Abstract
Introduction. Intravenous injection of mercury has seldom been reported, especially in cases of attempted suicide, and is associated with variable clinical outcomes. Case report. A young woman came to our attention after self-injecting and ingesting mercury drawn from 37 thermometers. The patient suffered lung embolization complicated by adult respiratory distress syndrome (ARDS), toxic dermatitis, anemia, mild hepato-renal impairment, and died after 30 days. Mercury was monitored in biological fluids (blood, plasma, urine, and bronchoalveolar fluid) to study its toxicokinetics and to evaluate dose–effect relationships. Its urinary clearance significantly increased after a chelation challenge test with meso-2,3-dimercaptosuccinic acid (DMSA) (median values of 2.48 and 8.85 before and after the test, respectively, p < 0.05). Conclusions. Mercury poisoning by intravenous injection is a clinical emergency, potentially leading to death. When injected, the element has a very slow clearance, mainly renal. Our data do not allow any conclusion about the effectiveness of chelation therapy.
Keywords:
Introduction
Mercury (Hg) is a toxic heavy metal that exists in three oxidation states: metallic elemental (Hg0), monovalent (mercurous, Hg+), and divalent (mercuric, Hg++) cations, the latter playing cytotoxic effects upon covalent binding to the sulfhydryl groups of proteins (Citation1). Inhaled Hg0 vapors readily cross biological membranes (Citation2) and may give rise to either acute or chronic neurotoxic, pneumotoxic (especially for high-dose acute exposures), or nephrotoxic effects (Citation3). Liquid Hg0 may enter the human body after accidental or deliberate intravenous (i.v.), intra-arterial (Citation4), or subcutaneous (Citation5) inoculation. Accidental injection happened as complication of either Hg-sealed syringes for arterial blood drawing (Citation6,Citation7) or contaminated venous blood supplies (Citation8). Deliberate i.v. injection occurred in suicidal (Citation9–28) as well as homicidal attempts (Citation29), in drug and alcohol addicts (Citation30–36), and in athletes wishing to improve their performances (Citation37–39). Injected Hg0 causes few signs of mercurialism and infrequent renal involvement (Citation40). Pneumotoxic effects dominate the clinical picture, diagnosis relying on the demonstration of mercury deposits at both the injection site and the pulmonary vessels (Citation41). We describe the toxicokinetic and toxicodynamic outcomes of a massive Hg0 poisoning by i.v. injection and ingestion.
Case report
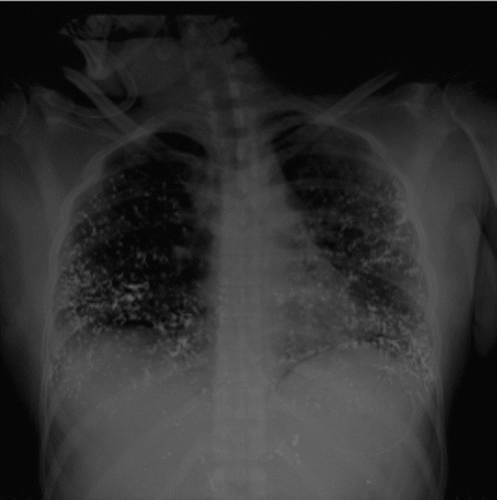
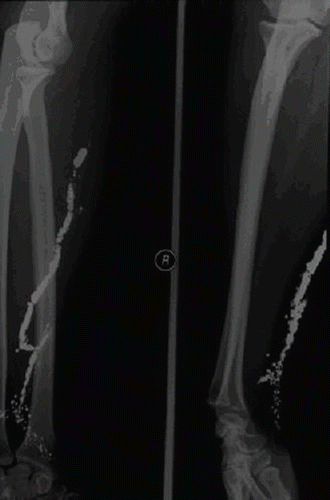
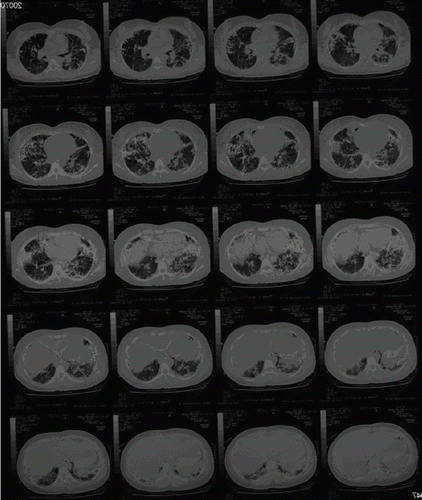
