Abstract
Objective. To report a case of serotonin syndrome associated with a single, 50 mg dose of sertraline in a child and discuss the findings in context with previous relevant literature involving other selective serotonin reuptake inhibitors used in children. Case Summary. A nine-year old male with chronic behavioral problems was prescribed oral sertraline 50 mg daily. After the first dose, the patient presented with abdominal pain, seizure-like activity, and change in mental status. He was admitted to a tertiary-care pediatric hospital and was treated for serotonin syndrome. Laboratory findings of elevated creatine kinase and serum creatinine were consistent with rhabdomyolysis as result of continued hypertonicity. Sertraline was discontinued and treatment with lorazepam and cyproheptadine was initiated. Clinical status, creatine kinase, and serum creatinine improved over 5 days of hospitalization. The Adverse Drug Reaction Probability Scale by Naranjo et al was applied to assess causality. The scale indicated the association of a single dose of sertraline and serotonin syndrome as “probable.” Conclusion. To our knowledge this is the first reported case of serotonin syndrome associated with a single dose of sertraline in a child using a validated causality scale. The sertraline 50 mg dose given to the child was higher than usual recommended initial doses (25 mg). This potential adverse reaction should be considered when selecting antidepressant therapy for children.
Keywords:
Introduction
The use of selective serotonin reuptake inhibitors (SSRIs) has increased over the past decade, in both adults and children. With an estimated 2–6% of children and adolescents having depression and the fact that suicide is the third leading cause of death among 10–19 year olds, the use of SSRIs and other antidepressant agents has increased in children (Citation1). Although currently the only FDA approved SSRI for children is fluoxetine, other agents in this class have been used off-label in pediatrics. As with any medication use, the potential for adverse effects exists. Serotonin syndrome (SS) is one possible effect from the use of SSRIs as well as other agents such as serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, opioids, over-the-counter cough/cold medicines, and illicit drugs. This condition is characterized by a combination of symptoms which includes changes in mental status, increased or abnormal neuromuscular tone, and autonomic dysfunction. Common causes for SS include overdose of SSRIs, combined use of SSRIs with MAOIs or other potentially offensive agents (Citation2).
Diagnosis of SS is made through physical exam and review of medication history. There currently is no laboratory test available to confirm the diagnosis of SS; however, one case report has suggested the use of urinary serotonin concentration as a biomarker (Citation3). Signs and symptoms of SS in children are similar to those seen in adults (). It may be difficult to diagnose SS in this population, as the condition has mostly been reported in adults, and because accuracy of medication history (including illicit drug use) from children and adolescents is questionable (Citation4).
Table 1. Signs and symptoms of serotonin syndrome in children as reported in the literature
The incidence of SS after drug interactions or overdose of SSRIs or other agents in children have been reported in the literature (Citation5–Citation7). There are two case reports of SS associated with the administration of a single, prescribed dose of SSRI; neither of these documented the association between the drug and SS by using a validated causality scale (Citation8,Citation9). We report the case of serotonin syndrome in a 9-year-old child resulting in tremors, agitation, and rigor, 30 minutes following a single, prescribed dose (50 mg) of sertraline, a SSRI, utilizing a validated causality scale to assess the association.
Case report
This case report was written in accordance with policies of the Institutional Review Board at our institution.
A 9-year-old male (31.2 kg) with chronic behavioral problems was prescribed oral sertraline 50 mg (1.6 mg/kg/day) by his primary care physician. He was on no medications prior to the initiation of sertraline. The child took the first sertraline dose that evening at approximately 5:30 p.m. and went outside to play. Within 30 minutes he began to complain of stomach pain and he became increasingly delirious and combative over the following two hours. According to his caregivers he had had no fever, cough, headache, vomiting, diarrhea, or rash preceding his mental status change. There were no known co-ingestions or injuries.
