Abstract
A review of US poison center data for 2004 showed over 9000 ingestions of valproic acid. A guideline that determines the conditions for emergency department referral and prehospital care could potentially optimize patient outcome, avoid unnecessary emergency department visits, reduce health care costs, and reduce life disruption for patients and caregivers. An evidence-based expert consensus process was used to create the guideline. Relevant articles were abstracted by a trained physician researcher. The first draft of the guideline was created by the lead author. The entire panel discussed and refined the guideline before distribution to secondary reviewers for comment. The panel then made changes based on the secondary review comments. The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial out-of-hospital management of patients with a suspected ingestion of valproic acid by 1) describing the process by which an ingestion of valproic acid might be managed, 2) identifying the key decision elements in managing cases of valproic acid ingestion, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to the acute ingestion and acute-on-chronic ingestion of immediate-release and extended-release dosage forms of valproic acid, divalproex, and valproate sodium alone. Co-ingestion of additional substances could require different referral and management recommendations depending on the combined toxicities of the substances. This review focuses on the ingestion of more than a single therapeutic dose and the effects of an overdose. Although therapeutic doses of valproic acid can cause adverse effects in adults and children, some idiosyncratic and some dose-dependent, these cases are not considered. This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions might be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment. Recommendations are in chronological order of likely clinical use. The grade of recommendation is in parentheses. 1) All patients with suicidal intent, intentional abuse, or in whom a malicious intent is suspected (e.g., child abuse or neglect) should be referred to an emergency department (Grade D). 2) Patients who are symptomatic (more than somnolence or exhibiting coma or seizures) after a valproic acid ingestion should be referred to an emergency department (Grade C). 3) Asymptomatic patients with an unintentional acute ingestion of 50 mg/kg or more or asymptomatic patients who are taking the drug therapeutically and who take an additional single acute ingestion of 50 mg/kg or more of any valproic acid formulation should be referred to an emergency department for evaluation (Grade C). 4) Patients with unintentional ingestions of immediate-release valproic acid formulations, who are asymptomatic, and more than 6 hours has elapsed since the time of ingestion, can be observed at home (Grade C). 5) Patients with unintentional ingestions of delayed-release or extended-release formulations of valproic acid who are asymptomatic, and more than 12 hours has elapsed since the time of ingestion, can be observed at home (Grade C). 6) Pregnant women who ingest below the dose for emergency department referral and do not have other referral conditions should be directed to their primary care obstetrical provider for evaluation of potential maternal and fetal risk. Routine referral to an emergency department for immediate care is not required (Grade D). 7) Do not induce emesis (Grade C). 8) Activated charcoal can be administered to asymptomatic patients who have ingested valproic acid within the preceding hour (Grade C). Prehospital activated charcoal administration, if available, should only be carried out by health professionals and only if no contraindications are present. Poison centers should follow local protocols and experience with its use. Do not delay transportation in order to administer activated charcoal (Grades D). 9) In patients who have ingested valproic acid and who are comatose, naloxone can be considered for prehospital administration in the doses used for treatment of opioid overdose, particularly if the patient has respiratory depression (Grade C). 10) A benzodiazepine can be administered by EMS personnel if convulsions are present and if authorized by EMS medical direction, expressed by written treatment protocol or policy, or if there is direct medical oversight (Grade C).
Introduction
Scope of the problem and importance of the guideline
According to the Toxic Exposure Surveillance System (TESS) of the American Association of Poison Control Centers, there were 9069 human ingestions of valproic acid reported to poison centers in the US in 2004, 6018 (66%) of which were evaluated in healthcare facilities. Children less than 6 years of age accounted for 922 (10%) ingestions. Major effects occurred in 366 of the 9069 cases and 13 resulted in death. None of the fatalities was the result of an acute ingestion by a child (Citation1). Eleven of the deaths involved suicide as a reason for the ingestion while the other two cases were adverse effects to chronic therapeutic use of the drug. In most fatalities, there were co-ingested substances that contributed to the toxicity, making the contribution of valproic acid to the outcome difficult to determine. Although the total number of reported cases of valproic acid is not large, the rate of major effects and mortality make this a substance of concern for poison center personnel.
Background on valproic acid
Valproic acid was approved by the US Food and Drug Administration in 1978 for the treatment of complex partial seizures and simple and complex absence seizures (Citation2). Two products have additional FDA approvals. The extended-release product (Depakote ER) is indicated for migraine headache prophylaxis, and the delayed-release product (Depakote) carries an indication for the short-term management of acute manic episodes associated with bipolar disorder. The off-label uses for this drug are many and include the management of aggression and agitation in elderly patients with dementia, alcohol withdrawal, personality disorders, catatonia, chorea, cluster headache, mood disorders, myelodysplastic syndrome, neuropathic pain, panic disorders, and social phobias (Citation3).
An exact mechanism for the action of the drug has yet to be determined. The net result of the actions of the drug is thought to be an increase in brain concentrations of γ-aminobutyric acid (GABA). Postulated mechanisms include enhanced synthesis of GABA, inhibition of GABA metabolism, and interruption of GABA reuptake into nerve endings. Valproic acid is also thought to inhibit neuronal firing by prolonging recovery from inactivation of voltage-sensitive sodium channels and to reduce the flow of calcium ions through T-type calcium channels, thus reducing neuronal pacemaker current (Citation4).
There are several oral dosage forms of valproic acid available for use in the US. These are listed in . Valproic acid is available as branded and generic capsules. Oral liquid dosage forms are labeled by the valproic acid content in each dosage unit; however, the actual chemical in the products is the sodium salt, sodium valproate. Divalproex sodium is a stable complex of one molecule of valproic acid and one molecule of sodium valproate that is marketed in delayed-release (enteric-coated) and extended-release tablets. Divalproex sodium dissociates to valproic acid in the gastrointestinal tract. Divalproex sodium, sodium valproate, and valproic acid products deliver similar quantities of the active valproate ion as they are all labeled as to the equivalent amounts of valproic acid in each product. Differences in the rate of dissolution and subsequent bioavailability of the tablet forms of divalproex sodium require different total daily doses of the drug to achieve comparable steady-state plasma concentrations of valproic acid. In general, the total daily dose for the extended-release dosage form is 8–20% higher than the daily dose of the delayed-release product. Peak plasma concentrations are achieved in 1–4 hours following oral administration of the sodium salt or valproic acid, in 3–5 hours for divalproex delayed-release, and in 4–17 hours following divalproex extended-release tablets. Several days of therapy might be required to see the full effects of a given dosage.
Table 1. Oral dosage forms of valproic acid marketed in the US (Citation2,Citation83)
For the management of complex partial seizures and simple and complex absence seizures, the starting dosage of all of the valproic acid products is 10–15 mg/kg/day with a maximum dosage of 60 mg/kg/day. For migraine prophylaxis, the dosage of divalproex sodium extended-release is 500–1000 mg/day. For the adjunctive management of mania associated with bipolar disorder, the starting dosage of divalproex sodium extended-release is 750 mg/day with a maximum dosage of 60 mg/kg/day (Citation2,Citation5,Citation6).
