Abstract
Introduction. Compared to other calcium channel blockers (CCBs), overdose with dihydropyridine CCBs are considered relatively benign due to their vascular selectivity. Although not a sustained-release preparation, amlodipine's prolonged duration of effect is concerning following overdose. In addition, angiotensin II receptor blocker blunting of vasoconstrictive and sympathetic compensatory responses could exacerbate calcium channel blocker toxicity. We describe severe toxicity associated with an overdose of amlodipine and valsartan. Case Report. A 75-year-old woman presented to the ED 45 minutes after a witnessed suicidal ingestion of a “handful” of amlodipine and valsartan tablets. Hypotension, which appeared two hours after ingestion, was refractory to crystalloids and colloids, calcium gluconate, epinephrine, norepinephrine, phenylephrine, and vasopressin infusions. High-dose insulin euglycemia (HIE) therapy, and treatment with glucagon and naloxone were successful in improving her hemodynamic status. In this combined overdose, right heart catheterization demonstrated both negative inotropic effects and decreased systemic vascular resistance. Conclusion. Co-ingestion of amlodipine with valsartan produced profound toxicity. Early institution of HIE therapy may be beneficial to reverse these effects.
Introduction
Calcium channel blockers (CCBs) lead fatalities among cardiovascular drug exposures, accounting for 30% of cardiovascular drug deaths reported to the AAPCC (Citation1). Amlodipine is often favored clinically over other CCBs for its vascular selectivity and relative lack of negative inotropy, once-daily dosing, and prolonged duration of effect (up to 72 hours) (Citation2). In overdose, the delayed onset and prolonged duration of effect are particularly concerning, as elevated blood concentrations can persist for days (Citation3). Additionally, selectivity can be lost in overdose (Citation4).
Angiotensin II receptor blocker (ARB) binding at the AT1 receptor inhibits angiotensin II–mediated vasoconstriction, sympathetic activation, peripheral noradrenergic transmission, baro-receptor desensitization, endothelin release, renal sodium reabsorption, adrenal cortical aldosterone release, and nitrous oxide destruction (Citation5,Citation6). ARBs and ACE inhibitors (ACE-Is) blunt the endogenous sympathetic and vasopressin response to hypotension and the vasopressor and vasoconstrictive effects of norepinephrine infusion (Citation7,Citation8). ARB effects on vasoconstriction and sympathetic activity could produce a synergistic toxicity in patients with an amlodipine overdose by limiting the effectiveness of both endogenous and exogenously administered catecholamines.
We report a case of severe amlodipine and valsartan toxicity that was refractory to conventional vasopressors, but responsive to high-dose insulin euglycemia (HIE).
Case report
A 75-year-old suicidal woman presented to the emergency department (ED) 45 minutes after a witnessed ingestion of a “handful” of amlodipine (10 mg) and valsartan (80 mg) tablets. She had a past medical history of hypertension and hypercholesterolemia. Initial vital signs were: blood pressure, 110/58 mm Hg; heart rate, 120 per minute; respiratory rate, 18 per minute; oral temperature, 98.3o F; room air pulse oximetry, 98%. Physical examination demonstrated normal mental status with a depressed affect. Her pupils were 4 mm, symmetric, and equally reactive to light. Lungs were clear to auscultation. Cardiac examination was significant for tachycardia. Her abdomen was soft, nondistended, and nontender, with normal active bowel sounds. Extremities were non-edematous, with a capillary refill of less than two seconds. Neurological examination was normal. An electrocardiogram (ECG) demonstrated normal sinus rhythm, with normal axis and intervals. Laboratory studies included a normal complete blood count (CBC) and chemistry panel notable for: potassium, 3.2 mEq/L; bicarbonate, 19 mEq/L; and glucose, 194 mg/dL. Neither acetaminophen nor salicylates were detected in her serum.
Activated charcoal, 1 gram/kg, was administered orally. Two hours after ingestion she vomited, and her blood pressure fell to 80/45 mm Hg, with a heart rate of 94/min. Hypotension was refractory to a total of 30 mL of 10% calcium gluconate, 5 L of intravenous normal saline, 500 mL of colloid, and concomitant epinephrine, norepinephrine, phenylephrine, and vasopressin infusions. Repeat ECG only demonstrated a QTc increase from 433 to 542 milliseconds.
