Abstract
Introduction. Tacrolimus is an immunosuppressant widely used in recipients of solid organ transplants to prevent rejection. Toxicity is usually reported in transplant patients. We report the first case of tacrolimus toxicity in a non-transplant patient. Case report. A 42 year-old, 48 kg woman complained of neck pain following a motor vehicle collision and was admitted for observation. On examination, her pulse was 112 beats/minute and her blood pressure 188/134 mmHg. Because the hypertension and tachycardia might be ethanol withdrawal, she was admitted and treated with multivitamins, folate, and thiamine in her maintenance fluids. She was discharged after 4 days in hospital. The day after her discharge, she was asked to return after it was discovered that she had inadvertently received tacrolimus (total of 400 mg) instead of thiamine. She was admitted with non-oliguric renal failure and metabolic acidosis. A tacrolimus concentration 27 hours after her last exposure was 96.8 ng/mL (therapeutic 5 to 20 ng/mL). Treatment was supportive and she was discharged after 4 days without sequellae. Discussion. Our patient's tacrolimus dose was 2.1 mg/kg/day for 4 days (therapeutic 0.03 to 0.05 mg/kg/day). Her tacrolimus elimination half-life was 16.5 hours, compared to a mean half-life in healthy volunteers of 34.2 ± 7.7 hours. Conclusion. Clinical toxicity, similar to that seen in transplant patients, can develop in non-transplant patients following intravenous administration of supra-therapeutic doses of tacrolimus.
Introduction
Tacrolimus is an immunosuppressant widely used in recipients of solid organ transplants. Adverse effects from use in the transplant population include nephrotoxicity, neurotoxicity, hypertension, disturbances in glucose metabolism, gastrointestinal disturbances, and increased susceptibility to infection and malignancy [Citation1–3]. However, there are no reports of significant toxicity from excessive dosing in a non-transplant patient. We report toxicity from excessive intravenous doses of tacrolimus given inadvertently to a non-transplant patient.
Case report
A 42 year-old, 48 kg woman complained of neck pain following a motor vehicle collision and was admitted for observation. The patient denied any significant past medical history, any current medications, and described tobacco and ethanol use as social and on weekends only. Her initial examination was unremarkable with the exception of tachycardia (112 beats/minute) and hypertension (188/134 mmHg) and she remained in hospital for treatment of her hypertension and persistent tachycardia. Clonidine and ramipril were prescribed for blood pressure control. Because the hypertension and tachycardia might represent ethanol withdrawal, a multivitamin, folate, and thiamine were added to the first bag of her daily maintenance fluids and administered at 75 mL/h and chlordiazepoxide (10 mg orally three times daily) was started. A contrast CT scan of the chest excluded pulmonary embolism as a cause of her tachycardia. Her blood pressure and heart rate stabilized. Her only set of routine laboratory tests were done the evening of hospital day 4, just before discharge. The electrolytes, BUN, creatinine and glucose were normal; her bicarbonate was 20 mmol/L (22 to 29 mmol/L).
The day after her discharge, the hospital asked the patient to return for evaluation after discovering that she had inadvertently received tacrolimus instead of thiamine. Based on pharmacy records, an estimated total of 400 mg of tacrolimus was given during her four day hospitalization. Since her discharge, the patient had developed fatigue and swelling of the upper and lower lips. Physical examination revealed only isolated upper and lower lip edema. Vital signs on re-admission were pulse 120 beats/minute and blood pressure 105/76 mmHg; temperature and respiratory rate were normal. Laboratory tests results were hemoglobin 15.5 g/dL, hematocrit 47.1%, sodium 135 mmol/L (135 to 145 mmol/L), potassium 4.9 mmol/L (3.5 to 5.5 mmol/L), chloride 104 mmol/L (95 to 105 mmol/L), bicarbonate 12 mmol/L (22 to 29 mmol/L), BUN 25 mg/dL (5 to 20 mg/dL), creatinine 4.0 mg/dL (0.6 to 1.4 mg/dL), glucose 106 mg/dL (70 to 200 mg/dL), arterial pH 7.25, arterial pCO2 35 mm Hg, and no myoglobin in the urine.