Following an acute ingestion of a large amount of valproic acid, central nervous system depression, ranging from drowsiness to coma is observed. Respiratory depression, although not a common finding, has also been reported (Citation7–9). Generalized tonic-clonic seizures occur rarely in valproic acid poisoning and seem to occur most frequently in patients with massive overdoses (Citation10–16). Metabolic acidosis, hypoglycemia, hypophosphatemia, hypocalcemia, and hypernatremia can occur. Hyperammonemia can occur after an acute-on-chronic overdose of the drug and also as an idiosyncratic adverse reaction with therapeutic use of the drug (Citation17,Citation18). It is unlikely to occur following an acute overdose in patients who have not been taking the drug chronically (Citation19,Citation20). The hyperammonemia appears to be primarily related to depletion of carnitine stores in the mitochondria resulting in interruption of the urea cycle and accumulation of ammonia, although other mechanisms might be involved (Citation17). Depletion of carnitine also shifts the metabolism of valproic acid to a pathway that results in accumulation of a 4-en-valproate metabolite that is thought to be associated with hepatotoxicity but is probably not the definitive hepatotoxin (Citation21). Hepatotoxicity is most commonly seen with therapeutic use of the drug but can also occur following a massive overdose. Following a massive overdose of the drug, such as in a suicide attempt, valproic acid also inhibits mitochondrial fatty acid ß-oxidation leading to auto-inhibition or saturation of its own metabolism. This leads to alternate metabolic pathways that might result in production of multiple hepatotoxic compounds (Citation22). On rare occasions, particularly with the idiosyncratic hepatotoxicity, liver injury can be severe and fatal. Bone marrow suppression presenting as thrombocytopenia and leucopenia 3–5 days following an acute ingestion has also been reported and it resolved within a few days to a week. Pancreatitis has been reported following both therapeutic use and acute overdoses of the drug (Citation10,Citation19,Citation23,Citation24).
Valproic acid formulations are FDA pregnancy category D drugs due to teratogenic effects seen in studies in experimental animals and human case reports. Additionally, there is a warning in the product literature that women taking valproic acid should be monitored during pregnancy for low fibrinogen and platelets and the potential for bleeding. There is reference, also in the product literature, to the death of a newborn following therapeutic use of valproic acid in the mother during pregnancy, although no specific information is provided (Citation6).
Definition of terms
For the purpose of this guideline, two age groups are defined as either children less than 6 years of age or older children and adults. The older age group is more likely to attempt self-harm and to conceal an ingestion. To be consistent with TESS definitions, acute ingestions are defined as those occurring over a period of up to 8 hours and chronic ingestions are those that occur over a period of more than 8 hours. Acute-on-chronic ingestion is an acute ingestion in a patient who has already been exposed to valproic acid for more than 8 hours.
Intended users of this guideline
The intended users of this guideline are personnel in US poison control centers. This guideline has been developed for the conditions prevalent in the US. While the toxicity of valproic acid is not expected to vary in a clinically significant manner in other nations, the out-of-hospital conditions could be much different. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of this guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial out-of-hospital management of patients with suspected ingestions of valproic acid by 1) describing the process by which an ingestion of valproic acid might be managed, 2) identifying the key decision elements in managing cases of valproic acid ingestion, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research.
This guideline applies to the acute ingestion and acute-on-chronic ingestion of immediate-release and extended-release dosage forms of valproic acid, divalproex, and valproate sodium alone. Co-ingestion of additional substances could require different referral and management recommendations depending on the combined toxicities of the substances. This review focuses on the ingestion of more than a single therapeutic dose and the effects of an overdose. Although therapeutic doses of valproic acid can cause adverse effects in adults and children, some idiosyncratic and some dose-dependent, these cases are not considered.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions might be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment.
Methodology
The methodology used for the preparation of this guideline was developed after reviewing the key elements of practice guidelines (Citation25,Citation26). An expert consensus panel was established to develop the guideline (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant US poison control center experience, and be an opinion leader with broad esteem. Two specialists in poison information were included as full panel members to provide the viewpoint of the end-users of the guideline.
Search strategy
Literature searches for relevant articles were performed by a single investigator. The National Library of Medicine's PubMed database was searched (through March 2006) using valproic acid as a MeSH term with the subheadings poisoning or toxicity, limited to humans. The PubMed database was further searched using valproic acid, valproate, and divalproex as textwords (title, abstract, MeSH term, CAS registry) plus either poison* or overdos* or intox* or toxic*, limited to humans. This process was repeated in International Pharmaceutical Abstracts (1970–March 2006, excluding abstracts of meeting presentations), Science Citation Index (1977–March 2006), Database of Abstracts of Reviews of Effects (accessed March 2006), Cochrane Database of Systematic Reviews (accessed March 2006), and Cochrane Central Register of Controlled Trials (accessed March 2006). Reactions (1980–March 2006), the valproic acid poisoning management in Poisindex, and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, NACCT abstracts published in the Journal of Toxicology Clinical Toxicology (1995–2004) and Clinical Toxicology (2005) were reviewed for original human data.
Six major toxicology textbooks were reviewed for recommendations on the management of valproic acid poisonings and for citations of additional articles with original human data in the chapter bibliographies. The Toxic Exposure Surveillance System (TESS) maintained by the American Association of Poison Control Centers was searched for deaths resulting from valproic acid poisoning. These cases were abstracted for review by panel members. All US poison control centers were surveyed in 2006 to ascertain their out-of-hospital management and triage practices for valproic acid poisonings.
Criteria used to identify applicable studies
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of these articles were reviewed, searching specifically for those that dealt with estimations of doses with or without subsequent signs or symptoms of toxicity and management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles that did not meet either of the preceding criteria, did not add new data (e.g., reviews, editorials), or that exclusively described inpatient-only procedures (e.g., dialysis) were excluded.
Data extraction process
All articles that were retrieved from the original search were reviewed by a single trained physician abstractor. The complete paper was reviewed for original human data regarding the toxic effects of valproic acid or original human data directly relevant to the out-of-hospital management of patients with valproic acid toxicity or overdose. Relevant data (e.g., dose, effects, time of onset of effects, therapeutic interventions or decontamination measures provided, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief description of each article was written. This evidence table is available at http://www.aapcc.org/DiscGuidelines/valproic%20acid%20evidence%20table%202006-6-8.pdf. The table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Every attempt was made to locate foreign language articles and have their crucial information extracted, translated, and tabulated. A written summary of the data was created and distributed by the abstractor. Copies of all of the abstracted articles were made available for reading by the panel members on a secure AAPCC website.
Criteria used to evaluate studies and assign levels of evidence
The articles were assigned level-of-evidence scores based on the Grades of Recommendation table developed by the Centre for Evidence-Based Medicine at Oxford University (Appendix 2). Single case reports and case series were classified as level 4.
Guideline writing and review
A guideline draft was prepared by the lead author (listed first). The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the expert consensus panel members were collected, anonymously copied into a table of comments, and submitted to the lead author for response. The lead author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the lead author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, and ACMT members and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through email communication to AAPCC staff. All submitted comments were stripped of any information that would identify their sources, copied into a table of comments, and reviewed by the expert consensus panel and the lead author. The lead author responded to each comment in the table and his responses and subsequent changes in the guideline were reviewed and accepted by the panel.