A 30-year-old woman was found in a hotel room where 37 broken fever thermometers and two empty syringes were also found. She was confused and denied i.v. drug abuse, but a needle puncture mark and a 5 × 3 cm erythematous, warm, and swollen area were apparent on the left forearm. After gastrointestinal decontamination (gastric lavage, activated charcoal, and cathartics) and anti-thrombosis prophylaxis (low-molecular weight heparin), the patient was admitted to the intensive care unit for respiratory support – endotracheal intubation and assisted ventilation (FiO2 about 50%). The past medical history included depression and previous suicidal attempts. The general physical examination showed mental confusion and agitation; blood pressure 110/80 mmHg; temperature 39°C; pulse rate 128 beats/min; respiration short, shallow, and frequent; bilaterally decreased breath sounds; and chest pain exacerbated by cough and deep ventilation. summarizes the main laboratory changes observed throughout the hospitalization period. Anemia (hemoglobinemia <7.1 g/dL) required blood transfusion on the 11th day. We also found raised C-reactive protein (C-RP) (30 mg/L, reference <10 mg/L) and γ-glutamyl transpeptidase (γ GT) levels (150 U/L, reference <65 U/L). Serum pseudocholinesterases, measured to monitor liver function, were consistently lower than the reference values (4500 U/L). Renal function was slightly impaired at admission (creatinine clearance = 73 mL/min), but increased plasma creatinine and urea values were recorded in the 4th week [1.5 (reference 0.6 to 1.3) and 90 (reference 10 to 50) mg/dL, respectively]. After stool release from the vagina, a gynecologist diagnosed a recto-vaginal fistula on the 3rd day that spontaneously healed by day 15. In the 2nd week, a diffuse erythema was apparent, and a dermatologist posed the diagnosis of mercurial toxic dermatitis. Standard chest X-ray on admission () showed scattered micronodular high-density opacities throughout the pulmonary vessels with an arborizing pattern. From the 8th day onward, bilateral progressively expanding alveolar-interstitial infiltrates were apparent. A radiograph of the left upper limb showed metallic opacities following the superficial forearm veins (). On the 11th day, a total body high-resolution computed tomography (HRCT) () revealed multiple hyperdense foci clustered in the periphery of both lungs, more so in the lower zones; bilateral diffuse alveolar infiltrates, sparing only the apical segment of the lower left lobe; and right pneumothorax and pneumomediastinum. Metallic deposits were apparent also in the right ventricular apex, in the portal vein, bilaterally in the urinary tracts, in the gastric fundus, and in the distal tract of right colon and rectum, but not in the brain.
Table 1. Distribution of laboratory parameters evaluated for the clinical assessment of the patient
The electrocardiogram on admission showed sinus tachycardia (128 beats/min) and unspecific repolarization anomalies (negative T waves in V1 to V4). Repeated examinations showed pulmonary hypertension (up to 46 mmHg) and less than 1 mm hyperechogenic images in the papillary muscles of the left ventricle.
The clinical course worsened because of iatrogenic complications (bilateral pneumothorax and tracheal-esophageal fistula) and the patient died on the 30th day.