Three hours after ingestion, EMS arrived and the patient was noted to have tremors, diaphoresis, and marked agitation. At the time of presentation to his local Emergency Department (ED), about 20 minutes after EMS arrival, his vital signs included a rectal temperature of 36.4°C, pulse of 162 beats per min (bpm), respiratory rate of 16 per minute, and blood pressure 109/71 mmHg. He was encephalopathic, with a Glascow Coma Scale (GCS) score of 10, and progressively severe agitation. Within 4.5 hours after ingestion his rectal temperature had increased to 38.8°C. His pupils were 4mm bilaterally and sluggishly reactive. During the child's stay in the referring ED, peripheral intravenous (IV) access was established and he was given three doses of IV lorazepam (0.25, 0.5, 1 mg) and one dose of haloperidol (2.5 mg) for treatment of combativeness. Vancomycin and ceftriaxone were given IV after blood cultures were obtained. Initial serum chemistries from the referring ED included serum BUN 21 mg/dL and creatinine 0.6 mg/dL. The patient's white blood cell count was 25.6 × 103/μL with 84% neutrophils, 11% lymphocytes, and 4% monocytes. Hemoglobin and platelet counts were normal. A venous blood gas demonstrated a pH of 7.42 with a pCO2 of 31 mmHg, a bicarbonate of 22 mmol/L, and a base deficit of −4 mmol/L. For airway protection, the patient underwent rapid sequence endotracheal intubation prior to transport to the pediatric intensive care unit (PICU).
Upon PICU admission (approximately 8.5 hours after ingestion, hospital day 1) his vital signs included an axillary temperature of 36.7°C, pulse 135 bpm, and blood pressure 90/42 mmHg. Physical exam at that time was significant for hypertonicity in all four extremities (lower ≫ upper) with 4+ deep tendon reflexes (DTR) in his legs, clonus bilaterally (lower extremities ≫ upper extremities), and bilaterally dilated pupils (5 mm) which were sluggishly reactive. As he emerged from sedation administered on transport, the patient was unable to follow commands and was intermittently agitated. Peripheral pulses, capillary refill, and lung compliance were normal. The patient's chest radiograph and computed tomography (CT) of the head at the time of admission were unremarkable.
After consultation with the toxicology service at the time of PICU admission, cyproheptadine treatment was initiated: 8mg via nasogastric tube (NG) followed by 2.5 mg via NG every 8 hours. As serotonin syndrome was viewed as a diagnosis of exclusion, the patient also underwent lumbar puncture, electroencephalogram (EEG), and magnetic resonance imaging (MRI) of the head on hospital day 1. The cerebrospinal fluid (CSF) was essentially unremarkable (10 RBC per mm3, 2 WBC per mm3, glucose 87 mg/dL, protein 27 mg/dL) with a negative Gram's stain. Intravenous (IV) vancomycin, ceftriaxone, and acyclovir were given until CSF bacterial cultures, herpes simplex virus PCR, and enteroviral PCR tests all proved negative. Arboviral serologies were similarly negative. The EEG showed slowing consistent with sedation but no epileptiform discharges or other focal findings. The patient's head MRI was normal. Serum and urine comprehensive toxicologic screens were negative except for medications administered at the referring hospital or on transport.
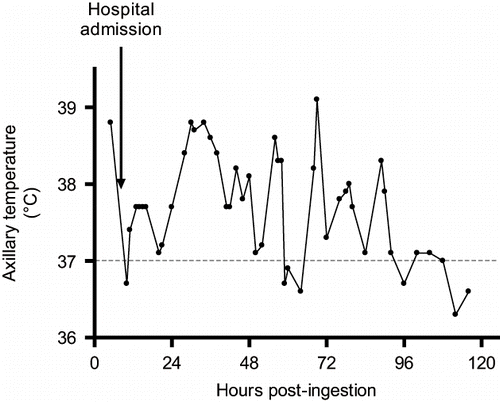
Intermittent IV lorazepam (3mg every 4 hours as needed) was used to treat agitation and spasticity. The patient's mental status improved throughout the first PICU day and he was successfully extubated that afternoon. He continued, however, to demonstrate marked hypertonicity, disorientation, and intermittent fevers (). These symptoms were managed with continued cyproheptadine therapy, intermittent lorazepam, and acetaminophen. It was well into the 4th hospital day before the patient became persistently afebrile. He had no clinical or microbiologic evidence of community acquired or nosocomial infection throughout his admission. Hemodynamics, urine output, and respiratory function remained normal.