Evaluation of evidence
Current poison control center practices
The expert consensus panel solicited referral and management guidelines for valproic acid from all US poison centers in 2006 and received documents from three poison centers. Twelve other centers indicated that they did not have written guidelines for valproic acid poisoning. The remaining centers did not respond to the request. One guideline provided an acute emergency department referral dose of greater than 50 mg/kg in children and any intentional ingestion in an adult or child. A second center's guideline stated that toxicity might develop following ingestion of more than 15–25 mg/kg, with ingestions of greater than 200 mg/kg causing a high risk of CNS depression. The third guideline provided no referral dose. For the time to onset of acute toxic effects, one center stated 8 hours, one stated 6–8 hours, and the third stated 6–17 hours. None of the guidelines suggested the use of ipecac syrup while all three recommended the use of activated charcoal if administered within 1–2 hours of ingestion.
Estimation of dose
The estimation of dose is based largely on the patient's history and the type of product. If precise data for the ingestion are unknown or unclear (package size, unit size, number of units ingested), poison centers often utilize a method in which the maximum potential dose is calculated. For example, if the actual dose ingested cannot be ascertained, the amount of the drug product that is missing from the container is multiplied by the concentration of the formulation.
When the mg/kg dose or a child's weight was not included in an article, the mg/kg dose was estimated by the use of pediatric growth charts (Citation27). The 95th percentile weight was used for a particular age and sex. When the sex of the child was not stated, the weight for boys was used. This approach errs on the side of estimating a lower mg/kg dose. Estimated mg/kg doses are italicized throughout the guideline whenever they are presented.
Review of textbooks
The review of valproic acid poisoning chapters in toxicology textbooks revealed substantial variation in recommendations. One commonly used reference provided no estimate of a toxic or referral dose but did state that “Clinical experience does not support the routine use of naloxone for reversing VPA-induced CNS depression and is not recommended” (Citation17). A second chapter provided no information on toxic dose and stated that “After an acute overdose, drowsiness progressing to coma occurs relatively rapidly but may progress more gradually, particularly with the divalproate form.” The author stated that there are no controlled studies assessing the effect of activated charcoal on clinical outcome. Concerning the use of naloxone, the author stated “Naloxone is relatively safe; its risk-to-benefit ratio is favorable for use in most patients with coma or ventilatory depression. Whether naloxone is truly effective in VPA poisoning is unknown” (Citation18). A third textbook also provided no information on a referral dose but did state that patients ingesting rapidly absorbed preparations should be observed for at least 6 hours, while those ingesting delayed-release or enteric-coated products should be observed for a minimum of 12 hours. The chapter referenced the use of naloxone in valproic acid overdose but stated that its effectiveness has not been consistently observed (Citation28). The fourth chapter also provided no information on a referral dose or on time to onset of symptoms. This author took a more favorable approach to the use of naloxone and stated that “High doses of naloxone should be administered if CNS depression is present” (Citation29). In a fifth chapter reviewed, no mention of a referral dose was made. The author stated that peak serum concentrations following ingestion of enteric-coated or delayed-release products can be delayed for 12–16 hours and that a patient should not be medically cleared prior to this time. This author stated that there is no evidence that gastric lavage and activated charcoal alter the clinical outcome and that high-dose naloxone has been reported to reverse CNS depression (Citation30). The sixth textbook examined made no mention of a referral dose or time to onset of symptoms. The authors advocated the use of activated charcoal and stated that gastric lavage might be of use if performed within 1 hour of ingestion. They went on to state that there is no antidote for valproic acid ingestion but that there are anecdotal reports that naloxone might be useful in reversing CNS and respiratory depression (Citation31).
Review of Poisindex
Poisindex, a computerized toxicology reference used by poison control centers, did not provide specific recommendations regarding doses at which emergency department referral is recommended. The monograph stated that coma has been associated with ingestion of more than 200 mg/kg and that doses less than 400 mg/kg are unlikely to cause severe toxicity. It then went on to state that 205 mg/kg caused coma in a 19-month-old boy (Citation32).
Review of TESS mortality data
An analysis of the American Association of Poison Control Centers' Toxic Exposure Surveillance System (TESS) database for deaths from ingestion of valproic acid during 2000–2005 revealed 53 cases in which valproic acid was listed as being involved. In 22 cases, valproic acid was listed as the sole substance, while the remainder involved multiple co-ingestants. Fifty-two of the cases were in adults and one case involved a child—a 5-year-old girl who was receiving valproic acid and sodium bromide to control a seizure disorder. She also had acute lymphocytic leukemia in remission. She presented with evidence of hepatotoxicity and died from hepatic failure and cerebral edema. There was no evidence of an overdose of either drug and the death appeared to be an adverse reaction to valproic acid.
Of the 52 adult cases, 51 were suspected or confirmed suicides. Death in these cases was the result of multi-organ system failure. In 37 cases, co-ingestants were involved and it was difficult to determine the contribution of each ingestant to the fatal outcome. One case appeared to be an adverse effect of therapeutic use of valproic acid resulting in fatal hepatotoxicity.
None of the valproic acid-associated deaths in the TESS database provided information useful in determining a referral dose for acute valproic acid ingestions or in determining the time to onset of symptoms following an acute ingestion.
Review of the literature
Acute ingestions in patients less than 6 years of age
There were no articles reviewed in which single doses of valproic acid were prospectively given to patients less than 6 year of age and were associated with subsequent toxicity. There were two level 6 abstracts of case series with some dose-response information. In an abstract describing 516 patients with valproic acid ingestions, the lowest dose resulting in any effect (drowsiness) was 20 mg/kg, the lowest dose associated with seizures was 100 mg/kg, and the lowest dose associated with loss of consciousness was 200 mg/kg. This paper included pediatric as well as adult cases, and an age breakdown was not provided (Citation33). In another abstract, a case series of 96 patients (aged 1–83 years) with valproic acid ingestions, doses ranging from 150 mg to 90 g were associated with effects in some patients, ranging from mild in severity to death (Citation34). There were also a number of level 4 articles (i.e., case reports, case series) with individual detailed case information on acute valproic acid toxicity. Specifically, there were 12 cases reported in 12 articles () (Citation8,Citation9,Citation11, Citation14,Citation20,Citation35–40,Citation84,Citation85). Among them, the lowest dose reported to result in adverse effects, regardless of the type of formulation ingested, was 500 mg (38 mg/kg) of valproic acid, which resulted in coma in a 3-year-old boy (Citation8). In addition, there was a description of 16 children aged 2–6 years with no dose information provided by the author. In this series, all 16 children had valproic acid serum concentrations higher than the upper limit of the therapeutic range. All of the children were drowsy, 11 had hypotonia, eight had ataxia, five had hypernatremia, and two had mild elevations in serum AST and ALT. None of the children was severely poisoned (Citation41).
