Abstract
Introduction. Stevens-Johnson syndrome (SJS) is an uncommon and potentially serious mucocutaneous disease. The most important step in the management of SJS is early recognition and immediate withdrawal of the causative agent. We present a patient with SJS associated with dimercaptopropane-1-sulfonate (DMPS) therapy. Case Report. An asymptomatic 11-year old boy who had been exposed chronically to mercury vapour had a 24-hour urine mercury concentration of 37 microgram/L (reference value <10 microgram/L). Exposure to the mercury vapour was stopped and treatment with oral DMPS was begun. After two weeks of therapy, he developed a disseminated cutaneous eruption of red pruritic macules on his chest and back, which three days later had spread all over his body with the discrete maculae becoming confluent; erosions and crusts developed on his lips and he had blisters in his mouth. The diagnosis of SJS was made, the DMPS was stopped, and the SJS resolved gradually. Discussion. Chelation agents like DMPS or DMSA are increasingly used and are available over the counter in some countries. These drugs are used in patients with complaints that are attributed to mercury-containing dental amalgams and in children with autism. Conclusion. The reported association suggests that SJS may be a potential complication of DMPS therapy, and this should be considered in the risk-benefit analysis of chelation. The reported association suggests that SJS may be a potential complication of DMPS therapy, and this should be considered in the risk-benefit analysis of chelation.
Introduction
Stevens-Johnson syndrome (SJS) is an uncommon mucocutaneous disease, belonging to the spectrum of SJS, SJS-TEN overlap syndrome, and toxic epidermal necrolysis (TEN). The spectrum is characterized by lesions of the skin and mucous membranes. SJS classically involves at least two mucous membranes or more and less than 10% involvement of the skin. In cases where more than 30% is involved, it is labelled as TEN. Between 10% and 30% SJS-TEN overlap is diagnosed. Most frequently SJS is caused by medications such as several antibiotics, anticonvulsants, non steroid anti-inflammatory drugs (NSAID), or allopurinol. SJS is characterized by specific skin lesions: dusky-red irregular macules (atypical targets), first becoming confluent, than often progressing to blisters and epidermal detachment. Other specific symptoms for SJS are hemorrhagic crusting of the lips, conjunctivitis, genital soreness and erosions, arthralgia and oesophageal or tracheal involvement, the latter two being very rare (Citation1).
The most important step in the management of SJS is early recognition and immediate withdrawal of the causative agent. Therefore, it is important to know which drugs can cause SJS. In this case report we present a patient with SJS most likely caused by dimercaptopropane-1-sulfonate (DMPS) therapy.
Case report
An 11-year old boy was treated in our out-patient clinic after chronic mercury exposure. High mercury levels in a 24-hour urine sample were found during family screening after the diagnosis of severe mercury intoxication in his twin sister. They were both chronically exposed to vaporized mercury after a mercury-containing thermostatic clock had fallen from the wall and the spilled mercury was vacuumed. His twin sister was the only family member who developed significant complaints, consisting of anorexia, weight loss, strange behaviour, hypertension and acrodynia (pink disease). As in his sister, a significantly elevated mercury level was found in the boy´s 24-hour urine sample (37 microgram/L; reference value <10 microgram/L), although he did not have any complaints. High mercury levels could not be explained by a high consumption of seafood.
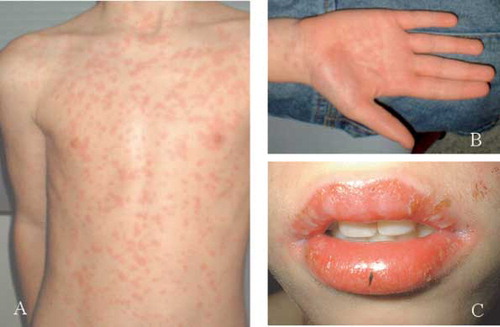
After immediate discontinuation of exposure to mercury, chelation therapy with DMPS was started to wash out mercury and prevent development of complications. Initial treatment started with 3 times per day 200 mg DMPS orally. After the first dose the boy complained of abdominal pain and nausea. No hypotension occurred. After two weeks the boy presented with an erythematous, pruritic rash on the chest and back. On consultation, he reported not feeling well the week before, he also had a slight fever and painful gums. Physical examination showed a disseminated cutaneous eruption of red pruritic macules on his chest and back. No abnormalities were seen in his mouth, his gingiva were not red or edematous. Differential diagnoses at that time included viral exanthema, allergic reaction to DMPS, or a symptom of mercury intoxication. Levocetirizine (5 mg once daily) was prescribed. However, three days later the rash had spread all over his body, the initially discrete maculae became confluent ( and and he had developed erosions and crusts on his lips with mucosal blisters in his mouth (. No cutaneous blisters or detachment of the skin were seen, nor any involvement of the eyes or genitals. There were no signs of infection. Serologic testing for Herpes simplex virus and Yersinia were negative. The cutaneous lesions together with the mucosal changes were consistent with the clinical diagnosis of Stevens-Johnson syndrome. We discontinued the DMPS immediately. During the following week the mucosal lesions gradually resolved; the skin lesions first turned into a confluent erythema with desquamation on the palms of both hands, followed by a gradual resolution of all skin lesions. This confirmed our diagnosis. The ophthalmologist did not find any corneal lesions.