Methods
We monitored Hg concentrations in blood and urine (HgB and HgU) from admission until the 19th day. Hg was determined also in plasma (HgP, days 1–3 and 8–19) and in the bronchoalveolar fluid drained from the endotracheal tube (HgBF) (days 3–19). On the 14th day, we performed a chelation challenge test with 300 mg of meso-2,3-dimercaptosuccinic acid (succimer, DMSA), in three equal doses every 8 h, and strictly monitored HgB, HgP, and HgU, after each dose. Hg was determined by inductively coupled plasma mass spectrometry (ICP-MS) using an ELAN DRC II instrument (Perkin Elmer, Sciex, Canada). Accuracy was checked by reference solutions (NIST 1640 trace element in water and 8AB occupational G-EQUAS for blood and urine). The detection limits and the coefficients of variability (CV%) for the different matrices were 0.1 μg/L and 5% for HgB and HgP, 0.001 μg/L and 5% for HgBF, and 0.05 μg/L and 4% for HgU, respectively. We used SPSS 15.0 for Windows for statistical analyses and Origin 7.5 for graphs. Parametric (Student's t test; Pearson's correlation analysis) and non-parametric (Mann Whitney U test) statistical tests were applied, according to the variables' distributions, assessed by the Kolmogorov Smirnov test. The cumulative Hg excretion by urine and BF was calculated as a cumulative sum extrapolated by the concentrations of Hg in the two fluids multiplied by the total volume, respectively. To fit data, we used a double Boltzmann's sigmoidal function with the general equation:
The t1/2 value for every term of the equation is calculable. In our case, the second term of the equation was unsaturated, so the t1/2 value was calculable only for the first term. The Hg urinary (or BF) clearance was calculated by the formula: HgU (or HgBF) (μg/L) × urine (or BF) flow rate (mL/min)/HgB (μg/L).
Results
displays the Hg levels in different matrices, over time. HgU (A) and HgB (C) largely exceeded the reference values (4.5 μg/L for both) for the Italian population (Citation42). The HgU concentrations [mean ± standard deviation: 2509.82 ± 2002.14 μg/L (range 422.50–7964.00 μg/L)] were about three times as high as the HgB ones [863.71 ± 218.03 (366–1202 μg/L)]. HgBF (B) was, on average, about 7% of HgU [145.61 ± 64.00 μg/L (50–240 μg/L)]. In the first week, both erythrocyte Hg (E) and HgP (D) progressively increased, but opposite trends were apparent thereafter. After DMSA, we observed a rise of HgU [4481.50 ± 2443.78 vs. 1664.82 ± 999.27 μg/L (before DMSA), p < 0.05] and a decrease of HgP levels [886.80 ± 300.18 vs. 1229.75 ± 293.89 μg/L (before DMSA), p < 0.05].
Fig. 4. Profiles of mercury (Hg) concentrations in different sampled matrices [(A) urine; (B) bronchoalveolar fluid (BF); (C) blood; (D) plasma; (E) red blood cells (RBC)] throughout the biomonitoring period (days 1–19) in hours (h). The hatched bar indicates the 24-h chelation challenge test.
![Fig. 4. Profiles of mercury (Hg) concentrations in different sampled matrices [(A) urine; (B) bronchoalveolar fluid (BF); (C) blood; (D) plasma; (E) red blood cells (RBC)] throughout the biomonitoring period (days 1–19) in hours (h). The hatched bar indicates the 24-h chelation challenge test.](/cms/asset/b99ab45d-4444-48c1-8dc5-f64a591a15f5/ictx_a_313790_f0004_b.gif)
The urinary Hg clearance ranged from 0.88 to 11.68 mL/min and increased after DMSA [medians of 8.85 vs. 2.48 (before DMSA) mL/min, p < 0.05)]. The bronchoalveolar Hg clearance showed a mean value of 0.12 ± 0.06 mL/min (range 0.04–0.27).
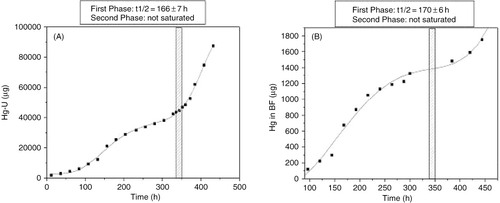
shows the cumulative HgU and HgBF excretion kinetics, respectively. In both cases, a biphasic kinetic was apparent, with similar t1/2 values for the first phases (166 ± 7 h and 170 ± 6 h, respectively), whereas the second phases, beginning after DMSA, were still in exponential growth when samplings ceased. Only HgB and HgP values were correlated (r = 0.94, p < 0.0001); no significant relationship was apparent among Hg levels in different matrices and any laboratory parameter.