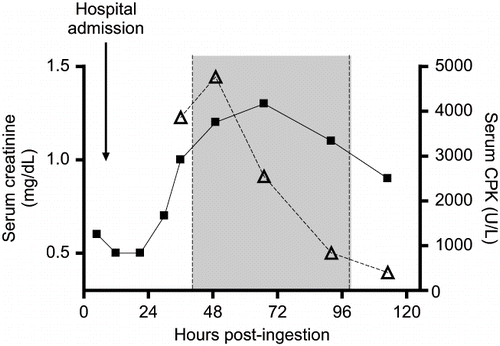
The following morning (hospital day 2), screening laboratories revealed a doubling of the patient's serum creatinine from hospital admission (0.5 to 1.0). Serum unfractionated creatine phosphokinase (CPK) at the time was 3859 U/L. Urine alkalinization was subsequently accomplished with the addition of sodium bicarbonate to the patient's maintenance IV fluids. Urine pH was maintained 7.0 – 8.5 until the serum creatinine improved. The CPK peaked in the evening of ICU day 2 (4763 U/L), while the serum creatinine reached its maximum (1.3 mg/dL) the following day (). Urine output and serum electrolytes were normal throughout. Urine alkalinization was stopped on hospital day 4.
Fig. 2. Time course of the patient's serum creatinine (▪) and creatine phosphokinase (Δ) levels. The shaded area represents the duration of urine alkalinization.
From a neurologic standpoint, by hospital day 2 the patient was awake and following commands, though still confused. He was still hypertonic (lower extremities ≫ upper extremities) though his DTRs were subjectively improved (3+). He was transferred to the regular ward on the afternoon of hospital day 2 on cyproheptadine and intermittent lorazepam. Over the next 48 hours his mental status normalized as did his peripheral muscle tone. Also of note, he developed mild, transient diarrhea typical of serotonin syndrome during this time. Cyproheptadine was discontinued on the morning of hospital day 5 and he was discharged to home later that day.
Causality in previous pediatric reports was not determined using an objective scale or scoring system. In order to analyze the likelihood of a causal link between our patient's single-dose sertraline ingestion and his subsequent adverse drug reaction (ADR), we utilized the scale described by Naranjo et al. The Adverse Drug Reaction Probability Scale by Naranjo et al. is a validated causality scale in which a user determines a score based on a series of questions pertaining to possible effects from other medications, timing of dose and event, laboratory evidence, or alternative causes for event. The scale ranges from 0 to 13; see for interpretation of scores. The “definite” causality generally requires a rechallenge to medication, which cannot be done in a drug overdose and is often associated with risks exceeding benefits. We assessed causality of our patient's condition of serotonin syndrome and his ingestion of a single dose of sertraline using this scale. The final, tabulated score was 6, indicating an association between the single dose of sertraline and SS in our patient as “probable” () (Citation10).
