Abstract
Introduction. There is little knowledge within the medical community of the existence of veterinary antibiotics in oil-based suspensions and the adverse effects that may occur with accidental human injection. Case report. A farmer injected an unknown quantity of Excenel RTU™ into her right thigh. Despite early debridement she developed a deep infection and recurrent chronic inflammation in the subcutaneous tissues and muscle secondary to the cottonseed oil suspension. Radical debridement and extensive split skin grafting was required but she still has had recurrences 12 months after injury. Discussion. Prompt surgical debridement should be performed as in cases of oil based veterinary vaccines. Despite being an antibiotic there is a significant risk of infection from a dirty needle following inoculation and multiple cultures should be taken and appropriate broad spectrum antibiotics used. Radical debridement and skin grafting necessitating specialist plastic surgical attention may be required.
Introduction
Veterinary antibiotics in suspensions of vegetable oil such as cottonseed oil are in common use around the world. This allows the antibiotic to be pre-mixed making it suitable for administration by the farmer when treating conditions such as footrot in large herds of cattle. There have been several reports of accidental human inoculation of oil-based veterinary vaccines that have required surgical debridement (Citation1–3). There is little knowledge within the medical community of the existence of oil suspensions of antibiotics and the adverse effects that may occur with accidental human injection.
Case report
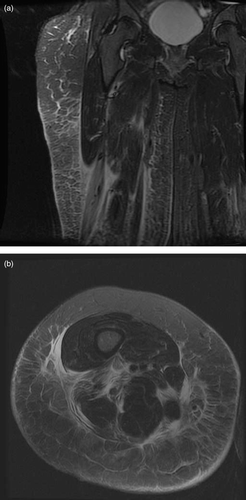
A 35 year old farmer injected an unknown quantity of Excenel™ RTU (ceftiofur hydrochloride, Pfizer Animal Health, Auckland, New Zealand) into the lateral aspect of her right thigh whilst working in a dairy shed. The syringe had originally contained 15 mL of the drug and the needle was contaminated with farmyard material. She presented 24 hours later with inflammation around the injection site and underwent formal debridement of the entry tract and soft tissue under general anaesthetic. Intravenous amoxicillin-clavulanic acid was commenced. Despite this over the next two days she developed further swelling and an area of necrosis in the subcutaneous fat. The wound was re-debrided and inflamed muscle found deep to the fascia lata. An MRI scan () showed changes extending down to the knee involving the deep fascia and lateral hamstring muscles. Over the next four days she developed areas of necrosis in the biceps femoris muscle and had further extensive debridement of muscle, skin and fat. A variety of organisms were cultured including Bacillus cereus, Corynebacterium, rhizobium radiobacter and Candida utilis. Blood tests showed a peak in creatinine kinase of 169 U/L at day four associated with a peak C-reactive protein of 345 mg/L. The hemoglobin fell from 120 g/L on admission to 75 g/L by day five, which appeared to be due to operative blood loss. There was no sign of hemolysis but a subsequent endoscopy revealed some gastric erosions. Liver function tests showed minor increases in alkaline phosphatase (135 U/L; reference 30 to 120 U/L) and gamma glutamyl transferase (75 U/L; reference 0 to 30 U/L).
Fig. 1a. and 1b. Coronal STIR and Axial T2 fat saturated MRI images through the thigh showing increased signal involving the subcutaneous fat (cellulitis), superficial muscle fascia (fasciitis) and biceps femoris muscle (myositis) and also extension of the signal to the neurovascular bundle.
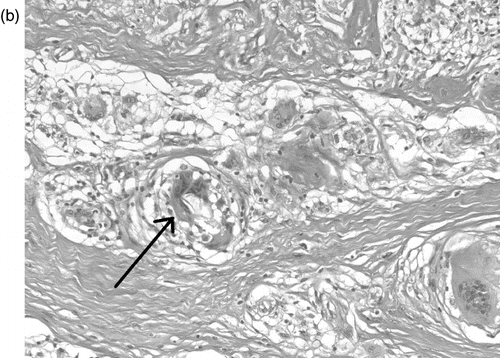
Histological examination initially showed a neutrophil infiltrate, fat necrosis with predominantly septal inflammation, and acutely inflamed, focally necrotic skeletal muscle, soft tissue and adipose tissue. Subsequently a chronic inflammatory infiltrate developed extending throughout the fibrofatty connective tissue. Non-necrotising granulomatous inflammation with giant cell formation developed, in which the centres of the larger granulomata contained foamy epithelioid macrophages, typical of a reaction to lipid material (). The giant cells contained fragments of foreign material and empty spaces where material had washed out during tissue processing ().
Fig. 2a. H&E, 100x magnification. A large granuloma within collagenous tissue contains a central area of foamy epitheliod macrophages and empty lipid-like spaces (arrow), surrounded by a rim of multinucleate giant cells admixed with epithelioid cells and lymphocytes.
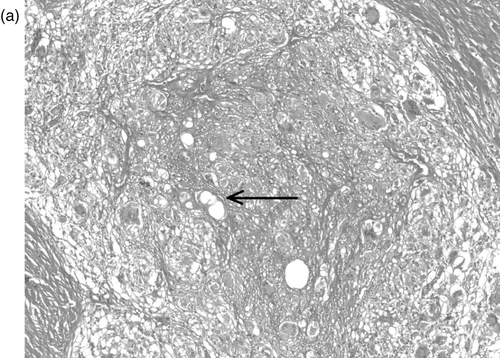
Fig. 2b. H&E, 200x magnification. A multinucleate giant cell contains polarisable foreign material (arrow) amongst the non-necrotising granulomata.