Discussion
The overall Hg0 intake assumed by our patient (about 500 g) is one of the largest reported in similar cases (Citation9–28). Fatal outcomes are rather uncommon after i.v. mercury injections; acute or subacute complications generally arise, sometimes lasting several years after the injection (Citation15,Citation24,Citation43). A man survived the acute phase (Citation15) after i.v. injection of about 40 mL of Hg0, whereas death occurred in a woman who injected as little as 1–2 mL (Citation16), usually not a life-threatening amount (Citation10,Citation30). Apart from ethanol (Citation3), no other acquired or genetic interfering factor is currently known.
Gastrointestinal HRCT scans demonstrated that an undetermined amount of Hg0 was ingested. Its relative contribution to the clinical picture was probably marginal, as less than 0.1% of Hg0 is absorbed through an intact gastrointestinal mucosa (Citation44–46). Rather, the recto-vaginal fistula was a probable complication of the passage of the element through the enteric tract (Citation47).
After i.v. injection, Hg0 coalesces into microemboli that, given the low solubility and chemical stability of the element, may last for years (Citation43,Citation48,Citation49). They occur at the injection site and then distribute to the right ventricle and the lung vessels. We found metallic deposits along the superficial veins of the forearm, with a retrograde pattern of deposition, probably due to both the higher specific gravity of the element compared to blood and the position of the forearm during the injection. Chest X-rays showed a typical pattern of hyperdense bilaterally scattered spherules, mostly in the lung periphery and following pulmonary vasculature (Citation41). Hg droplets may give rise to aseptic abscesses (Citation32), causing pleuritic chest pain and dyspnea in about 50% of cases (Citation21), usually in association with fever, leukocytosis, and raising of acute phase reactants (C-RP, fibrinogen) (Citation13), as recorded in our patient. Fibrosis surrounding Hg globules (Citation50) may give rise to transient or persisting (Citation43) lung-restrictive and/or interstitial alterations (Citation36,Citation37,Citation40,Citation51). Our patient developed an adult respiratory distress syndrome (ARDS) that, to our knowledge, has not been described. Metallic spots evidenced by HCRT in the right ventricular apex confirmed the i.v. route, whereas those in the left ventricle and bilaterally in the renal pelvis suggested the systemic arterial embolization and renal excretion (Citation32). Cardiac Hg deposits inducing inflammatory infiltrates with necrosis of myocardial fibers (Citation16,Citation17) and benign granulomatous fibrosis (Citation48) may be responsible for the ST changes (Citation10), as those we observed in our patient. In agreement with previous reports (Citation52), we found mild hepato-toxic effects. Although kidney is the second deposit organ after i.v. Hg0 injection (Citation16) and tubular epithelium is highly vulnerable to mercuric salts (Citation53); nephrotoxic effects, ranging from transient (Citation14) to progressive renal failure (Citation16), are uncommon. We observed a slight impairment of renal function in the last week only. Our patient may have suffered a mild toxic dermatitis. The cutaneous effects of mercury (Citation52,Citation54) may be related to the role of skin as minor excretion route (Citation30).
Typical mercurialism signs are not usually present in the early phases after i.v. Hg0 injection, due to its slow diffusion into the nervous system (Citation16,Citation55). In our case, HCRT excluded cerebral metal deposit and neurotoxic effects were lacking.
In the first week, circulating Hg accumulated in the erythrocytes, but thereafter tended to prevail in plasma, passing, on average, from 94.1 to 121.1% of HgB (p < 0.005). This could be due to the oxidation of Hg0 by the erythrocyte peroxide-catalase pathway into more soluble cationic species (Citation56), having also higher affinity for plasmatic proteins. Alternatively, it also could be ascribed to hemolysis possibly due to inhibition of anti-oxidant erythrocyte enzymes, production of reactive oxygen species by Hg++–hemoglobin complexes, and disruption of the cytoskeleton (Citation57). The progressive anemia with concomitant rise of serum bilirubin and lactate dehydrogenase levels supports this hypothesis.