Table 2. Published valproic acid poisoning cases in patients less than 6 years of age
Acute ingestions in patients 6 years of age and older
There was one article (level 1b) in which single doses of valproic acid were prospectively given and associated with subsequent toxicity. In this study, an oral dose of 300 mg of sodium valproate, followed by activated charcoal, was associated with nausea and vomiting in one of eight adult volunteers (Citation42).
There was one level 2b study, and two level 6 abstracts with dose and response information. Unfortunately, all three reported the doses of valproic acid and/or the patients' ages as ranges of values, making it impossible to determine what dose was associated with a given effect in a particular patient. In an abstract of a series of 516 pediatric and adult patients with valproic acid ingestion, the lowest dose resulting in any effects appears to have been 20 mg/kg (lethargy), while the lowest dose associated with seizures was 100 mg/kg and the lowest dose associated with loss of consciousness was 200 mg/kg (Citation33). In another abstract, a case series of 96 patients aged 1–83 years with valproic acid ingestions reported to range from 150 to 90 g, the effects ranged from mild in severity to death (Citation34). The third article was a level 2b study of 79 cases of acute valproate ingestion admitted to one toxicology service in which the median dose ingested was 6 g and was associated with effects in a number of patients, some of which were severe. The authors of this report concluded that doses of greater than 400 mg/kg could cause “severe” toxicity (Citation13).
There were also a number of level 4 or 6 articles with individual detailed case information on acute valproic acid toxicity (e.g., case reports, case series). Specifically, there were 38 cases reported in 33 articles () (Citation7,Citation19,Citation23,Citation34,Citation43–71). Among them, the lowest dose reported to result in adverse effects was 1200 mg, which resulted in severe toxicity (coma and cerebral edema) in a 19-year-old man who had also ingested 1500 mg aspirin (Citation55). The next lowest dose was an ingestion of 5 g of valproic acid, which resulted in moderate to severe toxicity (lethargy, hypoventilation) in a 22-year-old man with no reported co-ingestants (Citation7).
Table 3. Published acute valproic acid poisoning cases with dose information in patients 6 years of age and older
Acute-on-chronic ingestions
There were no papers that expressly examined the issue of acute-on-chronic poisonings with valproic acid. In many of the adult case reports, it appears that the patients might have taken their own medications, but the authors failed to confirm this fact.
Onset of effects after acute ingestion
The expert consensus panel members considered the time of onset for toxicity to develop after valproic acid ingestion to assist in making decisions about out-of-hospital management. All articles with toxicity information were searched for estimates of a time of onset. Unfortunately, the majority of articles reported times of hospital presentation, rather than times of symptom development. In such cases, it was only possible to establish an upper limit for the time to effect onset. Only a few reports reported a precise time of effect onset. Care should be taken to distinguish time to onset of initial effects from time to onset of serious or major effects, the time to onset of peak effects, or the time to onset of subsequent deterioration or complications.
There were six level 4 articles that specifically mentioned delayed onsets of clinical effects after valproic acid ingestion (Citation9,Citation50,Citation66,Citation72–74). On closer review, many of these patients had early symptoms followed by delayed deteriorations in their conditions. A few reports appeared to represent genuine cases of delayed onset of toxicity. One adult with a suicide attempt presented awake with a therapeutic serum concentration of valproic acid of 70 mg/L 3 hours after ingesting an unknown amount of the drug along with paroxetine, clonazepam, and ethanol. Nine to 11 hours after ingestion, he developed lethargy and poor arousability, followed by hypotension and hyperammonemia. His peak valproic acid serum concentration at 12 hours after the reported time of ingestion was 574 mg/L. The specific type of valproic acid formulation was not mentioned in this report (Citation74). In another case report, a 32-year-old woman with a history of multiple suicide attempts ingested 30 g of divalproex sodium along with 400 mg of chlorpheniramine and was noted to have elevated serum bilirubin and AST concentrations on admission, 3 hours after ingestion. She did not develop clinical symptoms of toxicity until 4½–8 hours after ingestion, when she became drowsy and went on to develop severe toxicity. Her valproic acid serum concentrations were 105 mg/L at 4½ hours after the reported time of ingestion, 825 mg/L at 14 hours and a peak of 1380 mg/L at 17 hours (Citation50). In a third case, a 24-year-old woman ingested divalproex sodium along with dimenhydrinate. At 8 hours after ingestion, she was lethargic and she became comatose at 13 hours after ingestion (Citation72). In both of the latter two cases, the patients had ingested either delayed-release or extended-release formulations and also ingested drugs known to slow gastrointestinal motility. This could be an explanation for the delayed onset of effect. In the only pediatric case report with delayed onset of effect, a 26-month-old boy who ingested 4.5 g of divalproex sodium (288 mg/kg) was asymptomatic on admission 1½ hours after ingestion and was noted to abruptly become obtunded and limp 4 hours after ingestion (Citation9).
In many cases, it was difficult to assess the rapidity of effect onset or deterioration because the patients were found or presented to a hospital with significant effects already present or the rapidity of their deterioration was not reported by the authors. In a number of cases, the onset of toxicity appeared to be progressive over the course of minutes to hours (Citation43,Citation57,Citation58,Citation72,Citation75,Citation76). Occasionally, the onset of symptoms was noted to be abrupt (Citation9,Citation51,Citation77).
Treatment measures
Gastrointestinal decontamination
There were a number of different gastrointestinal decontamination measures reported in case reports including activated charcoal, ipecac syrup, and gastric lavage. There were no controlled trials investigating the efficacy of these procedures in valproic acid overdose patients. Several uncontrolled case reports and series mentioned the use of these decontamination measures in individual patients, but it was impossible to determine the efficacy of any of these measures from these reports given the lack of controls, the concurrent use of other therapies, and the fact that decontamination procedures do not generally produce immediate clinical improvement.
A level 1b prospective trial investigated the efficacy of activated charcoal on valproic acid absorption in volunteers. In this study, both peak serum valproate concentration and AUC were significantly reduced (mean 65% reduction in absorption, P < 0.01) by 50 g activated charcoal administered 5 minutes after ingestion of 300 mg valproate sodium (Citation78). Another level 1b controlled trial looked at the efficacy of multiple-dose activated charcoal in enhancing valproate elimination after therapeutic doses of valproate sodium syrup and found the regimen tested did not significantly reduce the serum half-life of valproate; however, the first dose of activated charcoal was not administered until 4 hours after the sodium valproate (Citation42). In another paper, a 26-month-old boy with an ingestion of 4.5 g of extended-release valproic acid was given a continuous gastric infusion of activated charcoal at a rate of 0.25 g/kg/hour. The authors reported an elimination half-life of 4.8 hours versus an expected half-life of 10–16 hours (Citation9).
There were no prospective studies looking at the efficacy of ipecac syrup or gastric lavage in valproic acid exposures.
Naloxone
There were several level 4 case reports of patients improving significantly after naloxone administration with reversal of coma, respiratory depression, and pinpoint pupils (Citation7–9,Citation40,Citation65,Citation79,Citation80). There were a number of other level 4 or 6 reports in which naloxone had no effect on clinical status (Citation14,Citation19,Citation44,Citation48,Citation52,Citation66,Citation81,Citation82). The doses used were similar to those used in opioid overdose. No prospective clinical trials were found examining the use of naloxone in patients with valproic acid ingestions.