Poison control center (PCC) consultation was obtained 6 ½ hours after ingestion, at which time the patient's vitals were: BP, 81/41 mm Hg and pulse, 91 per minute. Arterial blood gas revealed pH, 7.23; PCO2, 40 mm Hg; PO2, 101 mm Hg; HCO3-, 16 mEq/L; lactate, 3.3 mmol/L. Vasopressor requirements had increased to epinephrine, 20 μg/min; norepinephrine, 64 μg/min; phenylephrine, 300 μg/min; and vasopressin, 0.04 units/min. Her mental status was preserved. Recommendations included: up to 30 mL rapid infusion of 10% calcium chloride followed by an infusion; high-dose insulin-euglycemia (HIE) therapy, consisting of 1 unit insulin/kg bolus followed by an initial infusion of 0.5–1.0 units/kg/hr with concurrent dextrose; glucagon and naloxone boluses (up to 10 mg and 2 mg respectively) followed by infusions; and possible increase in vasopressin infusion for persistently refractory hypotension, as well as additional activated charcoal and an assessment of cardiac function.
A bedside transthoracic echocardiogram documented a hyperdynamic left ventricle, normal right ventricle, absence of pericardial effusion, no evidence of valvular disease, and a normal inferior vena cava. Right heart catheterization demonstrated pulmonary capillary wedge pressures above 18 mm Hg (normal 2–10) for more than 40 hours during monitoring. Initial cardiac index CI fell from 4.89 L/min/m2 to 2.12 (normal 2.6-4.2) before recovering. Systemic vascular resistance was as low as 460 dynes/sec/cm-5 (normal 700–1600) 13 hours after ingestion. She ultimately received HIE therapy with insulin infusion rates as high as 2.64 units/kg/hr (). Glucagon, naloxone, and vasopressin infusions as high as 6 mg/hr, 1.64 mg/hr, and 0.12 units/min respectively did not produce significant benefit when sequentially added. Calcium chloride was administered incorrectly (0.03–0.05 grams/hr), and she actually was transiently hypocalcemic (ionized calcium 1.0 mmol/L, normal 1.12–1.30) on day 4, possibly secondary to concomitant mild pancreatitis. The patient was intubated for inadequate respiratory response to acidosis, respiratory difficulty, and to decrease her cardiac demand from work of breathing.
Fig. 1. Effect of insulin, epinephrine, and norepinephrine on blood pressure. Systolic (SBP, open triangle), mean arterial (MAP, open diamond), and diastolic (DBP, closed circle) blood pressures are in mm Hg. Insulin infusion (bars) is in units of regular insulin per kilogram per hour. Epinephrine infusion (asterisk) is indicated in micrograms per minute. Norepinephrine infusion (closed diamond) is indicated in micrograms × 10 per minute for figure clarity.
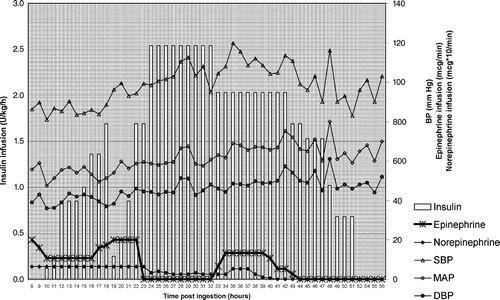
In total, she received 41 hours of HIE therapy and approximately 3573 units of insulin. Hyperglycemia (glucose maximum 1033 mg/dL) was noted during the onset of therapy, when a concurrent dextrose infusion had been started, but resolved promptly. She never experienced hypoglycemia. The remainder of her hospital course was complicated by thrombocytopenia, right lower lobe pneumonia, urinary tract infection, bilateral soleal deep venous thromboses, and mild gastrointestinal hemorrhage from suspected ischemic sigmoid colitis. She was discharged in good condition 37 days after initial hospitalization.
