Abstract
Introduction. Bites by Phoneutria spp. spiders are common in Brazil, although only 0.5–1% result in severe envenomation, with most of these occurring in children. Cases of systemic envenomation in adults are very unusual, and no serum venom levels have been previously quantified in these cases. Case report. A 52-year-old man was bitten on the neck by an adult female Phoneutria nigriventer. Immediately after the bite, there was intense local pain followed by blurred vision, profuse sweating, tremors, and an episode of vomiting; 1–2 h post bite the patient showed agitation and a blood pressure of 200/130 mmHg, and was given captopril and meperidine. Upon admission to our service 4 h post bite (time zero – T0), his blood pressure was 130/80 mmHg with a heart rate of 150 beats/min, mild tachypnea, agitation, cold extremities, profuse sweating, generalized tremors, and priapism. The patient was treated with antivenom, local anesthetic, and fluid replacement. Most of the systemic manifestations disappeared within 1 h after antivenom. Laboratory blood analyses at T0, T1, T6, T24, and T48 detected circulating venom by ELISA only at T0, before antivenom infusion (47.5 ng/mL; cut-off, 17.1 ng/mL); his serum blood sugar was 163 mg/dL at T0. The patient was discharged on the second day with a normal arterial blood pressure and a follow-up evaluation revealed no sequelae. Conclusion. This is the first report of confirmed moderate/severe envenoming in an adult caused by P. nigriventer with the quantification of circulating venom.
Introduction
Spiders of the genus Phoneutria, popularly known as wandering or banana spiders, live in Central and South America (Citation1,Citation2). Most of the clinically important bites involving this genus occur in Brazil (Citation1–5), where 2,687 cases were reported in 2006 (Citation6). Phoneutria species are nocturnal, aggressive spiders that do not construct webs. When molested, Phoneutria spiders assume a very characteristic defensive posture and can jump up to 40 cm (Citation1–3). Phoneutria nigriventer, which lives in central-western, southeastern, and southern Brazil, is responsible for most bites by this genus in humans (Citation1–5), and its venom is the most studied of the six Phoneutria species identified in Brazil (Citation5,Citation7–19).
Most bites by Phoneutria spp. cause mild envenoming, with only 0.5–1% being severe, mainly in children (Citation4,Citation5). Despite the medical importance of this genus, there are very few detailed descriptions of systemic envenoming by Phoneutria spp. in adults (Citation20,Citation21). In this report, we describe a case of moderate/severe envenoming caused by a large female P. nigriventer spider following a bite in the neck region. We also provide a quantification of the serum venom level.
Case report
A previously healthy 52-year-old man with no history of arterial hypertension (last measured 4 months earlier) was cleaning a public garden when he leaned against a tree and was bitten on the right side of the neck by an 8-cm-long female P. nigriventer spider that was captured and brought for identification (). Immediately after the bite, there was intense, non-radiating pain followed by blurred yellow vision, profuse sweating, tremors, and an episode of vomiting.
At the first medical facility, 1–2 h after the bite, the patient showed agitation and a blood pressure of 200/130 mmHg, and was treated with sublingual captopril (25 mg) and intravenous meperidine (50 mg). Upon admission to our service 4 h after the bite (time 0 – T0), the blood pressure was 130/80 mmHg with a heart rate of 150 beats/min, mild tachypnea, agitation, cold extremities, profuse sweating, generalized tremors, priapism, and local erythema at the bite site. The case was classified as moderate/severe (Citation3) and the patient received five vials of undiluted antiarachnid antivenom [Instituto Butantan, São Paulo, Brazil; F(ab´)2, 5 mL/vial, equine origin, 1 mL neutralizes 7.5 minimum lethal doses of P. nigriventer reference venom in guinea-pigs] infused intravenously over 15–20 min without pretreatment with antihistamines or corticosteroids; local anesthesia with 2% lidocaine (to control pain) and an intravenous infusion of Ringer lactate (500 mL) were also initiated. There were no early reactions to the antivenom and most of the systemic manifestations of envenomation disappeared within 1 h after antivenom; residual local pain was treated with one additional injection of lidocaine.