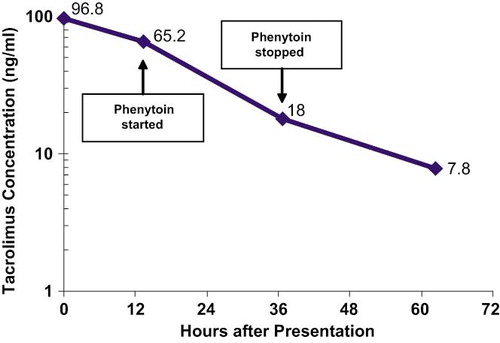
The patient was admitted for non-oliguric renal failure, metabolic acidosis, and angioedema, and started on dexamethasone, famotidine, and fluid hydration. The nephrologist discontinued the ramipril because of the probability of ACE inhibitor induced angioedema and began a bicarbonate drip the following day after her acidemia did not resolve with fluid resuscitation alone. A renal ultrasound revealed no abnormalities. An initial whole blood tacrolimus concentration measured by microparticle enzyme immunoassay was 96.8 ng/mL (therapeutic 5 to 20 ng/mL).
The evening of re-admission the patient was started on oral phenytoin (500 mg followed by three doses of 100 mg) in an attempt to induce tacrolimus metabolism. Serum creatinine and blood tacrolimus concentrations were followed, with creatinine normalizing (0.9 mg/dL), metabolic acidosis resolving, and tacrolimus concentrations declining four days after re-admission ( and ). The patient was discharged with normal laboratory values and asymptomatic on hospital day four after re-admission.
Table 1. Pertinent laboratory values
Discussion
Tacrolimus is an immunosuppressant agent used to prevent or reverse rejection in organ transplant patients [Citation1,Citation2] and for refractory auto-immune disorders [Citation4]. Tacrolimus is metabolized by hepatic CYP3A4 to inactive metabolites [Citation5,Citation6]. Post-transplant, tacrolimus is given as a continuous intravenous infusion at a rate of 0.03 to 0.05 mg/kg/24 hours with target whole blood trough concentrations of 5 to 20 ng/mL [Citation3]. Our patient received an estimated 2.1 mg/kg/day for 4 days. The initial tacrolimus concentration (96.8 ng/mL) was obtained 27 hours after the patient's last exposure. Review of the literature revealed patients with acute or acute on chronic toxicity developed symptoms consisting of nausea, tremor, elevations in BUN, creatinine and hepatic transaminases. [1, 7, 8, 10] Whole blood tacrolimus concentrations ranged from therapeutic levels to a maximum of 197 ng/mL [1, 7–10].
Tacrolimus toxicity is primarily derived from adverse effects at therapeutic doses in patients with organ transplants and includes nephrotoxicity, hypertension, nausea, vomiting, diarrhea, altered glucose metabolism, and increased risk of infection and malignancies [Citation1–12]. Anaphylaxis has been reported with IV administration, although it is thought to be due to the castor oil derivative in the injectable formulation [Citation13]. The description of tacrolimus toxicity is confounded by the concomitant use of other immunosuppressants or the co-existence of underlying pathology. Only one report describes tacrolimus ingestions in identified non-transplant patients: each of two non-transplant patients ingested 10 mg or less of tacrolimus and neither developed clinical toxicity [Citation7]. In another report of acute tacrolimus overdose, the transplant status of the patients was not clear [Citation1] so we believe our report is the first reported case of tacrolimus toxicity in a non-transplant patient.
Our patient received tacrolimus over four days due to a clerical transcription error that was not discovered until the pharmacy's stock of tacrolimus was significantly depleted. At the time of her initial hospital discharge, our patient had normal renal function and slightly low serum bicarbonate, findings previously reported with tacrolimus [Citation10]. On re-admission she had metabolic acidosis and non-oliguric renal failure, effects previously reported with tacrolimus toxicity in transplant patients [Citation3,Citation10].
The development of renal failure may have been multi-factorial in light of the patient receiving an ACE inhibitor and IV contrast. However, she had been given intravenous fluids and carried no other risk factors for radiocontrast-induced nephropathy outside of her hypertension. The patient's clinical presentation and rapid resolution is more consistent with acute tacrolimus toxicity as described in the literature [Citation1,Citation10].