Table 2. Score definitions of the Adverse Drug Reaction (ADR) Probability Scale by Naranjo et al
Table 3. Case score composition for sertraline using the Adverse Drug Reaction Probability Scale by Naranjo et al. (10)
Discussion
Serotonin syndrome incidence has been associated with overdose of SSRIs or drug-interactions with SSRIs resulting in excessive central serotonergic activity (Citation2). There are a few reports of toxicity after ingestion of sertraline alone in children; however, the dose of sertraline was either unknown or too high. Kaminski et al. reported a case of a 9-year-old male with a history of attention deficit hyperactivity disorder who presented with SS two hours after ingesting an unknown number of 50 mg sertraline tablets. Serum concentrations were followed throughout his hospital stay. Serum concentration of sertraline nine hours after ingestion was 68 ng/mL. He was previously taking methylphenidate until nine days prior to admission, stopping as a result of lack of medication supply. The patient's mother was currently taking sertraline for her history of depression. Upon admission to the emergency department, he presented with a blood pressure of 100/66 mm Hg, oral temperature of 38.5 C, an irregular pulse of 176 bpm, and respiratory rate of 38. He presented with change in mental status with hallucinations. His symptoms continued to persist and he was transferred to the pediatric critical care unit for further management. Treatment included activated charcoal, physostigmine, acetaminophen, lorazepam, and chloral hydrate. After four days of hospitalization, the patient's serum sertraline level was <10 ng/mL and he was discharged home (Citation11). Another case of reported sertraline toxicity involved a 5-year-old female who ingested at least eight, 50 mg sertraline tablets. Sertraline concentrations (99 ng/mL, 72 hours post-ingestion) reflected a large dose ingestion as it exceeded an expected concentration in an adult from a single, 100 mg dose of sertraline at four hours (54 ng/mL). She was treated with gastric lavage and charcoal in the emergency department and was discharged home 48 hours after ingestion. However, the patient was readmitted the next day due to symptoms of increased irritability, tachycardia, insomnia, agitation, and combative behavior; before she was discharged home after one week. No additional pharmacotherapy treatment was reported for her second admission (Citation6). Sertraline overdose was also attributed to a case involving a 24-month-old female, who accidentally ingested ten sertraline 50 mg tablets. Initially, the patient was asymptomatic for 3 hours while in the emergency department following gastric lavage and administration of activated charcoal. She later developed SS symptoms of hyperactivity, tremor, insomnia, hyperflexia, and unsteady ataxic gait 12 hours after ingestion. She was readmitted to the emergency department and after a toxicology consult, she was treated with scheduled oral cyproheptadine. The patient's symptoms subsided 40 minutes after starting cyproheptidine and she was discharged home with continued treatment (cyproheptidine 1mg PO Q8H) for two additional days. Based on telephone follow-up, the patient remained asymptomatic after discharge (Citation7).
There is, however, a lack of information in the literature describing SS following a single, prescribed dose of an SSRI. Gill et al. described an incident of SS after ingesting a single dose of fluvoxamine 50 mg in an 11-year-old boy with a history of attention-deficit disorder. One hour after ingestion he presented with symptoms of increasing agitation, tremor, and unresponsiveness to stimuli. In the emergency department, four hours after ingestion, he presented with blood pressure as high as 160/90 mm Hg, heart rate up to 190 bpm with jaw myoclonus and shivering. This patient was not treated with cyproheptadine, but with benzodiazepines, intravenous thiamine, and sodium bicarbonate. The patient was stabilized within 48 hours and was transferred to the inpatient psychiatry service for continued care. Of interest, the recommended starting dose of fluvoxamine is 25 mg daily with adjustments of 25 mg at 7 to 14 day intervals. The patient was also taking perphenazine and benztropine concurrently as well as valproic acid which had been discontinued one week prior to starting fluvoxamine. Also noteworthy, the authors reported the probability or definite causality of SS due to fluvoxamine as “unclear” (Citation8). Another case involved a 16-year-old female who presented with restlessness, tremor, and anxiety soon after a single dose of sertraline 100 mg. Upon admission to the emergency department four hours after ingestion, she had sinus tachycardia (105–120 bpm), blood pressure of 154/80 mm Hg, tremors, and increased motor tone. No previous medication history was discussed. She was treated with cyproheptadine (4 mg) and acetaminophen (650 mg) and was discharged home four hours after arrival (Citation9).
Our 9-year-old child had symptoms of stomach pain, delirium, and aggression within 30 minutes of ingestion, which was similar in timeframe as the previously described single, prescribed dose cases. No dose per body weight (mg/kg/dose) was described in the previous single, prescribed dose cases; however, all of them, including our patient, were prescribed a higher initial dose than those previously used in the literature for their age ranges (Citation12,Citation13). Severity of symptoms varied among the cases, ranging from moderate tremor and increased tone to continued hypertonicity resulting in rhabdomyolysis, with or without hyperthermia, and changes in mental status.