Blood analyses at T0, T1, T6, T24, and T48 revealed an elevated glucose level at T0 (163 mg/dL; reference value, 70–100 mg/dL), but with normal serum potassium, sodium, creatinine, and urea levels. Serum nitric oxide (NO), measured as nitrite using Griess reagent after the reduction of nitrate to nitrite (commercial kit; Cayman Chemical Co., Michigan, USA), was elevated at T0 (54.6 nmol/mL) but had decreased to 24.8 nmol/mL at T1, with no marked fluctuations thereafter. Circulating venom [measured by enzyme-linked immunosorbent assay (ELISA)] was detected only at T0 before antivenom infusion (47.5 ng/mL; the values for T1, T6, T24, and T48 were below the detection limit of 17.1 ng/mL of the assay). Arterial blood pressure was 130/80 mmHg at T0, with a slight elevation (160/90 mmHg) at T8 that normalized (120/80 mmHg) by the time the patient was discharged on the second day. A follow-up evaluation 3 days after discharge revealed a normal arterial blood pressure and no local or systemic sequelae.
Sandwich ELISA for the quantification of P. nigriventer venom
ELISA
The sandwich ELISA for the quantification of P. nigriventer venom was adapted from Chávez-Olórtegui et al. (Citation22). Phoneutria nigriventer F(ab´)2 antibodies were purified from commercial arachnid antivenom (Instituto Butantan) by immunoaffinity chromatography using P. nigriventer venom immobilized on a CNBr–Sepharose column. Ninety-six-well plates (high protein binding; Corning, NY, USA) were coated overnight at 4°C with 100 μL of P. nigriventer F(ab´)2 antibodies (20 μg/mL in 0.1 M sodium carbonate, pH 9.6) and then washed three times with washing buffer (WB, 0.9% NaCl containing 0.05% Tween-20; Sigma, Uppsala, Sweden). After blocking with 2% bovine casein (Sigma) in phosphate-buffered saline (PBS) for 1 h at room temperature and washing again, the serum samples, standards, and controls (100 μL) diluted at 1:1 in incubation buffer [PBS, containing 0.05% Tween-20, 0.02% normal horse serum (Sigma), and 0.25% bovine casein] were added followed by incubation for 1 h at room temperature. The plates were subsequently washed with WB and incubated with a P. nigriventer F(ab´)2–peroxidase conjugate (1:250, in incubation buffer). After further washes, the plates were incubated with substrate (100 μL of 0.2 mg of o-phenylenediamine/mL and 0.05% H2O2 in 0.15 M citrate buffer, pH 5.0) for up to 15 min in the dark at room temperature. The reactions were stopped by adding 50 μL of 5% H2SO4 and the final absorbances were read at 492 nm in a Multiskan MS multiwell plate reader (Labsystems, Helsinki, Finland). The data were fitted by linear regression using GraphPad Prism software (GraphPad Inc., San Diego, CA, USA). The appropriate antibody concentrations used in this assay and the quality of the conjugate were selected and evaluated essentially as described elsewhere (Citation23). In addition, normal horse serum (0.01%, final concentration) was used to minimize cross-reactivity of the antibodies with non-specific antigens (Citation24).
Assay parameters
The background absorbance values and detection limit for the ELISA were determined using sera samples from 23 healthy adult donors with no previous history of spider bites (or bites/stings by other venomous animals) and who had not previously been treated with antivenom for such accidents. The donors were recruited from persons attending the university hospital where the patient was treated and were from the same social (working) class as the patient. The sera were obtained after the patients had provided written consent and after approval by the Committee for Ethics in Human Research of the Faculty of Medical Sciences, UNICAMP. The absorbances of these samples ranged from 0.092 to 0.185 (mean ± SD = 0.116 ± 0.029).
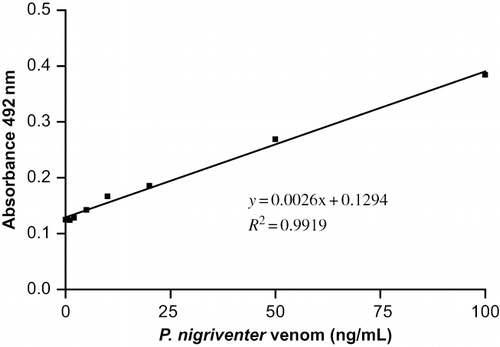
The assay cut-off value was defined as the mean absorbance of the 23 donor sera plus two standard deviations [cut-off absorbance = 0.116 + (2 × 0.029) = 0.174], and the limit of detection (LOD) was the venom concentration that corresponded to this cut-off absorbance (LOD = 17.1 ng/mL) (Citation25), as determined from a calibration curve with standards covering the concentration range of 0–100 ng/mL (0, 1, 2, 5, 10, 20, 50, 100 ng/mL, diluted in a pool of sera from healthy donors). At these concentrations, the standard curve was linear, with a high coefficient of determination (R2 = 0.9919) (). For each ELISA, a complete (new) standard curve was run concomitantly to allow accurate sample quantification. All standards and samples were assayed in duplicate.