Other treatment measures
There are no specific treatment measures that have been proven to be useful in the out-of-hospital environment to treat valproic acid poisoned patients. l-Carnitine has been given to patients with valproic acid poisoning with the intent of providing the cofactor required for ß-oxidation and to reduce interference with the urea cycle and the production of potentially hepatotoxic metabolites. Although frequently used, there is a lack of controlled studies confirming the efficacy of this treatment (Citation17,Citation18). l-Carnitine is available without prescription and theoretically could be used in the prehospital care of valproic acid poisoned patients. However, there is no published experience with the use of l-carnitine outside of a hospital.
Limitations of the literature
Unfortunately, the data on acute valproic acid poisoning generally suffered from a number of limitations: 1) much of the data was determined by retrospective observations and based on estimates of dose provided by patients or family members, raising questions about the accuracy of the dose estimates; 2) the dose-effect information was confounded in many cases by the presence of co-ingestants, differences in treatment measures provided, and concurrent medical conditions that could have altered the clinical presentation or outcome; 3) as there are several formulations of valproic acid and divalproex products, many authors failed to report exactly which product was involved; 4) among larger case series, many of the patients remained asymptomatic, and product formulations, ingestion doses, and frequency and severity of effects were typically reported as ranges of values, percentages, or means, so individual doses resulting in specific effects could not be determined; and 5) among the few prospective trials available, valproic acid was administered in therapeutic doses, which would be expected to be much smaller than doses likely to be seen in an overdose or poisoning.
Conclusions
Key decision points for triage
The expert consensus panel chose to emphasize the importance of information that would be needed in order to make a sound triage decision for a patient with a known valproic acid poisoning. These variables include the patient's intent, dose and formulation of the product, the presence of symptoms, and the time of ingestion. The expert consensus panel agreed that in each case, the judgment of the specialist in poison information, the poison center medical director, or other poison center-affiliated clinicians might override any specific recommendation from this guideline.
Patient intent
The panel concluded that all patients with suicidal intent or in whom a malicious intent was suspected (e.g., child abuse or neglect) should be expeditiously transported to an emergency department, regardless of the dose ingested. Patients without these characteristics (e.g., adults with definite unintentional ingestion or children below the age of 6 years in whom abuse is not suspected) are candidates for more selective referral to healthcare facilities.
Dose and formulation
The expert consensus panel concluded that home observation is suitable for both adult and pediatric patients who are asymptomatic, have an acute unintentional ingestion of up to 50 mg/kg of valproic acid, and have not ingested any other potentially toxic substances. This is based on very limited case report data. The panel selected this referral dose as cases in the literature reporting symptoms at amounts lower than 50 mg/kg are either poorly documented or the symptoms that developed were not severe. In addition, this amount is within the therapeutic dosing range of 10 to 60 mg/kg/day. From the literature, it appears that 100 mg/kg is the ingested dose at which moderate to severe symptoms can be expected to occur.
The expert consensus panel examined the issue of acute ingestions in patients taking the drug therapeutically and felt that the literature provided no assistance in determining if a different referral dose should be used. The panel felt that the 50 mg/kg referral dose provided a sufficient safety factor that this dose could be safely used in most of these situations as well.
This referral guideline applies to all of the formulations of valproic acid, divalproex sodium, and sodium valproate. Patients might be able to tolerate higher acute doses of the long-acting preparations, but there is insufficient evidence to establish an alternate referral dose.
Presence of symptoms
In a patient with a known unintentional valproic acid ingestion, medical evaluation in an emergency department is warranted if the patient is symptomatic with more than somnolence (lightly sedated but arousable with more than a speaking voice or irritating stimuli) or more severe symptoms such as coma (requiring painful stimuli to arouse or unarousable) or seizures.
Time of onset of toxicity after overdose
In the vast majority of reported cases, patients were symptomatic within 4–6 hours of ingestion. However, there is limited evidence to suggest that, on occasion and in situations in which delayed-release or sustained-release preparations have been ingested along with co-ingestants that decrease gastrointestinal motility, the onset of symptoms could be delayed up to 8–12 hours. Therefore, the panel concluded that an asymptomatic patient who unintentionally ingested an immediate-release valproic acid product is unlikely to develop symptoms if the interval between the ingestion and the call is longer than 6 hours. Patients who have ingested extended-release or delayed-release products, particularly with co-ingestants that decrease gastrointestinal motility, more than 12 hours before the first contact with a poison center and are still asymptomatic can be monitored at home. It is unlikely that these patients will become symptomatic after this time.
The expert consensus panel concluded that this triage guideline could be used when a capable caretaker is available to monitor the patient. During hours when a caretaker is normally asleep, it could be more difficult to adequately monitor the patient. Therefore, consideration should be made to refer these patients to an emergency department.
Pregnancy
All of the information related to teratogenicity, coagulopathy, and other effects on the newborn is derived from therapeutic use of valproic acid. No acute toxicity information in pregnant patients is available nor is any information available on the effects of single therapeutic doses. The panel concluded that pregnant women who ingest below the dose for emergency department referral and do not have other referral conditions should be directed to their primary care obstetrical provider for evaluation of potential maternal and fetal risk. Routine referral to an emergency department for immediate care is not required.
Potential out-of-hospital management
There were no controlled trials of ipecac syrup for valproic acid overdose. Nor were there any prospective volunteer studies examining the efficacy of ipecac syrup in reducing valproic acid absorption, even after therapeutic doses. Since altered mental status is a frequent complication of valproic acid overdose, the potential risk for aspiration following ipecac-induced emesis needs to be a consideration. The expert consensus panel concluded that there is no role for ipecac syrup in the treatment of patients who have ingested valproic acid.
The panel concluded that activated charcoal could be administered orally as part of the management of a valproic acid poisoned patient. However, the data to support the use of activated charcoal are extremely limited. It cannot be recommended for routine prehospital management at this time, although it might be considered in some regions in which prehospital activated charcoal is commonly administered by emergency medical personnel and there is a long transportation time to an emergency department. Also, the panel agreed that transportation to an emergency department should not be delayed in order to attempt activated charcoal administration.
The role of naloxone in the treatment of valproic acid poisoning is unclear. There are case reports in which it appeared that the patient responded to the drug and also case reports in which no response was apparent. Since naloxone has a low risk of adverse effects, the expert consensus panel concluded that the use of naloxone could be considered in patients who are comatose, particularly those patients with respiratory depression. The doses used in the case reports are the same doses commonly used to reverse opiate effects (adults and children over 20 kg: 0.4–2 mg IV, repeated every 2–3 minutes until response is achieved; children less than 20 kg: 0.01 mg/kg repeated every 2–3 minutes until response is achieved). As in any overdose situation in which naloxone is used, consideration must be given to the potential for precipitation of withdrawal symptoms in patients addicted to opiates. In these cases, the starting dose should be 0.05 mg in both adults and children.