The wound was closed secondarily when clean and she was discharged on flucloxacillin, metrionidazole, and ciprofloxacin. Three weeks from injury, however, the wound broke down and she required further debridement.
The lateral wound was split skin grafted directly onto muscle which took successfully. However, over the next 3 months she had multiple episodes where she developed erythema and induration in the fat and soft tissues extending down to the popliteal fossa. Traditional management of drainage debridement, and vacuum dressings failed to prevent recurrent areas of inflammation and fat necrosis developing (). A variety of organisms were cultured but many of the abscesses were sterile
Fig. 3. Clinical photograph of lateral aspect right thigh prior to wide excision following initial skin grafting and multiple debridements. There is extensive induration extending through the popliteal fossa.

An opinion was sought from a Plastic Surgeon who performed an extensive wide debridement of the right lateral thigh measuring 20 × 20cm down to and through the deep fascia in places. Subsequent treatment was with a suction dressing and split skin grafting. She required a further wide debridement (21 × 12 cm) and skin grafting of the posterior thigh for recurrent inflammation and abscess formation 5 months later. At one year following injury she continues to have recurrent problems.
Discussion
Excenel ™ (Ceftiofur hydrochloride, Pfizer Animal Health, Auckland, New Zealand) is a cephalosporin used in veterinary practice and is commonly used for the treatment of foot rot in cattle (Citation4). It is available in two preparations: a powder which can be made up in an aqueous solution and a pre-mixed cottonseed oil based suspension. This formulation, Excenel™ RTU (ready to use) is stable for longer periods of time and can be administered by farmers. The oil suspension is quite viscous and hard to inject. It is usually given intramuscularly or subcutaneously into the anterior half of the neck in cattle to avoid damage to move valuable parts of the carcass. Some local reaction can occur. Excenel™ RTU has a concentration of 50 mg/mL with a recommended dose of 2 mL/100 kg. In this case a potential dose of 15 mL sufficient for a 750 kg cow was given. There do not appear to have been any systemic effects from this dose, with no hepatic or renal problems identified. In contrast, acute rhabdomyolysis, renal failure, and death has been reported following human ingestion of monensin, a polyether ionophore veterinary antibiotic (Citation5). In our patient the rise in creatinine kinase was short lived and consistent with the degree of local muscle necrosis.
Despite early surgical debridement the suspension spread through the soft tissues and muscle of the posterior thigh and into the popliteal fossa region. While there was a superimposed infection with various farmyard organisms, it is important to note that the clinical course did not follow the typical one of abscess formation, drainage, and subsequent healing. Frequently sterile abscesses were found with liquefied fat and indurated skin with a tendency for areas of inflamed skin to recurrently flare-up over a period of months. A wide excision of all contaminated tissue was required with extensive skin grafting. Despite this, further recurrences of sterile abscesses and chronic granulomatous inflammation have occurred.
There have been a number of reports of inadvertent inoculation with oil based veterinary vaccines (Citation1–3). We have previously reported a case where injection of footrot vaccine resulted in extensive sterile abscess and granuloma formation within the quadriceps muscle requiring extensive debridement of that muscle (Citation2). More recently a case of injection into the shin with Gudair (Ovine Johne's Disease) vaccine was reported that required skin grafting of a necrotic area of approximately 2 cm in diameter (Citation3). The adjuvant mineral oil appears to be the main cause of the inflammation in these cases rather than a live or attenuated vaccine. It is well recognised that prompt surgical attention is required following inadvertent self inoculation with these vaccines and this advice is available at local Poison Centers and published by the manufacturers in their data sheets (Citation6).
Little information, however, is available for oil suspensions of veterinary antibiotics. The New Zealand National Poisons Centre has no reports of adverse reactions with these formulations. We believe that the large volume of the antibiotic and cottonseed oil injected in this case led to the extensive necrosis and chronic inflammation. Prompt surgical debridement should be performed as in cases of oil based vaccines. Despite being an antibiotic there is a significant risk of infection from a dirty needle following inoculation and multiple cultures should be taken and appropriate broad spectrum antibiotics used. Radical debridement and skin grafting necessitating specialist plastic surgical attention may be required in cases such as this when repeated flare-ups occur.
References
- CJ Patterson, M La Venture, SS Hurley, and JP Davis. Accidental self inoculation with Mycobacterium paratuberculosis bacterin (Johne's bacterin) by veterinarians in Wisconsin. J Am Vet Med Assoc 1988; 192:1197–1199.
- DPG Jones. Accidental self-inoculation with oil based veterinary vaccines. NZ Med J 1996; 109:363–365.
- GD Richardson, II Links, and PA Windsor. Gudair (OJD) vaccine self-inoculation: a case for early debridement. MJA 2005; 183 (3):151–152.
- Pfizer Animal Health. 2006. Excenel RTU [safety data sheet]
- C Caldeira, WS Neves, PM Cury, P Serrano, MASF Baptista, and EA Burdmann. Rhabdomyolysis, acute renal failure and death after monensin ingestion. AJKD 2001; 38 (5):1108–1112.
- Pfizer Animal Health. 2007. Gudair vaccine: Information for medical practitioners in case of accidental administration to humans [fact sheet].