Fig. 2. Standard curve for the ELISA used to quantify P. nigriventer venom. The points represent the means of duplicate determinations that differed from each other by < 5% in each assay. Similar results were obtained in four additional assays.
Quality controls (20, 40, and 80 ng/mL) were used to spike samples and to determine the assay coefficients of variation (CV). The accuracy and intra- and inter-assay precision were estimated at low (20 ng/mL, which was close to the LOD), medium (40 ng/mL), and high (80 ng/mL) concentrations of P. nigriventer venom diluted in a pool of sera from healthy donors. The intra-assay CV for each venom concentration was calculated from six determinations done simultaneously, and the inter-assay CV was calculated from three determinations done independently. The accuracy was calculated as the mean venom concentration that was detected with respect to the expected venom concentration, expressed as a percentage. As shown in , the assay showed high accuracy and good intra- and inter-assay precision (CV < 10%). The cross-reactivity of this ELISA with venoms from other arthropods, including the scorpion Tityus serrulatus and the spider Loxosceles gaucho, which, together with P. nigriventer venom, are used to produce the arachnid antivenom, was not examined here because the spider responsible for the bite was positively identified as P. nigriventer. The venom used in the standard curves was milked by electrical stimulation from the same spider that caused the accident.
Table 1. Accuracy and intra- and inter-assay variation for the ELISA used to quantify P. nigriventer venom
Discussion
Phoneutria nigriventer bites on the head and neck are very unusual but potentially serious. The quick onset and the severity of the clinical manifestations seen in our case probably resulted from rapid venom absorption from the bite site, close to major neck vessels.
Intense local pain is the major symptom reported after most P. nigriventer bites (Citation3–5) and was also observed in our case. Experimental studies have indicated that this hyperalgesia involves peripheral (tachykinin NK1 and NK2, and glutamate receptors) and central (spinal) mechanisms (neurokinins, excitatory amino acids, NO, proinflammatory cytokines, and prostanoids), all probably acting in synergism (Citation16,Citation17,Citation19).
Peptides present in P. nigriventer venom cause vascular smooth muscle contracture and increased skin vascular permeability by activating the tissue kallikrein system and tachykinin NK1 receptors (Citation9–12). The intraperitoneal injection of P. nigriventer toxin T×2–5 in adult mice produces priapism, hypersalivation, and death by pulmonary edema and respiratory distress (Citation18). These effects are abolished by pretreatment with 7-nitroindazole, a selective neuronal NO synthase inhibitor, indicating a role for NO in these responses (Citation18). Increased NO formation and the resulting relaxation of corpus cavernosum smooth muscle (Citation12,Citation14,Citation18) could possibly explain the long-lasting priapism (1–2 h) seen in the present case. Although priapism has been described in children with systemic envenoming by Phoneutria spp. (Citation4,Citation5,Citation26), this phenomenon has not previously been reported in adults.
The severe arterial hypertension recorded 1–2 h after the bite and that persisted up to T8 was consistent with studies in laboratory animals (Citation13,Citation15) (the normal blood pressure seen at T0 was probably related to the captopril administered at the first medical service). In anesthetized rats, the intravenous injection of P. nigriventer venom produces a biphasic response in arterial blood pressure characterized by short-lasting hypotension followed by sustained hypertension. The hemodynamic changes caused by P. nigriventer venom in rabbits involve central and peripheral components (Citation13,Citation15). The central component is mediated by the activation of cardiovascular centers that increase the sympathetic outflow to the periphery whereas the peripheral component involves either the direct activation of vascular α1-adrenergic receptors or catecholamine release from sympathetic nerve endings (Citation15). The tachycardia, profuse sweating, cold extremities, and transient hyperglycemia seen here suggest that the venom possibly increased sympathetic activity. However, we were unable to quantify the serum catecholamine levels to confirm this activation.
Antivenom has been used to treat envenoming by Phoneutria spp. in Brazil since 1925 (Citation20). Current guidelines of the Brazilian Ministry of Health recommend that antivenom be given only to patients who develop important systemic clinical manifestations such as severe arterial hypertension, diaphoresis, convulsions, priapism, pulmonary edema, and shock (Citation3); these manifestations occur in less than 3.3% of cases, including children (Citation4,Citation5). The clinical state of our patient improved rapidly following antivenom administration (within 1–2 h after infusion), in agreement with the outcome of early cases (Citation20,Citation21).
