ABSTRACT
Lead (Pb) exposure has adverse health effects and altered DNA methylation may contribute to Pb toxicity. LINE-1 is an interspersed repeated DNA that is used as a surrogate marker for estimating genomic DNA methylation levels, and GSTP1 is an isozyme that detoxifies xenobiotics like Pb, and its expression is inhibited by methylation. Thus, to assess the effects of Pb exposure on global hypomethylation and gene-specific promoter hypermethylation, we examined DNA methylation at LINE-1 repetitive elements and the GSTP1 promoter region. Blood samples were obtained from children (N = 123) living in Pb-polluted areas (as exposed children) and children (N = 63) living in Pb-unpolluted areas (as control children) in Kabwe, Zambia. ICP-MS was used to determine blood lead levels (BLLs), and pyrosequencing and a fluorescence-based polymerase chain reaction assay were used to determine levels of LINE-1 methylation and GSTP1 promoter methylation, respectively. Inverse association was found between BLLs and LINE-1 methylation (β = – 0.046, p = 0.006). The highest quartile of BLL had significant hypomethylation of LINE-1 (p for trend = 0.03), suggesting the higher the BLL, the lower LINE-1 methylation. GSTP1 methylation levels did not differ significantly between the two areas (p = 0.504), nor was it associated with Pb poisoning risk (OR = 1.03, p = 0.476), indicating GSTP1 methylation may not be a reliable biomarker of Pb exposure in healthy people. Therefore, Pb-related health problems could result from global DNA methylation changes due to high BLLs.
Introduction
Environmental lead (Pb) exposure among children is a global concern, especially in developing countries. In Zambia’s Kabwe town, the increasing health risk of Pb exposure is associated with Pb that originated from an old Pb mining site, which operated for almost a century before being ‘closed’ in 1994. The windblown mine dust from tailings left the town with dangerous levels of Pb [Citation1]. Moreover, unregulated activities such as illegal mining, playing around the dumpsite as well as crashing stones, and using soil from the site for income-generating are the sources of exposure for the population. Previous studies have found extremely high levels of Pb in soils [Citation1,Citation2], animal tissues and blood [Citation3–6], and human blood, faeces, and urine [Citation7,Citation8]. Recently, Yabe et al. [Citation9] reported clear evidence of Pb poisoning among families, especially children, from townships around the abandoned mine, with ingesting contaminated soil and inhaling dust being the main exposure sources.
Several studies have documented the deleterious effects of Pb on several major systems, including haematopoietic, cardiovascular, reproductive, and central nervous systems [Citation10,Citation11]. Especially in early childhood, exposure to Pb can alter neurochemistry and impair cognitive development [Citation12,Citation13]. In addition, the effect of Pb exposure on epigenetic mechanisms has been identified. Epigenetics can influence gene activity at both transcriptional and post-transcriptional levels, which can contribute to a wide range of biological processes [Citation14]. Among these mechanisms, DNA methylation, which consists of the addition of methyl to cytosine at cytosine-guanine nucleotide (CpG) sites, is the most studied. DNA methylation at these sites in the promoter region is related to transcriptional silencing and thus impedes the corresponding gene expression [Citation15]. Long Interspersed Nucleotide Element 1 (LINE-1) and Alu repetitive elements are major constituents of interspersed DNA repeats. Thus, their methylations have been shown to correlate with global genomic DNA methylation and can be used as proxy markers for determining the methylation level of genomic DNA in a wide range of diseases, including cancer [Citation16,Citation17]. The International Agency for Research on Cancer (IARC) classified the inorganic Pb as a ‘probable’ human carcinogen based on evidence from animals and human studies [Citation18]. Lead is a toxic environmental metal that may induce epigenetic changes after chronic exposure. Several studies in adults or patients looked at the effect of Pb exposure on global DNA methylation [Citation19–21], CpG site-specific DNA methylation [Citation22,Citation23], and differentially methylated regions (DMRs) [Citation24]. The hypomethylation of LINE-1 is considered a hallmark of most malignancies as it causes retrotransposon activation and, subsequently, genomic instability [Citation25,Citation26]. Glutathione-S-transferases (GSTs) are enzymes that detoxify hydrophobic and electrophilic compounds, such as Pb, by conjugation with reduced glutathione. GSTP1, the gene encoding the pi class GST, is found hypermethylated in many diseases, and its promoter hypermethylation is associated with a loss of expression [Citation27].