The expert consensus panel concluded that close monitoring of vital signs as well as respiratory, cardiovascular, and neurological status of patients with possible severe valproic acid poisoning is of critical importance. Supportive care should be provided as required.
Recommendations
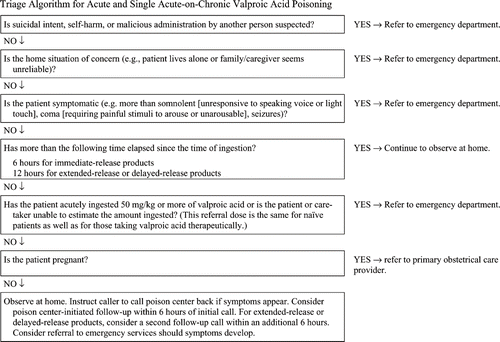
All patients with suicidal intent, intentional abuse, or in whom a malicious intent is suspected (e.g., child abuse or neglect) should be referred to an emergency department (Grade D).
Patients who are symptomatic (more than somnolence or exhibiting coma or seizures) after a valproic acid ingestion should be referred to an emergency department (Grade C).
Asymptomatic patients with an unintentional acute ingestion of 50 mg/kg or more or asymptomatic patients who are taking the drug therapeutically and who take an additional single acute ingestion of 50 mg/kg or more of any valproic acid formulation should be referred to an emergency department for evaluation (Grade C).
Patients with unintentional ingestions of immediate-release valproic acid formulations, who are asymptomatic, and more than 6 hours has elapsed since the time of ingestion, can be observed at home (Grade C).
Patients with unintentional ingestions of delayed-release or extended-release formulations of valproic acid who are asymptomatic, and more than 12 hours has elapsed since the time of ingestion, can be observed at home (Grade C).
Pregnant women who ingest below the dose for emergency department referral and do not have other referral conditions should be directed to their primary care obstetrical provider for evaluation of potential maternal and fetal risk. Routine referral to an emergency department for immediate care is not required (Grade D).
Do not induce emesis (Grade C).
Activated charcoal can be administered to asymptomatic patients who have ingested valproic acid within the preceding hour (Grade C). Prehospital activated charcoal administration, if available, should only be carried out by health professionals and only if no contraindications are present. Poison centers should follow local protocols and experience with its use. Do not delay transportation in order to administer activated charcoal (Grades D).
In patients who have ingested valproic acid and who are comatose, naloxone can be considered for prehospital administration in the doses used for treatment of opioid overdose, particularly if the patient has respiratory depression (Grade C).
A benzodiazepine can be administered by EMS personnel if convulsions are present and if authorized by EMS medical direction, expressed by written treatment protocol or policy, or if there is direct medical oversight (Grade C).
These recommendations are summarized in Appendix 4.
Implications for research
The panel identified the following topics where additional research is needed or analysis of existing databases might be useful.
Additional research needs to be performed to determine a more precise dose threshold for emergency department referral for valproic acid poisoning.
Additional research needs to be performed to determine if the different formulations of valproic acid affect the dose at which referral is required.
The role of prehospital administration of activated charcoal in the management of valproic acid poisoning needs additional clarification.
The role of prehospital administration of naloxone in the management of valproic acid poisoning needs additional clarification.
Disclosure
Dr. Booze's husband is employed by AstraZeneca. Dr. Erdman was employed by AstraZeneca at the time of his work on this guideline. There are no other potential conflicts of interest reported by the expert consensus panel or project staff regarding this guideline.
Notes
*Guideline for the Management of Poisoning, supported in full by Cooperative Agreement 8 U4BHS00084 between the American Association of Poison Control Centers and the Health Resources and Services Administration, Department of Health and Human Services.
References
- Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM. 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2005; 23: 589–666
- http://www.clinicalpharmacology.com, Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.; 2006. URL:
- Drugdex system [Internet database]. Thomson Micromedex, Greenwood Village, CO, Updated periodically. Accessed April 2006.
- McNamara JO. Goodman and Gilman's the Pharmacological Basis of Therapeutics11th, LL Brunton, JS Lazo, KL Parker. McGraw Hill, New York 2006; 501–525
- http://www.accessdata.fda.gov/scripts/cder/drugsatfda/, Depakene Product Label: Drugs@FDA website:
- http://www.accessdata.fda.gov/scripts/cder/drugsatfda/, Depakote Product Label. Drugs@FDA website:
- Alberto G, Erickson T, Popiel R, Narayanan M, Hryhorczuk D. Central nervous system manifestations of a valproic acid overdose responsive to naloxone. Ann Emerg Med 1989; 18: 889–891
- Espinoza O, Maradei I, Ramirez M, Pascuzzo-Lima C. An unusual presentation of opioid-like syndrome in pediatric valproic acid poisoning. Vet Hum Toxicol 2001; 43: 178–179
- Farrar HC, Herold DA, Reed MD. Acute valproic acid intoxication: enhanced drug clearance with oral-activated charcoal. Crit Care Med 1993; 21: 299–301
- Connacher AA, Macnab MS, Moody JP, Jung RT. Fatality due to massive overdose of sodium valproate. Scott Med J 1987; 32: 85–86
- Eeg-Olofsson O, Lindskog U. Acute intoxication with valproate. Lancet 1982; 1: 1306
- Franssen EJ, van Essen GG, Portman AT, de Jong J, Go G, Stegeman CA, Uges DR. Valproic acid toxicokinetics: serial hemodialysis and hemoperfusion. Ther Drug Monit 1999; 21: 289–292
- Isbister GK, Balit CR, Whyte IM, Dawson A. Valproate overdose: a comparative cohort study of self poisonings. Br J Clin Pharmacol 2003; 55: 398–404
- Lynch A, Tobias JD. Acute valproate ingestion induces symptomatic methemoglobinemia. Pediatr Emerg Care 1998; 14: 205–207
- Roodhooft AM, Van Dam K, Haentjens D, Verpooten GA, Van Acker KJ. Acute sodium valproate intoxication: occurrence of renal failure and treatment with haemoperfusion-haemodialysis. Eur J Pediatr 1990; 149: 363–364
- Singh SM, McCormick BB, Mustata S, Thompson M, Prasad GV. Extracorporeal management of valproic acid overdose: a large regional experience. J Nephrol 2004; 17: 43–49
- Doyon S. Anticonvulsants. Goldfrank's Toxicologic Emergencies8th, NE Flomenbaum, LR Goldfrank, RS Hoffman, MA Howland, NA Lewin, LS Nelson. McGraw Hill, New York 2006; 737–739
- Snodgrass WR. Critical Care Toxicology. Diagnosis and Management of the Critically Poisoned Patient, J Brent, SD Phillips, KL Wallace, JW Donovan, KK Burkhart. Elsevier Mosby, Philadelphia 2005; 565–570
- Mortensen PB, Hansen HE, Pedersen B, Hartmann-Andersen F, Husted SE. Acute valproate intoxication: biochemical investigations and hemodialysis treatment. Int J Clin Pharmacol Ther Toxicol 1983; 21: 64–68
- Murakami K, Sugimoto T, Woo M, Nishida N, Muro H. Effect of l-carnitine supplementation on acute valproate intoxication. Epilepsia 1996; 37: 687–689
- Siemes H, Nau H, Schultze K, Wittfoht W, Drews E, Penzien J, Seidel U. Valproate (VPA) metabolites in various clinical conditions of probable VPA-associated hepatotoxicity. Epilepsia 1993; 34: 332–346
- Dreifuss FE, Bryant AE, 3rd. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology 1996; 46: 465–469
- Eyer F, Felgenhauer N, Gempel K, Steimer W, Gerbitz KD, Zilker T. Acute valproate poisoning: pharmacokinetics, alteration in fatty acid metabolism, and changes during therapy. J Clin Psychopharmacol 2005; 25: 376–380
- Pinkston R, Walker LA. Multiorgan system failure caused by valproic acid toxicity. Am J Emerg Med 1997; 15: 504–506
- Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281: 1900–1905
- Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003; 139: 493–498
- http://www.cdc.gov/nchs/data/ad/ad314.pdf, Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Advance data from vital and health statistics; no. 314. Hyattsville, MD; National Center for Health Statistics, 2004.
