which have been reported to occasionally cause severe hepatotoxicity. Herbals, even though natural, can be toxic. There is a paucity of published data on the safety profile of these herbals. Therefore, the importance of an effective surveillance system for adverse effects associated with herbal use, similar to the one for western medicine, cannot be over-emphasized. Enhanced surveillance of the safety of herbs should go hand in hand with the developments and increasing use of herbal products in clinical practice.
References
- Working Party on Chinese Medicine Interim Report. Hong Kong, Government Printer, October 1991.
- EM Yoshida, CA McLean, ES Cheng, PD Blanc, KA Somberg, LD Ferrell, and JR Lake. Chinese herbal medicine, fulminant hepatitis, and liver transplantation. Am J Gastroenterol 1996; 91:2647–8.
- PP But, B Tomlinson, and KL Lee. Hepatitis related to the Chinese medicine Shou-wu-pian manufactured from Polygonum multiflorum. Vet Hum Toxicol 1996; 38:280–2.
- MF Yuen, S Tam, J Fung, DK Wong, BC Wong, and CL Lai. Traditional Chinese medicine causing hepatotoxicity in patients with chronic hepatitis B infection: a 1-year prospective study. Aliment Pharmacol Ther 2006; 24:1179–86.
- GM Woolf, LM Petrovic, SE Rojter, S Wainwright, FG Villamil, WN Katkov, P Michieletti, IR Wanless, FR Stermitz, JJ Beck, and JM Vierling. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med 1994; 121:729–35.
- D Melchart, K Linde, W Weidenhammer, S Hager, D Shaw, and R Bauer. Liver enzyme elevations in patients treated with traditional Chinese medicine. JAMA 1999; 282:28–9.
- S Itoh, K Marutani, T Nishijima, S Matsuo, and M Itabashi. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang). Dig Dis Sci 1995; 40:1845–8.
- JF Deng, TJ Lin, WF Kao, and SS Chen. The difficulty in handling poisonings associated with Chinese traditional medicine: a poison control center experience for 1991–1993. Vet Hum Toxicol 1997; 39:106–14.
- S Chitturi, and GC Farrell. Herbal hepatotoxicity: an expanding but poorly defined problem. J Gastroenterol Hepatol 2000; 15:1093–9.
- CK Lai, T Lee, KM Au, and AY Chan. Uniform solid-phase extraction procedure for toxicological drug screening in serum and urine by HPLC with photodiode-array detection. Clin Chem 1997; 43:312–25.
- CK Lai, and AY Chan. Tetrahydropalmatine poisoning: diagnoses of nine adult overdoses based on toxicology screens by HPLC with diode-array detection and gas chromatography-mass spectrometry. Clin Chem 1999; 45:229–36.
- R Liu, KW Yeung, and KY Lee. Study of the chemical ingredients of Teucrium viscidum. Chinese Herbal Medicine 1994; 25:234–5.
- ZX Zhuang, and NH Li. Chinese Medicinal Herbs of Hong Kong: The Commercial Press (H.K.) Limited, 1997.
- I Starakis, D Siagris, L Leonidou, E Mazokopakis, A Tsamandas, and C Karatza. Hepatitis caused by the herbal remedy Teucrium polium L. Eur J Gastroenterol Hepatol 2006 18:681–3.
- SP Dourakis, IS Papanikolaou, EN Tzemanakis, and SJ Hadziyannis. Acute hepatitis associated with herb (Teucrium capitatum L.) administration. Eur J Gastroenterol Hepatol 2002; 14:693–5.
- D Larrey, T Vial, A Pauwels, A Castot, M Biour, M David, and H Michel. Hepatitis after germander (Teucrium chamaedrys) administration: another instance of herbal medicine hepatotoxicity. Ann Intern Med 1992; 117:129–32.
- S Savvidou, J Goulis, I Giavazis, K Patsiaoura, P Hytiroglou, and C Arvanitakis. Herb-induced hepatitis by Teucrium polium L.: report of two cases and review of the literature. Eur J Gastroenterol Hepatol 2007; 19:507–11.
- A Castot, and D Larrey. Hepatitis observed during a treatment with a drug or tea containing Wild Germander. Evaluation of 26 cases reported to the Regional Centers of Pharmacovigilance. Gastroenterol Clin Biol 1992; 16:916–22.
- J Loeper, V Descatoire, P Letteron, C Moulis, C Degott, P Dansette, D Fau, and D Pessayre. Hepatotoxicity of germander in mice. Gastroenterology 1994; 106:464–72.
- M Lekehal, D Pessayre, JM Lereau, C Moulis, I Fouraste, and D Fau. Hepatotoxicity of the herbal medicine germander: metabolic activation of its furano diterpenoids by cytochrome P450 3A Depletes cytoskeleton-associated protein thiols and forms plasma membrane blebs in rat hepatocytes. Hepatology 1996; 24:212–8.
- V De Berardinis, C Moulis, M Maurice, P Beaune, D Pessayre, D Pompon, and J Loeper. Human microsomal epoxide hydrolase is the target of germander-induced autoantibodies on the surface of human hepatocytes. Mol Pharmacol 2000; 58:542–51.
- SA Kouzi, RJ McMurtry, and SD Nelson. Hepatotoxicity of germander (Teucrium chamaedrys L.) and one of its constituent neoclerodane diterpenes teucrin A in the mouse. Chem Res Toxicol 1994; 7:850–6.
- D Polymeros, D Kamberoglou, and V Tzias. Acute cholestatic hepatitis caused by Teucrium polium (golden germander) with transient appearance of antimitochondrial antibody. J Clin Gastroenterol 2002; 34:100–1.
- TY Chan, HP Tam, CK Lai, and AY Chan. A multidisciplinary approach to the toxicologic problems associated with the use of herbal medicines. Ther Drug Monit 2005; 27:53–7.
- DM Eisenberg, RB Davis, SL Ettner, S Appel, S Wilkey, M Van Rompay, and RC Kessler. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998; 280:1569–75.
- E Pak, KT Esrason, and VH Wu. Hepatotoxicity of herbal remedies: an emerging dilemma. Prog Transplant 2004; 14:91–6.
- CA Naranjo, U Busto, EM Sellers, P Sandor, I Ruiz, EA Roberts, E Janecek, C Domecq, and DJ Greenblatt. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45.
- WT Poon, CK Lai, and AYW Chan. Aristolochic acid nephropathy: the Hong Kong perspective. Hong Kong J Nephrol 2007; 9:7–14.