No studies have been conducted so far on the impact of lead exposure on epigenetic alterations among ‘apparently healthy’ children living in Kabwe Zambia, one of the most polluted cities in the world. Therefore, in the present study, we investigated the influence of Pb exposure on global DNA methylation using LINE-1 and CpG site-specific DNA methylation using GSTP1 in children exposed to environmental Pb in Kabwe, Zambia. Based on previous CpG island methylator phenotype studies in numerous types of malignancies, we chose LINE-1 as an indicative marker for global DNA methylation [Citation17], and GSTP1 for its ability to detoxify Pb and protecting cells against oxidative stress [Citation28].
Methods
Sampling strategy
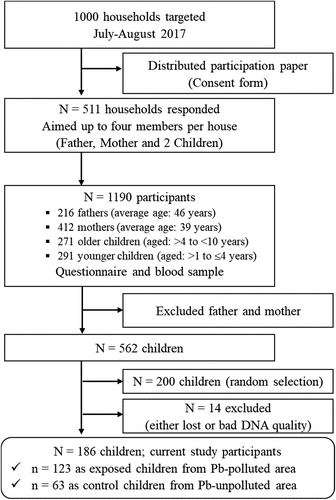
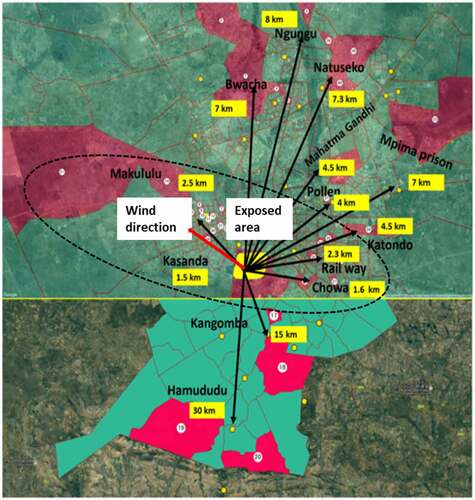
The present study is based on data from the KAMPAI (KAbwe Mining Pollution Ameliorative Initiative) project, which recruited 1190 participants. The participants (father, mother, and two children per household) and sampling areas are described in detail elsewhere [Citation9]. The detailed flowchart of KAMPAI data and the selection of 186 children in this study is depicted in . Blood samples were collected in 13 health centres in July and August 2017. The health centres were located from 1.5 to 30 km from the mine site, and we classified the area of sampling as an Pb-polluted area (exposed area: up to 3 km from the mine and the western side of the mine to the windward direction) and Pb-unpolluted area (control area: more than 3 km from the mine and opposite to the wind direction) (). This classification was made based on previous BLL data [Citation7,Citation9] and our knowledge of Kabwe town Pb pollution in environmental and biota samples [Citation2–5]. Weight (kg) and height (cm) were measured before blood samples were collected. Body mass index (BMI) was calculated by dividing weight by height squared and expressing the result in kilograms per square metre (kg/m2).
Blood collection and blood lead levels (BLLs) analysis
Approximately 3 mL peripheral venous blood samples were collected into heparinized blood collection tubes and then 200 µL (x2) of blood samples were kept in Eppendorf tubes (2 tubes per child) for Pb and molecular analysis. All samples were stored at – 20°C at the health centres, and then frozen samples were brought to the Laboratory of Toxicology, Faculty of Veterinary Medicine, Hokkaido University, Japan.
Analysis of Pb using inductively coupled plasma mass spectrometry (ICP-MS) and quality control analysis using certified reference material is described in our previous studies [Citation29]. Total BLLs were measured by using an Agilent 7700 series ICP-MS after micro-wave digestion of a 200 µL blood sample with 5 mL of two-fold diluted ultrapure 60% nitric acid and 1 mL of 30% hydrogen peroxide. 205Thallium and Seronorm™ Trace Elements Whole Blood L-2 certified reference material (Sero AS, Norway) were used as an internal standard and as quality control, respectively. Replicate analyses of this reference material showed mean recovery rate of 95% ± 4.5 with a detection limit of 0.01 μg/L.