Discussion
This case demonstrates the occurrence of SJS in association with the therapeutic use of DMPS in response to elevated urinary mercury levels. Other causes of SJS were considered initially. An infectious cause was unlikely as there were no signs of infection and serologic testing for Herpes simplex virus, mycoplasma, coxsackie, adenovirus, and Yersinia were negative. Besides DMPS, no other medication was taken. DMPS was, therefore, by far the most likely cause of SJS in this patient, as the rash developed about ten days after starting of DMPS therapy and readily resolved after its discontinuation.
Mercury itself could have caused SJS in our patient. However, to our knowledge, mercury has never been reported as a possible cause of SJS and TEN, and moreover the exposure to mercury was reduced supposedly by the treatment with DMPS, which makes mercury not a likely candidate for causing SJS in our patient. Mercury has been reported to cause a skin disease with some similarities with SJS and TEN: acute generalized exanthematous pustulosis (AGEP), but as no pustules were seen and blistering and mucosal involvement was present in our patient, this diagnosis was less likely (Citation2). For this reason and because of the rapid improvement after discontinuation of DMPS, we did not perform a skin biopsy.
Initially our patient presented with a pruritic rash, because of this presentation we first thought of an allergic reaction. After developing mucous lesions and atypical target lesions and the rapid improvement after discontinuation, SJS was the most likely diagnosis.
The most frequently reported side effects of DMPS are gastro-intestinal complaints and hypotension. SJS has never been reported, but as DMPS contains a sulfonate group, which is also present in sulfamethoxazole and glibenclamide, this side effect can be expected. The pathogenesis of SJS is still not fully understood. However, exposure to increased amounts of reactive metabolites and a decreased ability to detoxify reactive metabolites have been suggested. Different metabolizing abilities have been found amongst patients with SJS compared to controls, such as a significantly lower N‐acetylating capacity in patients hospitalized with SJS (Citation3).
Lately, chelation agents like DMPS or DMSA are increasingly used and are available over the counter in some countries. These drugs are used in patients with complaints that are attributed to mercury-containing dental amalgams and in children with autism, because of the suggested correlation with chronic mercury intoxication. Currently available studies however do not demonstrate a direct relationship between any of these diseases and mercury poisoning (Citation4–7).
This case report suggests that chelation therapy with DMPS is not harmless medication; it can have serious side effects and should therefore only be used in patients with proven intoxication.
References
- Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994; 331(19)1272–1285
- Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001; 28(3)113–119
- Dietrich A, Kawakubo Y, Rzany B, Mockenhaupt M, Simon JC, Schopf E. Low Nacetylating capacity in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Dermatol 1995; 4(5)313–316
- Soden SE, Lowry JA, Garrison CB, Wasserman GS. 24-h provoked urine excretion test for heavy metals in children with autism and typically developing controls, a pilot study. Clin Toxicol 2007; 45(5)476–481
- Risher JF, Amler SN. Mercury exposure: Evaluation and intervention. The inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. NeuroToxicol 2005; 26: 691–699
- Ip P, Wong V, Ho M, Lee J, Wong W. Mercury exposure in children with autistic spectrum disorder: case-control study. J Child Neurol 2004, 19: 431–434
- Muran PJ. Mercury elimination with oral DMPS, DMSA, vitamin C, and glutathione: an observational clinical review. Altern Ther Health Med 2006; 12(3)70–75