Treatment also varied among the reported cases. Some patients were given agents such as physostigmine or intravenous thiamine whereas others, including our patient, were given cyproheptadine. Cyproheptadine, an antihistamine, has antagonistic properties against serotonin (5-HT2A). This action combined with anticholinergic effects appears to help treat symptoms of SS such as diarrhea (Citation2,Citation7). Dose of cyproheptadine ranged from adult dose (4 mg) as seen used in a 16-year old female to that of our dose of 2.5 mg by mouth every 8 hours (0.24 mg/kg/day). The treatment of SS with cyproheptadine is based on anecdote and experience, since clinical trials are not available. Recommended adult dosing includes an initial dose of 12 mg followed by 2 mg every two hours during symptoms. Pediatric dosing advice has been published, with a recommended dose of 0.25 mg/kg/day, divided into two to three doses but this regimen does not begin with a large loading dose which has been suggested as necessary for treatment of SS in adults; therefore we started treatment with an 8 mg dose in our case (Citation2,Citation14,Citation15). In addition to cyproheptadine, benzodiazepines were also used among other cases, including our patient, to reduce hyperflexia and tremor (Citation8,Citation11).
SS has previously been attributed to overdoses of SSRIs, tricyclic antidepressants, MAOIs, etc. or combinations thereof. Our experience suggests it should be considered a possible adverse effect of a single, prescribed dose of such medications. Although rare with the use of a single drug, at a prescribed dose, this adverse effect can lead to critical illness requiring emergency department admissions and even hospitalization in the intensive care unit among children. Health care professionals should be aware of this possible adverse effect and its treatment with various medications. Our findings are important as the use of antidepressants in particular, SSRIs, is on the rise among children with depression and other mental illnesses.
References
- CJ Whittington, T Kendall, P Fonagy, D Cottrell, A Cotgrove, and E Boddington. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004; 363:1341–5.
- EW Boyer, and M Shannon. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
- M Brvat, D Stajer, G Kozelj, J Osredkar, M Mozina, and M Bunc. Urinary serotonin level is associated with serotonin syndrome after moclobemide, sertraline, and citalopram overdose. J Toxicol Clin Toxicol 2007; 45:458–460.
- M Ganetsky, and E Brush. Serotonin syndrome-what have we learned?. Clin Pediatr Emerg Med 2005; 6:103–108.
- DO Lee, and CD Lee. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999; 19:894–6.
- M Pao, and T Tipnis. Serotonin syndrome after sertraline overdose in a 5-year-old child. Arch Pediatr Adolec Med 1997; 151:1064–7.
- BZ Horowitz, and ME Mullins. Cyproheptadine for serotonin syndrome in an accidental pediatric sertraline ingestion. Pediatr Emerg Care 1999; 15:325–7.
- M Gill, F LoVecchio, and B Selden. Serotonin syndrome in a child after a single dose of fluvoxamine. Ann Emerg Med 1999; 33:457–9.
- ME Mullins, and BZ Horowitz. Serotonin syndrome after a single dose of fluvoxamine. Ann Emerg Med 1999; 34:806–7.
- CA Naranjo, U Busto, EM Sellers, P Sandor, I Ruiz, EA Roberts, E Janecek, C Domecq, and DJ Greenblatt. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45.
- CA Kaminski, MS Robbins, and RE Weibley. Sertraline intoxication in a child. Ann Emerg Med 1994; 23:1371–1374.
- SM Cheer, and DP Figgitt. Fluvoxamine: a review of its therapeutic potential of anxiety disorders in children and adolescents. Paediatr Drugs 2001; 3:763–81.
- Product information, package insert: Zoloft® (sertraline hydrochloride). New York: Pfizer Inc., 2007.
- BA Spirko, and JF Wiley. Serotonin syndrome: a new pediatric intoxication. Pediatr Emerg Care 1999; 15:440–443.
- A Graudins, A Strearman, and B Chan. Treatment of serotonin syndrome with cyproheptadine. J Emerg Med 1998; 615–9.