DNA methylation analysis
A NucleoSpin Blood Kit was used to extract genomic DNA from a 200 μL blood sample. After extraction, DNA was quantified using a Nanodrop 1000A Spectrophotometer (Delaware, USA). An approximate 1 μg of genomic DNA from each subject was bisulphite-treated using the Qiagen EpiTect Fast Bisulphite Kit.
Analysis of LINE-1 methylation was performed by pyrosequencing as described by Pilsner et al. [Citation20]. The primers used to produce a 98-amplicon product are presented in . PCR was performed using the PyroMark PCR Kit (Qiagen) with the following cycling settings: 95°C for 15 min followed by 40 cycles at 94°C for 30s, 55°C for 20sec, and 72°C for 20s. The final extension step was done at 72°C for 10 min. The integrity of the PCR product (146 bp) was visualized on a 1.5% agarose gel. Pyrosequencing was achieved using the PyroMark Q24 pyrosequencing system. After PCR, the biotinylated product was bound to Streptavidin Sepharose beads and single-stranded using the Pyrosequencing Vacuum Prep Tool. Then, it was annealed with the pyrosequencing primer (). The assay measured DNA methylation at three CpG sites adjacent to each other, and the average methylation was used for statistical analysis. For each batch, twenty samples, one each of EpiTect 100% bisulphite-converted methylated and unmethylated control DNA samples, and two blank (Milli Q) samples were run to monitor contamination, precision, and accuracy of the analysis.
Table 1. Primer sequences used for the analysis.
The GSTP1 promoter methylation level was assessed by fluorescence-based real-time PCR using two sets of primers and a probe, one for GSTP1 (140 bp amplicon, gene of interest) and one for β-actin (ACTB) (133 bp amplicon, an internal reference) [Citation30]. The primers and probe sequences used are listed in . PCR was performed with 1 U of Invitrogen Platinium Taq DNA polymerase, 1x PCR buffer, 2.0 mM MgCl2, 200 µM of each dNTP, 600 nM of each primer, 200 nM probe, RNase-free water, and 40 ng bisulphite-modified DNA in a 20 µL total reaction mixture. Amplification was performed with an initial PCR activation at 95°C for 3 min, followed by 45 cycles at 95°C for 15s and 60°C for 1 min. The quantitative PCR analysis was carried out using the StepOne Plus Real-Time PCR system. Each sample was run in duplicate. The relative methylation level in each sample was described as the percentage of methylated reference (PMR). The PMR was computed by dividing the GSTP1:ACTB of a sample by the GSTP1:ACTB of EpiTect 100% bisulphite-converted methylated DNA and multiplying by 100. Samples were categorized as ‘methylated’ if the cycle threshold (Ct) number for replicate analysis was below 40 Ct. For verification, samples with Ct values of less than 40 were re-run. Milli Q water was used as no template control.
Statistical analysis
All statistical analysis was done using JMP pro 14.0 software at a significance level of p < 0.05. Continuous variables were analysed by mean (± standard deviation) and categorical variables by numbers. To improve normality, BLLs were transformed by square roots while the mean methylation level of LINE-1 followed a normal distribution. For continuous variables, we used the t-test between pairs and the Kruskal-Wallis non-parametric test among pairs, while the chi-square test was used for categorical variables. We performed a linear mixed-effects regression to examine the effects of BLL, area, age, gender, height, weight, and BMI on average LINE-1 methylation. We considered average LINE-1 methylation across the three evaluated CpG sites. Then, for trend testing, we examined the global DNA methylation in relation to the quartiles of BLLs. For analysing the association between GSTP1 promoter methylation status and risk of Pb toxicity, logistic regression analysis was used to obtain adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs). Age, gender, and BMI were employed as confounding variables.