- Normann SA, Speranza VC, Gaar GG. Valproate and selected newer anticonvulsants. Clinical Toxicology, MD Ford, KA Delaney, J Ling, T Erickson. WB Saunders, Philadelphia 2001
- Seger D. Anticonvulsants. Clinical Management of Poisoning and Drug Overdose3rd, LM Haddad, MW Shannon, JF Winchester. WB Saunders, Philadelphia 1998
- Seger D. Anticonvulsant medications. Medical Toxicology3rd, RC Dart. Lippincott, Williams & Wilkins, Philadelphia 2004
- Barrueto F, Nelson LS. Antiepileptic agents. Pediatric Toxicology: Diagnosis & Management of the Poisoned Child, TR Erickson, WR Ahrens, SE Aks, CR Baum, LJ Ling. McGraw-Hill, New York 2005
- Valproic acid management. Poisindex system. Greenwood Village (CO): Thomson Micromedex; edition expires, RK Klasco, March, 2006
- Garnier R, Fournier E. Intoxication aiguë par le valproate de sodium. Nouv Presse Med 1982; 11: 678
- von Bardeleben RS, Stürer A, Weilemann LS. Valproic acid intoxications in 96 documented cases 1995–1998: the role of hemoperfusion in therapy [abstract]. J Toxicol Clin Toxicol 1998; 36: 471–472
- Dharnidharka VR, Fennell RS, 3rd, Richard GA. Extracorporeal removal of toxic valproic acid levels in children. Pediatr Nephrol 2002; 17: 312–315
- Dupuis RE, Lichtman SN, Pollack GM. Acute valproic acid overdose. Clinical course and pharmacokinetic disposition of valproic acid and metabolites. Drug Saf 1990; 5: 65–71
- Ishikura H, Matsuo N, Matsubara M, Ishihara T, Takeyama N, Tanaka T. Valproic acid overdose and L-carnitine therapy. J Anal Toxicol 1996; 20: 55–58
- Janssen F, Rambeck B, Schnabel R. Acute valproate intoxication with fatal outcome in an infant. Neuropediatrics 1985; 16: 235–238
- Manabe Y, Uriu M, Kanamori Y, Furukawa F. Acute valproate intoxication. Pharm Mon 1987; 29: 657–659
- Steiman GS, Woerpel RW, Sherard ES, Jr. Treatment of accidental sodium valproate overdose with an opiate antagonist. Ann Neurol 1979; 6: 274
- Lifshitz M, Gavrilov V. Acute valproic acid poisoning in young children. J Pharm Technol 2001; 17: 151–153
- A al-Shareef, Buss DC, Shetty HG, Ali N, Routledge PA. The effect of repeated-dose activated charcoal on the pharmacokinetics of sodium valproate in healthy volunteers. Br J Clin Pharmacol 1997; 43: 109–111
- Al Aly Z, Yalamanchili P, Gonzalez E. Extracorporeal management of valproic acid toxicity: a case report and review of the literature. Semin Dialysis 2005; 18: 62–66
- Andersen GO, Ritland S. Life threatening intoxication with sodium valproate. J Toxicol Clin Toxicol 1995; 33: 279–284
- Azaroual N, Imbenotte M, Cartigny B, Leclerc F, Vallee L, Lhermitte M, Vermeersch G. Valproic acid intoxication identified by 1H and 1H-(13)C correlated NMR spectroscopy of urine samples. MAGMA 2000; 10: 177–182
- Blayac D, Roch A, Michelet P, De Francheschi E, Auffray JP. Acidose lactique majeure secondaire à une intoxication volontaire par valproate. Ann Fr Anesth Reanim 2004; 23: 1007–1010
- Camilleri C, Albertson T, Offerman S. Fatal cerebral edema after moderate valproic acid overdose. Ann Emerg Med 2005; 45: 337–338
- Fernandez MC, Walter FG, Kloster JC, Do SM, Brady LA, Villarin A, Ruffenach SJ, Prosnitz EH, Salmon JV. Hemodialysis and hemoperfusion for treatment of valproic acid and gabapentin poisoning. Vet Hum Toxicol 1996; 38: 438–443
- Frifelt JJ, Wanscher MC, Wienholtz G, Horwitz N, Brockhattingen A. Akut valproatforgiftning. Ugeskr Laeger 1987; 149: 3255–3257
- Graudins A, Aaron CK. Delayed peak serum valproic acid in massive divalproex overdose--treatment with charcoal hemoperfusion. J Toxicol Clin Toxicol 1996; 34: 335–341
- Guillaume CP, Stolk L, Dejagere TF, Kooman JP. Successful use of hemodialysis in acute valproic acid intoxication. J Toxicol Clin Toxicol 2004; 42: 335–336
- Johnson LZ, Martinez I, Fernandez MC, Davis CP, Kasinath BS. Successful treatment of valproic acid overdose with hemodialysis. Am J Kidney Dis 1999; 33: 786–789
- Karlsen RL, Kett K, Henriksen O. Intoxication with sodium valproate. A case report. Acta Med Scand 1983; 213: 405–406
- Kay TD, Playford HR, Johnson DW. Hemodialysis versus continuous veno-venous hemodiafiltration in the management of severe valproate overdose. Clin Nephrol 2003; 59: 56–58
- Khoo SH, Leyland MJ. Cerebral edema following acute sodium valproate overdose. J Toxicol Clin Toxicol 1992; 30: 209–214
- Kielstein JT, Woywodt A, Schumann G, Haller H, Fliser D. Efficiency of high-flux hemodialysis in the treatment of valproic acid intoxication. J Toxicol Clin Toxicol 2003; 41: 873–876
- Kroll P, Nand C. Hemodialysis in the treatment of valproic acid overdose. J Clin Psychiatry 2002; 63: 78–79
- Lakhani M, McMurdo ME. Survival after severe self poisoning with sodium valproate. Postgrad Med J 1986; 62: 409–410
- Lee WL, Yang CC, Deng JF, Chen YF, Lin HD, Wang PH. A case of severe hyperammonemia and unconsciousness following sodium valproate intoxication. Vet Hum Toxicol 1998; 40: 346–348
- Lokan RJ, Dinan AC. An apparent fatal valproic acid poisoning. J Anal Toxicol 1988; 12:35–37.