We calculated urinary Hg clearance values similar to previously reported (Citation30) and consistent with high HgU levels lasting several years after i.v. Hg0 injection (Citation43). Oxidation of Hg0 into cations, whose salts are excreted by kidneys, colon, and salivary glands, is a slow process (Citation58). The total amount of Hg eliminated through the BF was about 1.8 mg (about 95 μg/day), a lower figure than previously reported (about 228 μg/day) for exhaled Hg0 vapors (Citation30) that were probably lost by our collection system. The very low HgBF levels and the delay in the time course profile compared to HgB and HgU (showing parallel profiles, because of fast exchanges between blood and urine; ) seem to indicate that the BF is a minor excretion route for Hg. We have recently demonstrated that levels of pneumotoxic metals in exhaled breath condensate, sharing some commonality with BF, are promising markers of effective dose at the target organ in exposed workers (Citation59).
Different chelating agents, including penicillamine, dimercaprol, 2,3-dimercaptopropane-1-sulphonate (DMPS), and DMSA, have been administered in cases of i.v. Hg injection (Citation10). The beneficial role of these molecules relies on covalent binding and removal of Hg cations from sensitive tissues in critical organs (Citation60,Citation61). The efficacy of these therapies on the disappearance of Hg0 deposits would be negligible (Citation23,Citation44). After DMSA administration, we observed a rise in Hg urinary excretion (and a parallel decrease of HgP), higher than previously reported (Citation10), but our results are inadequate to draw any definitive conclusion.
Conclusions
I.v. Hg poisoning is a serious clinical emergency, potentially leading to death. The main excretion route is urinary with a kinetic picture that is at least biphasic.
Acknowledgements
The authors thank Dr. G. Marchitelli for his skilled comments to X-ray and HRCT films.
References
- WL Hughes. A physicochemical rationale for the biological activity of mercury and its compounds. Ann N Y Acad Sci 1957; 65:454–460.
- L Magos, and TW Clarkson. Overview of the clinical toxicity of mercury. Ann Clin Biochem 2006; 43:257–268.
- JB Hursh. Partition coefficients of mercury (203 Hg) vapor between air and biological fluids. J Appl Toxicol 1985; 5:327–332.
- NC Cowan, P Kane, and J Karani. Case report: metallic mercury embolism-deliberate self-injection. Clin Radiol 1992; 46:357–358.
- EH Kayias, GI Drosos, D Hapsas, and GA Anagnostopoulou. Elemental mercury-induced subcutaneous granuloma. A case report and review of the literature. Acta Orthop Belg 2003; 69:280–284.
- RH Gadsden, GB Bradham, JT BuxtonJr, and JC Hewitt. Metallic mercury embolism: report of cases. J Am Med Assoc 1965; 193:573–575.
- HB Devlin, and M Sudlow. Peripheral mercury embolization occurring during arterial blood sampling. Br Med J 1967; 1:347–348.
- E Schulz, and H Beskind. Systemic deposition of metallic mercury. J Pediatr 1960; 57:733–737.
- R Chitkara, NS Seriff, and HY Kinas. Intravenous self-administration of metallic mercury in attempted suicide. Chest 1978; 73:234–236.
- F Eyer, N Felgenhauer, R Pfab, G Drasch, and T Zilker. Neither DMPS nor DMSA is effective in quantitative elimination of elemental mercury after intentional IV injection. Clin Toxicol (Phila) 2006; 44:395–397.
- RJ Giombetti, DH Rosen, AR Kuczmierczyk, and DO Marsh. Repeated suicide attempts by the intravenous injection of elemental mercury. Int J Psychiatry Med 1988; 18:153–167.
- M Díaz-Sánchez, FJ Martínez-Lagares, WR Castaned, A Givica Pérez, and JM Santana Montesdeoca. Deliberate, repeated self-administration of metallic mercury injection: case report and review of the literature. Eur Radiol 2001; 11:1351–1354.
- JW Gulden, F Christ, E Hauser, and HJ Kramer. Circulatory distribution of intravenously injected metallic mercury. Rontgenblatter 1987; 40:401–405.
- HT Haffner, J Erdelkamp, E Goller, F Schweinsberg, and V Schmidt. Morphological and toxicological findings after intravenous injection of metallic mercury. Dtsch Med Wochenschr 1991; 116:1342–1346.