Discussion
This case adds to a growing number of reports of amlodipine poisoning (Citation3,Citation4,Citation9–21). The lowest reported ingested dose in the five fatal adult cases was 70 mg (100 mg in the absence of co-ingestants). There are two cases of survival following 1000 mg ingestion (Citation3,Citation12). The lowest oral dose producing hypotension was 30 mg in an adult (Citation10). When reported, onset of symptoms occurred as early as 2 hours (Citation11). Pulmonary edema complicated several cases (Citation3,Citation4,Citation10,Citation16,Citation17), and renal failure was a feature of a number of others (Citation3,Citation4,Citation11,Citation14,Citation15,Citation18,Citation20).
Unlike diltiazem or nifedipine, amlodipine can also induce nitric oxide-dependent vasodilatation in coronary and peripheral arteries, and may inhibit angiotensin converting enzyme itself (Citation22,Citation23). In conjunction with an ACE-I or ARB, these complex effects might worsen toxicity.
Refractory hypotension during therapeutic dosing of ARBs is described during anesthesia induction, when sympathetic tone is lost (Citation7,Citation24). Despite an initial hyperdynamic left ventricle and normal right ventricle, right heart catheterization in this patient demonstrated significant persistent loss of peripheral vasomotor tone, which is consistent with possible contributive effects of valsartan. We are unaware of previously published case reports of valsartan overdose alone causing significant hemodynamic compromise.
The calcium dose provided in this case was inadequate. The patient experienced late transient hypocalcemia, possibly secondary to concomitant pancreatitis. Some authors have advocated high-dose calcium therapy, achieving up to 30 grams of calcium salts in 12 hours and serum calcium concentrations of 23.8 mg/dL (5.94 mmol/L) without apparent ill effect (Citation25,Citation26). Others recommend a bolus dose followed by continuous infusion, with maintenance of physiologic calcium concentrations (Citation27). Additionally, our patient was refractory to large volume IV crystalloid resuscitation (5 L), which has been observed in previous patients with amlodipine overdose (Citation12).
Glucagon bolus and infusion produced a minimal effect in our patient. Its use is recommended based on animal data and limited human case reports. Beta-adrenergic receptor-independent increase in myocyte adenyl cyclase-mediated phosphorylation of calcium channels and arachadonic acid-mediated increases in sarcoplasmic reticulum calcium stores are proposed as mechanisms of action (Citation27,Citation28).
The basis for high-dose insulin euglycemia (HIE) therapy in CCB overdose was first demonstrated in dogs (Citation29). There are several case reports which describe HIE therapy in the successful management of CCB and specifically amlodipine overdose (Citation3,Citation9,Citation12,Citation15,Citation21,Citation30). As can be seen in , early in therapy, when the insulin infusion was decreased, the epinephrine requirements rose markedly; later, when the infusion was decreased again, both epinephrine and norepinephrine requirements increased. In the absence of human trials of high-dose insulin use in CCB overdose, data on efficacy, possible mechanism (inotropic effect and metabolic function), and safety come from trials in patients with analogous, though non-toxicological compromised cardiac function. In a randomized study of 40 patients undergoing cardiovascular surgery, insulin at 1 unit/kg/hr improved lactate clearance, increased glucose utilization, lowered dobutamine requirements, and tended to improved cardiac indices (Citation31). Doses of 2.5 units/kg/hr insulin were safely tolerated without excessive insulin-induced potassium dysregulation (Citation32). In combination with dopamine, doses of 7 units/kg insulin were safely tolerated and significantly improve cardiac output in post-CABG patients without increasing in oxygen demand (Citation33). Since both mathematical models and direct interstitial measurements indicate that exogenous insulin administration follows saturation kinetics, with Km's for transport and action in picomolar concentrations (Citation34,Citation35), additional mechanisms must be implicated. These mechanisms may include counteracting CCB-mediated insulin resistance, inhibiting insulin release, and improving myocardial substrate utilization (Citation29,Citation31,Citation36,Citation37). Our recommendations regarding bolus and maintenance insulin dosing were initially incompletely followed, reflecting the experience of others who have reported 46% noncompliance with HIE recommendations (Citation38).