Results
General characteristics and BLLs
The participants’ demographic characteristics (age, height, weight, and BMI), and BLLs are presented in . A total of 186 children (92 females and 94 males) with an average age of 5.30 ± 2.45 years were enrolled in this study. Based on gender and area, the study groups did not significantly differ in terms of age, height, weight, and BMI. The overall mean BLL was 24.1 ± 18.0 µg/dL (ranged from 1.62 to 74.1 µg/dL), with a significant difference between the exposed area (32.4 ± 15.9 µg/dL) and the control area (6.23 ± 4.25 µg/dL) (p < 0.001). The BLLs varied considerably by gender, with female children having a mean BLL (26.4 ± 18.3 µg/dL) that was significantly higher than male children (21.6 ± 17.4 µg/dL) (p = 0.035). According to the Kruskal-Wallis test, BLLs significantly differed by age among the age groups (x2 = 9.29, p = 0.025). Further evaluation using the Wilcoxon analysis for each pair revealed that children in the first group (≤ 2 years old) have significantly higher levels of Pb in their blood, with an average of 33.5 µg/dL.
Table 2. Characteristics of study subjects and blood Pb levels (µg/dL) for exposed (n = 123) and control (n = 63) children enrolled for this study, July-August 2017.
Methylation level of LINE-1
The overall average LINE-1methylation level among the studied children was 76.8% (ranging from 69.8% to 83.6%), with no significant difference between areas (control children: 77.1 ± 2.34 vs exposed children: 76.6 ± 3.26; p = 0.568). To assess the relationship between global DNA methylation and variables such as BLL and demographics, we utilized a multiple regression analysis (). The result showed no significant associations between LINE-1 methylation level and demographic characteristics such as area, gender, age, height, weight, and BMI. On the contrary, an inverse association with coefficient values of – 0.046 between LINE-1 methylation and BLL (t ratio = – 2.76, p = 0.006) was found.
Table 3. Mixed-effects regression analysis of LINE-1 methylation level with blood Pb level and characteristics of the study subjects.
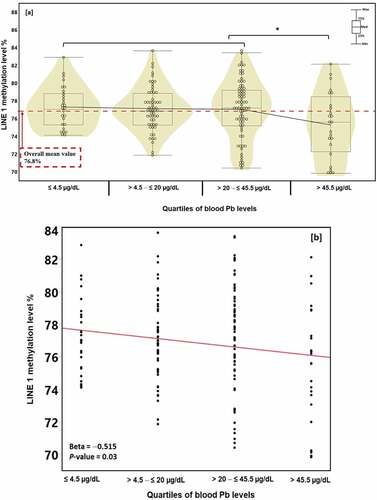
Thus, we divided the participants into four groups based on their quartile BLL: Q1: ≤ 4.5 µg/dL (N = 27), Q2: > 4.5 – ≤ 20 µg/dL (N = 57), Q3: > 20 – ≤ 45.5 µg/dL (N = 73), and Q4: > 45.5 µg/dL (N = 24) groups (). The average LINE-1 methylation levels of these groups were 77.3%, 77.1%, 77.0%, and 75.2%, respectively. As shown in , the methylation level at the fourth quartile BLL was inversely associated with LINE-1 methylation (p for trend = 0.03). The nonparametric Wilcoxon analysis showed that the difference between this methylation level and the other three quartile methylation levels was statistically significant (p < 0.03). Furthermore, shows an inverse relationship between quartiles of BLL and LINE-1 methylation levels with a beta value of – 0.515 (p = 0.03).
GSTP1 promoter methylation level
We examined the GSTP1 promoter methylation status in children exposed to environmental Pb using quantitative methylation-specific PCR. The GSTP1 methylation level in whole blood samples stratified by area and gender is illustrated in . The Kruskal-Wallis one-way analysis showed no statistical difference in the GSTP1 methylation level between areas (x2 = 0.446, p = 0.504). Blood samples from eight (12.7%) of 63 children from the control area and sixteen (13%) of 123 children from the exposed area showed GSTP1 methylation. The chi-square test showed no methylation frequency difference between the areas (x2 = 0.004, p = 0.952). Based on detection numbers (methylated: control 8 vs exposed 16; unmethylated: 55 vs 107), children with the methylated GSTP1 gene were not associated with the risk of Pb poisoning (adjusted OR = 1.03, 95% CI:0.42–2.55; p = 0.476) (). A gender-based comparison revealed no differences in methylation frequency (female: unmethylated N = 82 and methylated N = 10; male: unmethylated N = 80 and methylated N = 14) or susceptibility to Pb toxicity (female vs male: adjusted OR = 0.69, 95% CI:0.29–1.66; p = 0.207) ().