- H Hohage, B Otte, G Westermann, J Witta, U Welling, W Zidek, and S Heidenreich. Elemental mercurial poisoning. South Med J 1997; 90:1033–1036.
- HRM Johnson, and O Koumides. Unusual case of mercury poisoning. Br Med J 1967; 1:340–341.
- A Kedziora, and J Duflou. Attempted suicide by intravenous injection of mercury: a rare cause of cardiac granulomas. A case report. Am J Forensic Med Pathol 1995; 16:172–176.
- V Maniatis, G Zois, and K Stringaris. IV mercury self-injection: CT imaging. AJR Am J Roentgenol 1997; 169:1197–1198.
- RB McFee, and TR Caraccio. Intravenous mercury injection and ingestion: clinical manifestations and management. J Toxicol Clin Toxicol 2001; 39:733–738.
- P Nalepa, T Lech, and W Kotucha. Intravenous injection of metallic mercury: case report. Pneumonol Alergol Pol 1996; 64:88–92.
- C Papadopoulos, N Vasile, JP Richard, and JL Renard. Mercury pulmonary embolism. Two case reports. Rev Pneumol Clin 1999; 55:43–46.
- N Peterson, W Harvey-Smith, and CA Rohrmann. Radiographic aspects of metallic mercury embolism. AJR Am J Roentgenol 1980; 135:1079–1081.
- IM Rodrigues, ND Hopkinson, and RI Harris. Pulmonary embolism associated with self-administration of mercury. Hum Toxicol 1986; 5:287–289.
- L Garza-Ocanas, A Pineyro-Lopez, and O Torres Alanis. Intravenous self-administration of metallic mercury: report of a case with a 5-year follow-up. J Toxicol Clin Toxicol 1997; 35:83–87.
- F Umber. Quecksilber-Embolien der lebenden Lunge durch intravenose Injektion von metallischem Quecksilber. Med Klin 1923; 19:35.
- E Walter. Long-term observation of a case of intravenous injection of elemental mercury. Radiologe 1986; 26:40–46.
- G Wedekind, and D Beyer. Multiple microembolization caused by elemental mercury. Radiologe 1994; 34:483–486.
- R Winker, AW Schaffer, C Konnaris, A Barth, P Giovanoli, W Osterode, HW Rudiger, and C Wolf. Health consequences of an intravenous injection of metallic mercury. Int Arch Occup Env Health 2002; 75:581–586.
- A Kumar, R Jain, S Sawhney, AK Goel, and K Chattopadhyay. Intravenous administration of metallic mercury with homicidal intent. J Assoc Physicians India 1992; 40:640–641.
- JJ Ambre, MJ Welch, and CW Svare. Intravenous elemental mercury injection: blood levels and excretion of mercury. Ann Intern Med 1977; 87:451–453.
- Z Chodorowski, and JS Anand. Intravenous self-administration of metallic mercury by two alcohol abusers. Przegl Lek 2000; 57:585–587.
- ME Conrad, JP Sanford, and JA Preston. Metallic mercury embolization; clinical and experimental. Arch Intern Med 1957; 100:59–65.
- MA De Ruggieri, E Pampiglione, B Annicchiarico Petruzzelli, and A Aurizi. A case of embolism caused by metallic mercury in a drug addict. Ann Ig 1989; 1:673–678.
- E Naidich, D Bartelt, P Wheeler, and W Stern. Metallic mercury emboli. Am J Roentgenol Radium Ther Nucl Med 1973; 117:886–891.
- H Ochs, I Boldt, W Messerschmidt, and U Boldt. Intravenous injection of thermometer mercury. Munch Med Wochenschr 1975; 117:1117–1120.
- MG Stahl, HW Bonekat, and JW Shigeoka. Concomitant pulmonary thromboembolism and metallic mercury embolism. Chest 1985; 88:787–789.
- B Celli, and MA Khan. Mercury embolization of the lung. New Engl J Med 1976; 295:883–885.
- Z Chodorowski, and J Sein Anand. Repeated metallic mercury intravenous injection for doping. Przegl Lek 2002; 59:377–378.