There are no clinical reports on the circulating levels of P. nigriventer venom, although Chávez-Olórtegui et al. (Citation22) found levels of 11 and 26 ng/mL in two patients envenomed by P. nigriventer. However, these authors provided no clinical details of their patients and gave no indication of whether antivenom was administered in the two cases, so direct comparison with the findings for the case reported here is difficult. Using the ELISA described here, we have also measured a similar venom level (67.8 ng/mL) in a fatal case of a 4-year-old boy bitten by a Phoneutria spider in the southern Brazilian state of Santa Catarina (unpublished case attended at the State Poison Control Center of Santa Catarina, in Florianópolis, SC, Brazil). Although the venom level observed in our case was higher than those reported by Chávez-Olórtegui et al. (Citation22), it was nevertheless compatible with that reported for other arachnids, particularly scorpions such as Androctonus mauretanicus mauretanicus and Buthus occitanus (venom levels 15.9–49.7 ng/mL) (Citation27), Androctonus australis (venom levels 17–29 ng/mL) (Citation28), and Tityus serrulatus (venom levels 4.0–50.0 ng/mL) (Citation29).
No venom was detected by ELISA at various times after antivenom. It is unclear whether this lack of detection reflected the inability of the assay to detect very low venom concentrations after neutralization by antivenom (Citation30) or the rapid clearance of the venom from the circulation (independently of the presence of antivenom). In mice, P. nigriventer venom levels are highest during the first 30 min following subcutaneous inoculation with no venom being detected beyond 4 h post injection (Citation22).
Conclusion
Bites by P. nigriventer in the neck region can produce moderate/severe envenoming in adults. The early onset of clinical manifestations following such bites may be facilitated by the rapid absorption of venom in this region and result in circulating venom that can be quantified by ELISA. However, further studies, including a large prospective case series of patients bitten by these spiders, are needed to adequately assess the contribution of circulating venom, NO, catecholamines, and other mediators, such as cytokines, to the range and severity of clinical manifestations seen after envenoming by Phoneutria spp.
Acknowledgments
The authors thank Thomaz A. A. Rocha e Silva for photographing and milking the spider. E.A., M.H.S.L.B., and S.H. are supported by research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- SM Lucas. Spiders in Brazil. Toxicon 1988; 26:759–772.
- SM Lucas. Aranhas de interesse médico no Brasil. JLC Cardoso, FOS França, FH Wen, CMS Málaque, and V HaddadJr. Animais peçonhentos no Brasil. Biologia, clínica e terapêutica dos acidentes. São Paulo, Brazil: Sarvier/FAPESP, 2003: 141–149.
- Anon. Ministério da Saúde. Fundação Nacional da Saúde. Manual de diagnóstico e tratamento de acidentes por animais peçonhentos. Brasília: Ministério da Saúde do Brasil, 1998.
- F Bucaretchi, CR Deus Reinaldo, S Hyslop, PR Madureira, EM De Capitani, and RJ Vieira. A clinico-epidemiological study of bites by spiders of the genus Phoneutria. Rev Inst Med Trop. São Paulo 2000; 42:17–21.
- E Antunes, and CMS Málaque. Mecanismo de ação do veneno de Phoneutria e aspectos clínicos do foneutrismo. JLC Cardoso, FOS França, FH Wen, CMS Málaque, and V HaddadJr. Animais peçonhentos no Brasil. Biologia, clínica e terapêutica dos acidentes. São Paulo, Brazil: Sarvier/FAPESP, 2003: 150–159.
- Ministério da Saúde. Sistema de Informação de Agravos de Notificação – SINAN. Acidentes por animais peçonhentos – notificaÇões registradas no SINAN. Available at: http://dtr2004.saude.gov.br/sinanweb/novo/. Accessed 22 April 2008.
- MD Fontana, and O Vital-Brazil. Mode of action of Phoneutria nigriventer spider venom at the isolated phrenic nerve-diaphragm of the rat. Braz J Med Biol Res 1985; 18:557–565.
- O Vital-Brazil, GB Bernardo-Leite, and MD Fontana. Modo de ação da peçonha da aranha armadeira, Phoneutria nigriventer (Keiserling, 1891), nas aurículas isoladas de cobaia. Ciênc Cult 1988; 40:181–185.
- E Antunes, RA Marangoni, SD Brain, and G De Nucci. Phoneutria nigriventer (armed spider) venom induces increased vascular permeability in rat and rabbit skin in vivo. Toxicon 1992; 30:1011–1016.
- E Antunes, RA Marangoni, NCC Borges, S Hyslop, MD Fontana, and G De Nucci. Effects of Phoneutria nigriventer venom on rabbit vascular smooth muscle. Braz J Med Biol Res 1993; 26:81–91.
- RA Marangoni, E Antunes, SD Brain, and G De Nucci. Activation by Phoneutria nigriventer (armed spider) venom of the tissue kallikrein-kininogen-kinin system in rabbit skin in vivo. Br J Pharmacol 1993; 109:539–543.