- Matsumoto J, Ogawa H, Maeyama R, Okudaira K, Shinka T, Kuhara T, Matsumoto I. Successful treatment by direct hemoperfusion of coma possibly resulting from mitochondrial dysfunction in acute valproate intoxication. Epilepsia 1997; 38: 950–953
- Meyer S, Kuhlmann MK, Peters FT, Limbach HG, Lindinger A. Severe valproic acid intoxication is associated with atrial tachycardia: secondary detoxication by hemoperfusion. Klin Padiatr 2005; 217: 82–85
- Minari M, Maggiore U, Tagliavini D, Rotelli C, Cabassi A, David S, Fiaccadori E. Severe acute valproic acid intoxication successfully treated with hemodiafiltration without hemoperfusion. Ann Emerg Med 2002; 39: 204–205
- Minville V, Roche Tissot C, Samii K. Epuration extrarénale, supplémentation en lcarnitine et intoxication à l'acide valproïque. Ann Fr Anesth Reanim 2004; 23: 357–360
- Montero FJ. Naloxone in the reversal of coma induced by sodium valproate. Ann Emerg Med 1999; 33: 357–358
- Payen C, Frantz P, Martin O, Parant F, Moulsma M, Pulce C, Descotes J. Delayed toxicity following acute ingestion of valpromide. Hum Exp Toxicol 2004; 23: 145–148
- Poklis A, Poklis JL, Trautman D, Treece C, Backer R, Harvey CM. Disposition of valproic acid in a case of fatal intoxication. J Anal Toxicol 1998; 22: 537–540
- Robinson P, Abbott C. Severe hypothermia in association with sodium valproate overdose. N Z Med J 2005; 118: U1681
- Thabet H, Brahmi N, Amamou M, Ben Salah N, Hedhili A. Hyperlactatemia and hyperammonemia as secondary effects of valproic acid poisoning. Am J Emerg Med 2000; 18: 508
- van Kuelen JG, van Wijk JA, Touw DJ, van der Deure J, Markhorst DG, Gemke RJ. Effectiveness of hæmofiltration in valproic acid intoxication. Acta Pædiatr 2001; 90: 958–959
- Wasserman GS, Aldridge SC, Kelly JC, Abdel-Rahman SM. A unique valproic acid ingestion. J Toxicol Clin Toxicol 2001; 39: 419–422
- Brubacher JR, Dahghani P, McKnight D. Delayed toxicity following ingestion of enteric-coated divalproex sodium (Epival). J Emerg Med 1999; 17: 463–467
- Ingels M, Beauchamp J, Clark RF, Williams SR. Delayed valproic acid toxicity: a retrospective case series. Ann Emerg Med 2002; 39: 616–621
- LoVecchio F, Thole D, Bagnasco T. Delayed absorption of valproic acid, resulting in coma. Acad Emerg Med 2002; 9: 1464
- Hintze G, Klein HH, Prange H, Kreuzer H. A case of valproate intoxication with excessive brain edema. Klin Wochenschr 1987; 65: 424–427
- Ruskosky D, Holmes C, Schauben J. Utilization of hemodialysis to enhance valproic acid (VPA) elimination [abstract]. J Toxicol Clin Toxicol 1999; 37: 636–637
- Kane SL, Constantiner M, Staubus AE, Meinecke CD, Sedor JR. High-flux hemodialysis without hemoperfusion is effective in acute valproic acid overdose. Ann Pharmacother 2000; 34: 1146–1151
- Neuvonen PJ, Kannisto H, Hirvisalo EL. Effect of activated charcoal on absorption of tolbutamide and valproate in man. Eur J Clin Pharmacol 1983; 24: 243–246
- Houghton BL, Bowers JB. Valproic acid overdose: a case report and review of therapy. MedGenMed 2003; 5: 5
- Roberge RJ, Francis EH, 3rd. Use of naloxone in valproic acid overdose: case report and review. J Emerg Med 2002; 22: 67–70
- Hicks LK, McFarlane PA. Valproic acid overdose and haemodialysis. Nephrol Dial Transplant 2001; 16: 1483–1486
- Williams SR, Clark RF. Hemodialysis of a valproic acid poisoning [abstract]. J Toxicol Clin Toxicol 1995; 33: 491
- http://online.factsandcomparisons.com, Drug Facts and comparisons 4.0 [database online]. Wolters Kluwer Health; 2006. URL:
- Schnabel R, Rambeck B, Janssen F. Fatal intoxication with sodium valproate. Lancet 1984; 1: 221–222
- Koelliker P, Koelliker D, Toerne T. Bullous skin lesions associated with severe valproic acid overdose in a four year-old child [abstract]. J Toxicol Clin Toxicol 1999; 37: 638
Appendix 1
Expert Consensus Panel Members
Lisa L. Booze, PharmD
Certified Specialist in Poison Information
Maryland Poison Center
University of Maryland School of Pharmacy
Baltimore, Maryland
E. Martin Caravati, MD, MPH, FACMT, FACEP
Professor of Surgery (Emergency Medicine)
University of Utah
Medical Director
Utah Poison Center
Salt Lake City, Utah
Gwenn Christianson, RN, MSN
Certified Specialist in Poison Information
Indiana Poison Center
Indianapolis, Indiana
Peter A. Chyka, PharmD, DABAT, FAACT
Professor, Department of Clinical Pharmacy
College of Pharmacy
University of Tennessee Health Science Center
Knoxville, Tennessee
Daniel J. Cobaugh, PharmD, FAACT, DABAT
Director of Research
ASHP Research and Education Foundation
Bethesda, Maryland
Former Associate Director, American Association of Poison Control Centers
Daniel C. Keyes, MD, MPH, FACEP, FACMT
Medical Director
Pine Bluff Chemical Demilitarization Facility
Associate Professor, Southwestern Toxicology Training Program
Dallas, Texas
Anthony S. Manoguerra, PharmD, DABAT, FAACT
Professor of Clinical Pharmacy and Associate Dean
School of Pharmacy and Pharmaceutical Sciences
University of California San Diego
Former Director, California Poison Control System, San Diego Division
San Diego, California
Lewis S. Nelson, MD, FACEP, FACMT, FACCT
Associate Professor of Emergency Medicine
New York University School of Medicine
Associate Medical Director
New York City Poison Control Center
New York, New York
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT
Director, West Virginia Poison Center
Professor, West Virginia University School of Pharmacy
Department of Clinical Pharmacy
Charleston, West Virginia
Paul M. Wax, MD, FACMT
Attending Toxicologist
UT Southwestern Medical Center
Dallas, Texas
Alan D. Woolf, MD, MPH, FACMT
Director, Program in Environmental Medicine
Children's Hospital, Boston
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Appendix 3
Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Pharmacists Association
American Public Health Association
American Society of Health-System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control and Prevention – National Center for Injury Prevention and Control
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety
Appendix 4