The elimination half-life of tacrolimus was calculated using non-linear regression analysis for a one-compartment open model. By this calculation, our patient had an elimination half-life of 16.5 hours (r2 = 0.99) compared to a mean half-life in healthy volunteers of 34.2 ± 7.7 hours [Citation16]. The explanation for this finding is not clear.
Administering a CYP3A4 inducer, such as phenytoin, may increase the metabolism of tacrolimus [Citation1,Citation8,Citation9,Citation14] but the time necessary for enzyme induction makes it unlikely that phenytoin played a major role in the elimination of tacrolimus in our patient [Citation15]. There was no observable change in elimination over the period of phenytoin administration, although the over-all elimination rate appears to have been enhanced, compared to controls. Hypothetically, the patient's increased elimination rate might be due to an unrecognized CYP3A4 polymorphism.
Conclusion
Renal failure and metabolic acidemia may develop in patients without solid organ transplants who receive large doses of tacrolimus, and this toxicity is similar to that seen in transplant patients. Treatment is primarily symptomatic and supportive.
References
- CF Curran, PC Blahunka, and ID Lawrence. Acute overdoses of tacrolimus. Transplantation 1996; 62 (9):1376–7.
- JJ Fung, M Alessiani, K Abu-Elmagd, S Todo, R Shapiro, A Tzakis, D Van Thiel, J Armitage, A Jain, and J McCauley. Adverse effects associated with the use of FK 506. Transplant Proc 1991; 23 (6):3105–3108.
- GL Plosker, and RH Foster. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs 2000; 59 (2):323–389.
- D Politt, B Heintz, J Floege, and PR Mertens. Tacrolimus- (FK 506) based immunosuppression in severe systemic lupus erythematosus. Clin Nephrol 2004; 62 (1):49–53.
- T Shiraga, H Matsuda, K Nagase, K Iwasaki, K Noda, H Yamazaki, T Shimada, and Y Funae. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol 1994; 47 (4):727–735.
- K Nagase, K Iwasaki, K Nozaki, and K Noda. Distribution and protein binding of FK506, a potent immunosuppressive macrolide lactone, in human blood and its uptake by erythrocytes. J Pharm Pharmacol 1994; 46 (2):113–117.
- R Mrvos, M Hodgman, and EP Krenzelok. Tacrolimus (FK 506) overdose: a report of five cases. Clin Toxicol 1997; 35 (4):395–399.
- Z Karasu, A Gurakar, J Carlson, S Pennington, B Kerwin, H Wright, N Bakr, and A Sebastian. Acute tacrolimus overdose and treatment with phenytoin in liver transplant recipients. J Okla State Med Assoc 2001; 94 (4):121–123.
- CN Yeh, CH Hsieh, CM Hung, LB Jeng, TC Chao, and MF Chen. Acute overdoses of tacrolimus (FK 506). Digestive Diseases and Sciences 1999; 44 (8):1650–1652.
- LL Hardwick, and TD Batiuk. Severe prolonged tacrolimus overdose with minimal consequences. Pharmacotherapy 2002; 22 (8):1063–1066.
- S Takahashi, K Sugimoto, S Hishikawa, K Mizuta, A Fujimura, and H Kawarasaki. Recurrence of hepatic artery thrombosis following acute tacrolimus overdose in pediatric liver transplant recipient. Pediatr Transplantation 2005; 9 (6):809–12.
- JJ Fung. Tacrolimus and transplantation: a decade in review. Transplantation 2004; 77 (9 Suppl):S41–S43.
- A Hisatomi, M Kimura, M Maeda, M Matsumoto, K Ohara, and H Noguchi. Toxicity of polyoxyethylene hydrogenated castor oil 60 (HCO–60) in experimental animals. J Toxicol Sci 1993; 18 (Suppl 3):1–9.
- M Su, RS Hoffman, and LS Nelson. Acute tacrolimus overdose without significant toxicity. Clin Toxicol 2002; 40 (2):205–206.
- KE Thummel, DD Shen, TD Podoll, KL Kunze, WF Trager, CE Bacchi, CL Marsh, JP McVicar, DM Barr, JD Perkins, and RL Carithers. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther 1994; 271 (1):557–566.
- I Bekersky, D Dressler, A Alak, GW Boswell, and QA Mekki. Comparative tacrolimus pharmacokinetics: normal versus mildly hepatically impaired subjects. J Clin Pharmacol 2001; 41 (6):628–635.