Abstract
Introduction. Sibutramine is an amphetamine-like drug used for its weight reducing effect. Sibutramine-induced acute coronary syndrome has rarely been reported. We report a case of myocardial infarction associated with the use of sibutramine. Case Report. A 37-year-old woman presented to an Emergency Department (ED) with intermittent retrosternal chest pain, nausea, and sweating for 3 days. She reported taking one sibutramine tablet each day for 3 days. Blood pressure was 128/89 mm Hg and pulse 66 beats/min. An electrocardiogram revealed ST elevation over the inferior leads and ST depression over leads AVR and V1, the other leads were normal. Serum troponin T was 0.65 μg/L, and sibutramine was identified in her urine. Echocardiography revealed mild hypokinesia over the inferior wall without evidence of acute aortic dissection. The ST segment changes resolved spontaneously within 24 h of cardiac care unit (CCU) admission, a coronary angiogram performed 1 week later was unremarkable, and echocardiography performed 4 weeks after the event showed normal resting regional wall motion. Discussion. Seventeen medications containing sibutramine as an active ingredient were registered in Hong Kong in 2007. Sibutramine was introduced in the United States in 1997 and in Australia, United Kingdom, and Italy in 2001. Hypertension, tachycardia, dry mouth, and headache are the most commonly reported adverse reactions. Cardiovascular toxicities include tachycardia, palpitation, hypertension, and tachyarrhythmia. Conclusions. We postulate that the myocardial infarction was the result of coronary vasospasm associated with the therapeutic use of sibutramine-containing slimming pills.
Introduction
Hong Kong is a city that integrates Chinese and western culture. Such atmosphere also extends to the health-seeking behavior of the local Hong Kong people. It is not uncommon for people here to seek formal or informal medical advice from mainland China. With the new era of healthy body image, people may use various modalities, including medications to help lose weight, and it is not uncommon for people to take medications from over the border for a such purpose.
We report a young woman who consumed medications from over the border for their slimming effects, which resulted in a potentially life-threatening condition, acute myocardial infarction.
Case report
A 37-year-old woman presented to an Accident and Emergency Department (ED) in Hong Kong in the middle of the night with intermittent retrosternal chest pain, nausea, and sweating for 3 days. The patient was not obese [body mass index (BMI) of 22], did not smoke, and was not known to have hypertension or diabetes. However, she did have depression and was taking fluoxetine 60 mg daily and an occasional diazepam. During the ED assessment, her initial vital signs included a blood pressure of 128/89 mmHg and peripheral pulse of 66 beats/min. An electrocardiogram revealed ST elevation over the inferior leads and ST depression over leads AVR and V1, the other leads were normal (). Her chest pain subsided in the ED and she was not given any thrombolytic agent. She was admitted to the cardiac care unit (CCU) for further management and close monitoring. She reported later to have taken some non-prescription herbal capsules 3 days prior this admission, which was bought in mainland China for weight reduction. She took three tablets, one tablet per day, in total before she developed the presenting symptoms.
Fig. 1. The electrocardiogram of the patient when she presented to the Accident and Emergency Department showing ST elevation over the inferior leads and ST depression over lead V1.
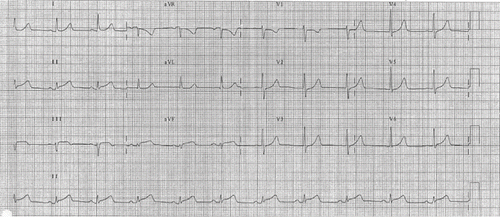
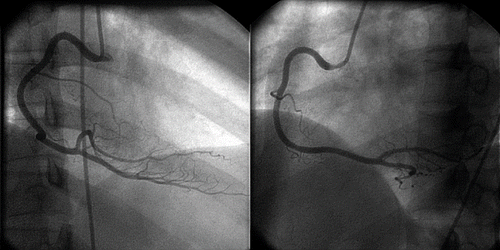
Investigations revealed an elevated serum troponin T, with a maximum level of 0.65 μg/L. Her lipid profiles were unremarkable except mildly elevated triglycerides. There was no evidence of autoimmune vasculitis. Echocardiography revealed mild hypokinesia over the inferior wall without evidence of acute aortic dissection. She received standard acute coronary syndrome care (aspirin, diltiazem, low-molecular weight heparin, and intravenous nitrates). The ST segment changes resolved spontaneously within 24 h of CCU admission. A coronary angiogram performed 1 week later was unremarkable (). A second echocardiography performed 4 weeks after the event showed normal resting regional wall motion.
Fig. 2. The coronary angiogram of the patient which was performed 1 week after her presentation showing normal right coronary artery.
Detailed history and routine laboratory urine drug screening did not identify use of cocaine, amphetamine, or amphetamine-like substances. However, sibutramine was identified in her urine sample by liquid chromatography mass spectrometry. The patient had no further chest pain after stopping the herbal slimming pills.
The diagnosis was acute coronary syndrome secondary to transient coronary spasm. Her acute coronary syndrome had a “probable” association with the use the sibutramine tablets, based on the Naranjo score of 5 points (Citation1).