Figure 4. GSTP1 promoter’s methylation levels in whole blood and adjusted odd ratios (a) per area [control area: >3 km far away from the mine site; exposed area: within 3 km of the mine area and windward direction] and (b) per gender. PMR: percent methylation reference; solid bars indicate mean (m), and median (m) within a group of children; #indicates samples with PMR values ≤ 0.01. [a]: adjusted for age, gender, and BMI: [b]: adjusted for area and BMI.
![Figure 4. GSTP1 promoter’s methylation levels in whole blood and adjusted odd ratios (a) per area [control area: >3 km far away from the mine site; exposed area: within 3 km of the mine area and windward direction] and (b) per gender. PMR: percent methylation reference; solid bars indicate mean (m), and median (m) within a group of children; #indicates samples with PMR values ≤ 0.01. [a]: adjusted for age, gender, and BMI: [b]: adjusted for area and BMI.](/cms/asset/0da73f16-6acf-4ff4-acdb-db67a0df5d90/kepi_a_2123924_f0004_b.gif)
Discussion
Lead exposure in Kabwe town is evident and the risk of Pb poisoning must be emphasized. The overall result showed the overall mean/median BLL were 24.1/22.4 µg/dL. Of all the samples, 164 (88.2%) had BLL that exceeded the updated blood Pb reference value of 3.5 µg/dL [Citation31]. In the present study, female gender was an important factor of higher Pb exposure though previous studies reported that male gender was an important determinant [Citation32,Citation33]. The BLL in females (mean: 26.4 µg/dL) was significantly higher than that in males (21.6 µg/dL) and showed an average increment of 4.8 µg/dL. One possible reason for this outcome could be exposure to Pb contaminated dust as female children participate in house chores like sweeping the floor more than their male counterparts. Previous studies reported pronounced Pb-induced effects on haem synthesis and neurological and reproductive impairment are more severe in females than in males even at lower blood Pb concentrations [Citation34,Citation35]. Therefore, this result suggests that Pb poisoning could be prevalent among female children in Kabwe. More research is needed to clarify the inconsistencies of gender related Pb toxicity.
Globally, ‘early’ childhood Pb poisoning, which can be prevented, is a major challenge. Concurrent with previous studies [Citation7,Citation36], the under 2 years old age group had the highest average BLL (33.5 µg/dL). This result is expected, as younger children are more vulnerable to nondietary Pb exposure than older ones because of their frequent hand-to-mouth activities. Moreover, they spent most of their playtime on the floor, which might have been contaminated by Pb in the case of Kabwe town. Previous studies from Kabwe reported very high concentrations of Pb in soil [Citation1,Citation2]. Calabrese et al. [Citation37] reported that some children can ingest 25 to 60 g soil/day which is 600 times higher than the recommended soil ingestion values for children [Citation38]. Generally, this finding emphasized the risk of Pb poisoning among younger children at which immediate countermeasures should be taken in Kabwe town.
Various epidemiological and toxicological evidence shows that early life and childhood Pb exposure have an impact on human health without clear signs of toxicity [Citation10]. In the human body, DNA methylation plays a major role in the regulation and development of disease by changing the patterns and levels of global and regional DNA methylation. Predominantly, it either undergoes global DNA hypomethylation of LINE-1 and Alu retroelements or hypermethylation in the CpG-rich promoter regions of genes, such as GSTP1. Thus, in the present study, we examined the impact of environmental Pb exposure on global DNA methylation of LINE-1 and gene-specific methylation of GSTP1 gene in 186 children’s blood samples.