The recommendation for naloxone was based on human and animal experimental evidence and case reports. Naloxone partially antagonized the endomorphine-1 and -2 inhibition of phenylephrine- and angiotensin II-induced contractile response in a rat thoracic aortic ring model (Citation39). In dogs, 0.1 mg/kg naloxone reversed enalapril and enalaprilic acid inhibition of the vagal-stimulation pressor response (Citation40). Co-administration of 0.2 mg/kg naloxone mitigated captopril-related decreases in systolic and diastolic blood pressure in healthy men (Citation41). Another double-blind, placebo-controlled study of healthy men found that naloxone pretreatment with 10 mg followed by 2.46 mg/hr infusion eliminated captopril-induced systolic blood pressure drop (Citation42). In one patient 1.6 mg bolus of naloxone followed by repeat 2 mg bolus reversed hypotension due to overdose with 500 mg captopril (Citation43). Another author, however, found naloxone ineffective in overdose of 750 mg captopril and “possibly intravenous narcotics” (Citation44). Naloxone has been previously utilized in combined amlodipine and benazepril coingestion (Citation19).
Because ACE inhibition or ARB blockade prevents vasopressin-mediated response, several authors have successfully used vasopressin or vasopressin analogues in cases of significant ACE-I- or ARB-induced hypotension. Bolus terlipressin reversed shock following induction of anesthesia in a patient taking irbesartan (Citation7). A prospective study noted 32 of 51 patients taking ACE-I or ARBs developed significant hypotension upon anesthesia induction, despite having stopped their medications 12–24 hours prior to surgery. Ten patients (31%) failed rescue with ephedrine or phenylephrine, but responded to terlipressin (Citation24). A second randomized study in which 20 of 42 patients chronically on ACE-I/ARBs had ephedrine-refractory shock upon anesthesia induction demonstrated the superiority of terlipressin to norepinephrine (Citation45). A 54-year-old woman with mixed ingestion including irbesartan, HCTZ, diazepam, acetaminophen, and alcohol responded to terlipressin after therapy with fluids, ephedrine, phenylephrine, and norepinephrine failed to improve her severe hypotension (Citation46). In contrast to effectiveness in ACE-I/ARB toxicity, in one canine model of CCB toxicity vasopressin as a single intervention failed to return blood pressure to within 20% of baseline (Citation47). Nevertheless, others have incorporated vasopressin analogues in the successful resuscitation of amlodipine (Citation3,Citation19) or felodipine (Citation48) overdose in humans.
Conclusions
Clinicians should anticipate that patients who overdose on amlodipine may have profound, prolonged hypotension, with loss of vascular selectivity. Coingestants which adversely affect cardiovascular reflex mechanisms, vasomotor tone, inotropy, or chronotropy may increase the severity of the toxicity of amlodipine. HIE therapy should be considered early in course of treatment of severe CCB overdose, especially with coingestants, as its effects are not immediately realized.
References
- Lai MW, Klein-Schwartz W, Rodgers GC, Abrams JY, Haber DA, Bronstein AC, Wruk KM. 2005 Annual Report of the American Association of Poison Control Centers’ National Poisoning and Exposure Database. Clin Toxicol 2006; 44: 803–932
- Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology 1992; 80(Suppl 1)31–6
- Vogt S, Mehlig A, Hunziker P, Scholer A, Jung J, Baranda A, González A, Weinmann W, Marsc S. Survival of severe amlodipine intoxication due to medical intensive care. Forensic Sci Int 2006; 161: 216–20
- Adams BD, Browne WT. Amlodipine overdose causes prolonged calcium channel blocker toxicity. Am J Emerg Med 1998; 16: 527–8
- McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 2003; 91: 30H–37H
- Black HR. Evolving role of aldosterone blockers alone and in combination with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in hypertension management: A review of mechanistic and clinical data. Am Heart J 2004; 147: 564–72
- Brabant SM, Eyraud D, Bertrand M, Coriat P. Refractory Hypotension After Induction of Anesthesia in a Patient Chronically Treated with Angiotensin Receptor Antagonists. Anesth Analg 1999; 89: 887–8
- Licker M, Neidhart P, Lustenberger S, Valloton MB, Kalonji T, Fathi M, Morel DR. Long-term Angiotensin-converting Enzyme Inhibitor Treatment Attenuates Adrenergic Responsiveness without Altering Hemodynamic Control in Patients Undergoing Cardiac Surgery. Anesthesiology 1996; 84: 789–800
- Boyer EW, Shannon M. Treatment of calcium-channel-blocker intoxication with insulin infusion. N Engl J Med 2001; 344: 1721–2
- Cosbey SH, Carson DJ. A fatal case of amlodipine poisoning. J Anal Toxicol 1997; 2: 221–2
- Feldman R, Glinska-Serwin M. Gleboka hipotensja z przemijajaca oliguria oraz ciezka niewydolnosc serca w przebiegu ostrego zamierzonego zatrucia amlodypina. [Deep hypotension with transient oliguria and severe heart failure in course of acute intentional poisoning with amlodipine]. Pol Arch Med Wewn 2001; 105: 495–9
- Harris NS. Case records of the Massachusetts General Hospital. Case 24–2006. A 40-year-old woman with hypotension after an overdose of amlodipine. N Engl J Med 2006; 355: 602–11
- Johansen SS, Genner J. A fatal case of amlodipine poisoning. J Clin Forensic Med 2003; 10: 169–172
- Koch AR, Vogelaers DP, Decruyenaere JM, Callens B, Verstraete A, Buylaert WA. Fatal intoxication with amlodipine. J Toxicol Clin Toxicol 1995, 33: 253–6
- Rasmussen L, Husted SE, Johnsen SP. Severe intoxication after an intentional overdose of amlodipine. Acta Anaesthesiol Scand 2003; 47: 1038–40
- Saravu K, Balasubramanian R. Near-fatal amlodipine poisoning. J Assoc Physicians India 2004; 52: 156–7
- Stanek EJ, Nelson CE, DeNofrio D. Amlodipine overdose. Ann Pharmacother 1997; 31: 853–6
- Tovar JL, Bujons I, Ruiz JC, Ibanez L, Salgado A. Treatment of severe combined overdose of calcium antagonists and converting enzyme inhibitors with angiotensin II. Nephron 1997; 77(2)239
- Weisgerber K, Link A, Hammer B, Bohm M. Vasopressinanaloga als ultima ratio bei einer schweren intoxikation mit vasodilatanzien. [Vasopressin analogue injection as ultimate measure for counteracting severe catecholamine-refractory poisoning by several vasodilators taken with suicidal intent]. Dtsch Med Wochenschr 2003; 128: 2189–92
- Wood DM, Wright KD, Jones AL, Dargan PI. Metaraminol (Aramine) in the management of a significant amlodipine overdose. Hum Exp Toxicol 2005; 24: 377–81
- Yuan TH, Kerns WP, 2nd, Tomaszewski CA, Ford MD, Kline JA. Insulin-glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol 1999; 37: 463–74
- Lenasi H, Kohlstedt K, Fichtlscherer B, Mulsch A, Busse R, Fleming I. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser1177 and Thr495. Cardiovasc Res 2003; 59: 844–53
- Xu B, Xiao-hong L, Lin G, Queen L, Ferro A. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. Br J Pharmacol 2002; 136: 375–82
- Eyraud D, Brabant S, Nathalie D, Fleron MH, Gilles G, Bertrand M, Coriat P. Treatment of intraoperative refractory hypotension with terlipressin in patients chronically treated with an antagonist of the renin-angiotensin system. Anesth Analg 1999; 88: 980–4
- Howarth DM, Dawson AH, Smith AJ, Buckley NA, Whyte IM. Calcium channel blocking drugs in overdose: an Australian series. Hum Exp Toxicol 1994; 13: 161–6
- Buckley NA, Whyte IM, Dawson AH. Overdose with Calcium Channel Blockers BMJ. 1994; 308: 1639