- RAB Lopes-Martins, E Antunes, MLV Oliva, CA Sampaio, J Burton, and G De Nucci. Pharmacological characterization of rabbit corpus cavernosum relaxation mediated by the tissue kallikrein-kinin system. Br J Pharmacol 1994; 113:81–86.
- SKP Costa, H MorenoJr, SD Brain, G De Nucci, and E Antunes. The effect of Phoneutria nigriventer (armed spider) venom on arterial blood pressure of anaesthetized rats. Eur J Pharmacol 1996; 298:113–120.
- E Rego, AC Bento, AB Lopes-Martins, E Antunes, JC Novello, S Marangoni, JR Giglio, B Oliveira, and G De Nucci. Isolation and partial characterization of a polypeptide from Phoneutria nigriventer spider venom that relaxes rabbit corpus cavernosum in vitro. Toxicon 1996; 34:1141–1147.
- V Estato, E Antunes, B Machado, G De Nucci, and E Tibiriçá. Investigation of the haemodynamic effects of Phoneutria nigriventer venom in anaesthetized rabbits. Toxicon 2000; 38:841–853.
- EM Zanchet, and Y Cury. Peripheral tachykinin and excitatory amino acid receptors mediate hyperalgesia induced by Phoneutria nigriventer venom. Eur J Pharmacol 2003; 467:111–118.
- EM Zanchet, I Longo, and Y Cury. Involvement of spinal neurokinins, excitatory amino acids, proinflammatory cytokines, nitric oxide and prostanoids in pain facilitation induced by Phoneutria nigriventer spider venom. Brain Res 2004; 1021:101–111.
- CM Yonamine, LRP Troncone, and MAP Camillo. Blockade of neuronal nitric oxide synthase abolishes the toxic effects of Tx2–5, a lethal Phoneutria nigriventer spider toxin. Toxicon 2004; 44:169–172.
- SKP Costa, A Starr, S Hyslop, D Gilmore, and SD Brain. How important are NK1 receptors for influencing microvascular inflammation and itch in the skin? Studies using Phoneutria nigriventer venom. Vascul Pharmacol 2006; 45:209–214.
- V Brazil, and J Vellard. Contribuição ao estudo do veneno das aranhas. Mem Inst Butantan 1926; 3:243–299.
- J Vellard. Les araignées vraies. Les ctènes. J Vellard. Le venin des araignées. Monographies de L'Institut Pasteur. Paris, France: Masson et Cie, 1936: 169–184.
- C Chávez-Olórtegui, K Bohórquez, LM Alavarenga, E Kalapothakis, D Campolina, WS Mari, and CR Diniz. Sandwich-ELISA detection of venom antigens in envenoming by Phoneutria nigriventer spider. Toxicon 2001; 39:909–911.
- D Catty, and C Raykundalia. ELISA and related enzyme immunoassays. D Catty. Antibodies – a practical approach. Vol. II. Oxford, England: IRL Press, 1989: 97–154.
- M Ho, MJ Warrell, DA Warrell, D Bidwell, and A Voller. A critical reappraisal of the use of enzyme-linked immunosorbent assays in the study of snake bite. Toxicon 1986; 24:211–221.
- MN Krifi, and M el Ayeb. An equilibrium ELISA for the dosage of Androctonus australis garzonii (Aag) and Buthus occitanus tunetanus (Bot) scorpion venoms: set up and calibration. Arch Inst Pasteur Tunis 1998; 75:185–194.
- S Schenberg, and FA Pereira-Lima. Pharmacology of the polypeptides from the venom of the spider Phoneutria fera. Mem Inst Butantan 1966; 33:627–638.
- N Ghalim, B El-Hafny, F Sebti, J Heikel, N Lazar, R Moustanir, and A Benslimane. Scorpion envenomation and serotherapy in Morocco. Am J Trop Med Hyg 2000; 62:277–283.
- MN Krifi, F Amri, H Kharrat, and M El Ayeb. Evaluation of antivenom therapy in children severely envenomed by Androctonus australis garzonii (Aag) and Buthus occitanus tunetanus (Bot) scorpions. Toxicon 1999; 37:1627–1634.
- NA Rezende, C Chávez-Olórtegui, and CFS Amaral. Is the severity of Tityus serrulatus scorpion envenoming related to plasma venom concentrations?. Toxicon 1996; 34:820–823.
- MN Krifi, S Savin, M Debray, C Bon, M El Ayeb, and V Choumet. Pharmacokinetic studies of scorpion venom before and after antivenom immunotherapy. Toxicon 2005; 45:187–198.