Discussion
Obesity is a growing pandemic worldwide. Weight reduction pills are gaining growing acceptance in the era of self-image awareness. In Hong Kong, medications containing sibutramine, orlistat, phentermine, amfepramone, and acomplia are registered as oral weight reduction agents. However, it is not uncommon to encounter banned substances like fenfluramines and N-nitroso-fenfluramine marketed as proprietory Chinese medicine products from mainland.
In 2007, 17 medications containing sibutramine as an active ingredient were registered in Hong Kong. The effectiveness of sibutramine in weight reduction has been demonstrated in several randomized, double blind studies (Citation2,Citation3). The recommended doses are 10–15 mg/day for obese patients with BMI more than 30 without co-morbidities and for patients with BMI more than 27 with diabetes mellitus, dyslipidemia, or hypertension (Citation4).
Sibutramine was introduced in the United States in 1997 and in Australia, United Kingdom, and Italy in 2001. It was temporarily withdrawn in 2002 in Italy after 103 serious adverse reactions including two deaths that were reported in the United Kingdom (Citation5). There have been 397 serious adverse reactions reported to the FDA since it was first marketed in February 1998 up through the end of September 2001 (Citation6). Hypertension, tachycardia, dry mouth, and headache are the most commonly reported adverse reactions. One hundred fifty-two of these 397 patients were hospitalized; there were 29 deaths, of which 19 were attributed to cardiovascular etiologies (Citation7).
Sibutramine is an amphetamine-like anorexiant and inhibits the pre-synaptic reuptake of serotonin, norepinephrine, and dopamine. The anorectic effect probably is mediated through its noradrenergic activity, and it has serotoninergic and sympathomimetic activities even in therapeutic doses. Adverse effects in therapeutic doses include xerostomia, insomnia, anorexia, and constipation. Cardiovascular toxicities include tachycardia, palpitation, hypertension, and tachyarrhythmia (Citation8). The mechanism of sibutramine-induced acute coronary syndrome is not established, but sibutramine may behave like amphetamine or amphetamine-like substance through reversible coronary narrowing or vasospasm.
The manufacturer recommends not prescribing sibutramine to patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. Sibutramine use should also be avoided in patients taking other serotonergic medications, including selective serotonin reuptake inhibitors, serotonin agonists, lithium, meperidine, fentanyl, monoamine oxidase inhibitors, and dextromethorphan. Laboratory studies have suggested that sibutramine's action may be partially inhibited by noradrenergic antagonists (alpha and beta blockers) or serotonergic antagonists (Citation9). Despite the concurrent use of fluoxetine in our patient, there was no evidence of a serotonin syndrome. Our patient was slightly different from a recent case report of a Malaysian young woman suffering from an acute myocardial infarction after 3 months use of sibutramine (Citation10), in that our patient had only taken the medication for 3 days.
Conclusion
In the new era of healthy body image, weight reduction is becoming increasingly popular. It is not uncommon to encounter more patients taking anti-obesity medications and presenting with adverse drug effects. Sibutamine is a registered drug in Hong Kong that requires a physician's prescription. However, the drug is readily obtained via the Internet or over-the-counter and may be marketed as herbal products. We report an unusual case of myocardial infarction in a young patient taking sibutramine-containing slimming pills. Physicians should be aware that this agent may have significant side effects and drug interactions even in therapeutic doses. A detailed drug history should be taken in all patients presenting with an acute coronary syndrome.
References
- CA Naranjo, U Busto, EM Sellers, P Sandor, I Ruiz, EA Roberts, E Janecek, C Domecq, and DJ Greenblatt. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Therap 1981; 30 (2):239–245.
- MEJ Lean. Sibutramine: a review of clinical efficacy. Int J Obes Relat Metab Disord 1997; 21:30–36.
- PJ Leo, JE Hollander, and RD Shih. Phenylpropanolamine and associated myocardial injury. Ann Emerg Med 1996; 28:359–362.
- American Heart Association: Medical Treatment of Obesity. http://www.americanheart.org/presenter.jhtml?identifier=1818. Accessed 9 December 2007.
- NE Flomenbaum, LR Goldfrank, RS Hoffman, MA Howland, NA Lewin, and LS Nelson. Dieting agents and regimens. Toxicologic Emergencies. 8th ed. New York, USA: McGraw-Hill Professional; 2006: 623–624.
- Health Research Group: Protecting Health, Safety and Democracy. http://www.citizen.org/publications/release.cfm?id=7160 Accessed 29 October 2007.
- E Wooltorton. Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias. CMAJ 2002; 166 (10):1307–1308.
- LL Ioannides-Demos, J Proietto, AM Tonkin, and JJ McNeil. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf 2006; 29 (4):277–302.
- GA Bray, DH Ryan, D Gordon, S Heidingsfelder, F Cerise, and K Wilson. A double-blind randomized placebo-controlled trial of sibutramine. Obes Res 1996; 4:263–270.
- SM Azarisman, YA Magdi, S Noorfaizan, and M Oteh. Myocardial infarction induced by appetite suppressants in Malaysia. NEJM 2007; 357 (18):1873–1874.