- Salhanick SD, Shannon MW. Management of calcium channel antagonist overdose. Drug Saf 2003; 26: 65–79
- Sauvadet A, Rohn T, Pecker F, Pavoine C. Arachidonic acid drives mini-glucagon action in cardiac cells. J Biol Chem 1997; 272: 12437–45
- Kline JA, Raymond RM, Leonova ED, Williams TC, Watts JA. Insulin improves heart function and metabolism during non-ischemic cardiogenic shock in awake canines. Cardiovasc Res 1997; 34: 289–98
- Marques M, Gomes E, de Oliveira J. Treatment of calcium channel blocker intoxication with insulin infusion: case report and literature review. Resuscitation 2003; 57: 211–3
- Koskenkari JK. Metabolic and hemodynamic effects of high-dose insulin treatment in aortic valve and coronary surgery. Ann Thorac Surg 2005; 80: 511–7
- Doenst T, Bothe W, Beyersdorf F. Therapy with insulin in cardiac surgery: controversies and possible solutions. Ann Thorac Surg 2003; 75: S721–8
- Svedjeholm R, Ekroth R, Joachimsson PO, Tyden H. High-dose insulin improves the efficacy of dopamine early after cardiac surgery. A study of myocardial performance and oxygen consumption. Scand J Thorac Cardiovasc Surg 1991; 25: 215–21
- Prigeon RL, Roder ME, Porte D, Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest 1996; 97: 501–7
- Mokshagundam SP, Peiris AN, Stagner JI, Gingerich RL, Samols E. Interstitial insulin during euglycemic-hyperinsulinemic clamp in obese and lean individuals. Metabolism 1996; 45: 951–6
- Kline JA, Leonova E, Williams TC, Schroeder JD, Watts JA. Myocardial metabolism during graded intraportal verapamil infusion in awake dogs. J Cardiovasc Pharmacol 1996; 27: 719–26
- Kline JA, Raymond RM, Schroeder JD, Watts JA. The diabetogenic effects of acute verapamil poisoning. Toxicol Appl Pharmacol 1997; 145: 357–62
- Miller AD, Maloney GE, Kanter MZ, Clifton JC, DesLauriers CA. Are poison center recommendations for high-dose insulin treatment in calcium-channel blocker poisoning patients followed [Abstract]. Clin Toxicol 2006; 44: 711
- Qi YM, Yang DJ, Duan X, Yang F, Li SR, Shen JM, Wang R. Endomorphins inhibit contractile responses of rat thoracic aorta rings induced by phenylephrine and angiotensin II in vitro. Acta Pharmacol Sin 2002; 23: 40–4
- Montastruc P, Dang-Tran L, Carvajal A, Rostin M, Montastruc JL. Naloxone reverses the effects of enalapril and enalaprilic acid on the pressor responses to afferent vagal stimulation. Neuropeptides 1985; 6: 537–42
- Millar JA, Sturani A, Rubin PC, Lawrie C, Reid JL. Attenuation of the antihypertensive effect of captopril by the opioid receptor antagonist naloxone. Clin Exp Pharmacol Physiol 1983; 10: 253–9
- Ajayi AA, Campbell BC, Rubin PC, Reid JL. Effect of naloxone on the actions of captopril. Clin Pharmacol Ther 1985; 38: 560–5
- Varon J, Duncan SR. Naloxone reversal of hypotension due to captopril overdose. Ann Emerg Med 1991; 20: 1125–7
- Barr CS, Payne R, Newton RW. Profound prolonged hypotension following captopril overdose. Postgrad Med J 1991; 67: 953–4
- Boccara G, Ouattara A, Godet G, Dufresne E, Bertrand M, Riou B, Coriat P. Terlipressin Versus Norepinephrine to Correct Refractory Arterial Hypotension after General Anesthesia in Patients Chronically Treated with Renin-Angiotensin System Inhibitors. Anesthesiology 2003; 98: 1338–44
- McNamee JJ, Trainor D, Michalek P. Terlipressin for refractory hypotension following angiotensin-II receptor antagonist overdose. Anaesthesia 2006; 61: 408–9
- Sztajnkrycer MD, Bond GR, Johnson SB, Weaver AL. Use of vasopressin in a canine model of severe verapamil poisoning: a preliminary descriptive study. Acad Emerg Med 2004; 11: 1253–61
- Leone M, Charvet A, Delmas A, Albanese J, Martin C, Boyle WA. Terlipressin: a new therapeutic for calcium-channel blockers overdose. J Crit Care 2005; 20: 114–5