So far, no research in the study area has correlated Pb burden with genomic DNA methylation in seemingly healthy children who were exposed to environmental Pb. Previous studies reported the impact of Pb exposure on epigenetic marks mainly in adults exposed to occupational Pb [Citation19,Citation39,Citation40]. In the present study, an inverse correlation between LINE-1 methylation and BLL was observed at higher BLL. This result is similar to a previous report that showed a lower methylation LINE-1 level at a high BLL [Citation19]. Moreover, Li et al. [Citation39] reported significant LINE-1 hypomethylation with increased BLLs. These results showed that LINE-1 hypomethylation seemed to follow a dose-dependent pattern, especially at higher BLLs. The higher the BLL, the lower the LINE-1 methylation level (hypomethylation). Experimental models using animal and human cells also showed Pb disturbances in epigenetic status. For instance, Sanchez et al. [Citation41] reported reduced global DNA methylation levels of 13% at 10 µg/dL and 29% at 50 µg/dL Pb exposure using zebrafish. Moreover, Senut et al. [Citation42] reported the impact of Pb exposure on the disruption of global DNA methylation in human embryonic stem cell lines and altered their differentiation. The basis for this demethylation might be caused by the high affinity of Pb for the DNA methyltransferase (DNMT) enzyme, which initiates DNA hypomethylation by inactivating the enzyme [Citation41]. However, previous studies by Pilsner et al. [Citation20] using cord blood samples and by Wright et al. [Citation21] using frozen buffy coats showed no association between LINE-1 methylation and BLLs, but with patella Pb levels. This could be explained by the low Pb concentration in blood samples. In these studies, they reported an association of LINE-1 methylation with higher patella Pb levels; >21 µg/g for maternal patella Pb [Citation20] and > 29 µg/g for adults’ patella Pb level [Citation21]. Taken together, our study adds further understanding of global DNA hypomethylation with increasing BLLs. LINE-1 hypomethylation has been routinely used as an alternate measure of genomic DNA methylation levels. A review and meta-analysis by Barchitta et al. [Citation43] evaluated the role of LINE-1 hypomethylation in human cancer and revealed that LINE-1 hypomethylation is evident in cancer patients. Thus, as inorganic Pb is classified as a ‘probable’ carcinogen chemical, children with high BLL might be at risk now or at a later stage of their life. The worst scenario for Kabwe is that the whole population is in danger of Pb exposure. This finding, however, should be interpreted with caution due to the inability of the bisulphite treatment to distinguish between 5-methylcytosine (5mC) and 5-hydroxymethylcytosine (5hmc). DNA methylation analyses based on bisulphite conversion would likely show excessive levels of 5mC and 5hmC. Based on these methods, there might be under-or overestimations of densely hydroxymethylated DNA regions.
GSTP1 is a metabolic enzyme that detoxifies and bio-transforms a variety of electrophilic compounds, including Pb. Lead exposure can cause oxidative damage to several tissues by interfering with the generation and removal of reactive oxygen species, promoting lipid peroxidation, and reducing the antioxidant defence system [Citation11]. However, genetic variation and epigenetic modifications in GSTP1 increase interindividual differences in susceptibility to xenobiotic toxicity. Hypermethylation of the GSTP1 promoter region has been linked with different types of cancer [Citation44–46]. Furthermore, a single nucleotide polymorphism in this gene can impair enzyme function and be linked to diseases [Citation46,Citation47]. In our study, we compared the GSTP1 methylation status between control and exposed areas. The average BLLs in these areas were 6.23 ± 4.25 µg/dL for control and 32.4 ± 15.9 µg/dL for the exposed area. No significant change in the GSTP1 methylation level (control: mean of 0.020% vs. exposed: mean of 0.027%; p = 0.504) was detected between areas. In agreement with the present result, Li et al. [Citation23] assessed GSTP1 promoter methylation status in cell models and occupational Pb exposed adults and found no significant changes in GSTP1 CpG methylation levels. In our previous studies, we reported hypermethylation of ALAD and p16 genes [Citation48], and polymorphism of ALAD, VDR, and GST genes associated with Pb susceptibility in children from Kabwe [Citation49,Citation50]. Thus, GSTP1 methylation status is not a good biomarker for Pb-risk susceptibility in healthy subjects.