- SV Manoukian, and NK Wenger. Mercury in the heart. Am J Cardiol 1991; 67:317–318.
- F Deschamps, C Strady, G Deslee, B Menciere-Faroy, and S Deschamps. Five years of follow-up after elemental mercury self-poisoning. Am J Forensic Med Pathol 2002; 23:170–172.
- SE Rossi, PC Goodman, and T Franquet. Nonthrombotic pulmonary emboli. AJR Am J Roentgenol 2000; 174:1499–1508.
- P Apostoli, A Colombi, M Buratti, G Elia, C Flore, P Carta, A Ibba, I Cortesi, A Mangili, and L Alessio. Evaluation of the dose of mercury in exposed and control subjects. Med Lav 2002; 93:159–175.
- M Dell'Omo, G Muzi, A Bernard, S Filiberto, RR Lauwerys, and G Abbritti. Long-term pulmonary and systemic toxicity following intravenous mercury injection. Arch Toxicol 1997; 72:59–62.
- JL Lin, and PS Lim. Massive oral ingestion of elemental mercury. J Toxicol Clin Toxicol 1993; 31:487–492.
- N Wright, WB Yeoman, and GF Carter. Massive oral ingestion of elemental mercury without poisoning. Lancet 1980; 1:206.
- SJ Balk. Resources for pediatricians. How do I answer questions from parents, patients, teachers, and others?. Pediatr Clin North Am 2001; 48:1099–1111.viii.
- M Berlin. Handbook of the Toxicology of Metals. L Friberg, GF Nordberg, and VB Vouk. Specific metals. Amsterdam/New York/Oxford: Elsevier; 1990: II387–445.
- P Davey, and M Benson. A young man with a heavy heart. Hearth 1999; 82:e11.
- T Konopka, P Nalepa, and E Rzepecka-Wozniak. Long-term survival after a suicidal intravenous injection of mercury. Arch Med Sadowej Kryminol 2006; 56:267–270.
- CR Larios, S Gonzalo Tropez, O Raudales, and C Barahona. Pulmonary hydrargyrosis. Bull Am Coll Physicians 1970; 9:22.
- BG Hanningan. Self-administration of metallic mercury by intravenous injection. Br Med J 1978; 2:933.
- KM Murray, and JC Hedgepeth. Intravenous self-administration of elemental mercury: efficacy of dimercaprol therapy. Drug Intell Clin Pharm 1988; 22:972–975.
- BA Fowler. Mechanisms of kidney cell injury from metals. Environ Health Perspect 1993; 100:57–63.
- EM Souza, ML Cintra, VG Melo, RJ Vieira, EM De Capitani, and FA Zambrone. Subcutaneous injection of elemental mercury with distant skin lesions. J Toxicol Clin Toxicol 2000; 38:441–443.
- NI McNeil, HC Issler, RE Olver, and OM Wrong. Domestic metallic mercury poisoning. Lancet 1984; 1:269–271.
- TW Clarkson. Mercury. J Am Coll Toxicol 1989; 8:1291–1296.
- L Zolla, G Lupidi, A Bellelli, and G Amiconi. Effect of mercuric ions on human erythrocytes. Relationships between hypotonic swelling and cell aggregation. Biochim Biophys Acta 1997; 1328:273–280.
- HB Gerstner, and JE Huff. Clinical toxicology of mercury. J Toxicol Environ Health 1977; 2:491–526.
- M Goldoni, S Catalani, G De Palma, P Manini, O Acampa, M Corradi, R Bergonzi, P Apostoli, and A Mutti. Exhaled breath condensate as a suitable matrix to assess lung dose and effects in workers exposed to cobalt and tungsten. Environ Health Perspect 2004; 112:1293–1298.
- J Aaseth, J Alexander, and N Raknerud. Treatment of mercuric chloride poisoning with dimercaptosuccinic acid and diuretics: preliminary studies. J Toxicol Clin Toxicol 1982; 19:173–186.
- S Suzuki, and N Ozaki. The protective effects of thiol-containing compounds on mercuric chloride-induced acute inhibition of enzymes from mouse kidney. Toxicology 1984; 29:207–220.