The following limitations are recognized in this study: (i) a cross-sectional design with small sample size, which may make determining the incidence of Pb poisoning difficult and reduce statistical power; (ii) a single point blood sample where Pb has a short half-life in blood; (iii) only Pb is investigated; and (iv) only LINE-1 and GSTP1 gene-specific methylation were investigated. Thus, it is difficult to comprehensively credit the direct influence of Pb exposure on DNA methylation with health outcomes. Therefore, in the future, (i) prospective studies with large sample sizes, not only children but also adults, are recommended; (ii) besides Pb, other heavy metals such as zinc and cadmium should be investigated since they are discharged as secondary products; (iii) to associate the effect of environmental pollutants, including Pb exposure, on epigenetic markers, more genes such as differentially methylated region (DMR) genes and nervous system regulatory genes, as well as gene expression levels, should be investigated; and (iv) since Pb is a ‘probable’ carcinogenic compound, a case-control study is suitable to study the burden of Pb exposure using patients, especially those with cancer.
In conclusion, the findings of our study demonstrate that children exposed to Pb experienced hypomethylation of their global DNA. The results show that hypomethylation of LINE-1 occurs at high BLLs, providing further evidence of global DNA hypomethylation. In turn, this could impair gene regulation, which can lead to negative health consequences. Meanwhile, GSTP1 methylation is not a good potential methylation biomarker of Pb exposure in healthy subjects. Further studies are needed to examine the mechanisms behind the relationship between Pb exposure and DNA methylation at the global and gene-specific levels.
Ethics approval
Study protocol approval and permission to conduct the research were obtained from the University of Zambia Research Ethics Committee (UNZAREC; REF. No. 012-04-16) and the Ministry of Health Zambia, respectively. Material transfer agreement (MTA, Approval No. E00417) has been issued by the Ministry of Health, Zambia, for transporting frozen samples to Japan.
Consent to participate
Participation in this study was voluntary, and all individual participants enrolled after getting signed informed consent from their parents.
Consent for publication
All the co-authors have read the manuscript and agreed to its publication.
Data availability
Data is available from the corresponding author on reasonable request.
Acknowledgments
We are very grateful to the children that participated in this study and the laboratory technicians and nurses at the health centers in Kabwe. We are also grateful to the Kabwe District Health Office, Kabwe Municipal Council, and the Ministry of Health, Zambia for facilitating our work. Our appreciation is extended to Takahiro Ichise and Nagisa Hirano for their technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bose-O’Reilly S, Yabe J, Makumba J, et al. Lead intoxicated children in Kabwe, Zambia. Environ Res. 2018;165:420–424.
- Nakayama SMM, Ikenaka Y, Hamada K, et al. Metal and metalloid contamination in roadside soil and wild rats around a Pb-Zn mine in Kabwe, Zambia. Environ Pollut Barking Essex. 1987;159(1):175–181.
- Yabe J, Nakayama SMM, Ikenaka Y, et al. Metal distribution in tissues of free-range chickens near a lead-zinc mine in Kabwe, Zambia. Environ Toxicol Chem. 2013;32(1):189–192.
- Toyomaki H, Yabe J, Nakayama SMM, et al. Factors associated with lead (Pb) exposure on dogs around a Pb mining area. Vol. 247. Kabwe: Zambia: Chemosphere; 2020. p. 125884.
- Doya R, Nakayama SMM, Nakata H, et al. Land use in habitats affects metal concentrations in wild lizards around a former lead mining site. Environ Sci Technol. 2020;54(22):14474–14481.
- Yabe J, Nakayama SM, Ikenaka Y, et al. Uptake of lead, cadmium, and other metals in the liver and kidneys of cattle near a lead-zinc mine in Kabwe, Zambia. Environ Toxicol Chem. 2011;30(8):1892–1897.
- Yabe J, Nakayama SMM, Ikenaka Y, et al. Lead poisoning in children from townships in the vicinity of a lead-zinc mine in Kabwe, Zambia. Chemosphere. 2015;119:941–947.
- Yabe J, Nakayama SMM, Ikenaka Y, et al. Lead and cadmium excretion in feces and urine of children from polluted townships near a lead-zinc mine in Kabwe, Zambia. Chemosphere. 2018;202:48–55.
- Yabe J, Nakayama SM, Nakata H, et al. Current trends of blood lead levels, distribution patterns and exposure variations among household members in Kabwe, Zambia. Chemosphere. 2020;243:125412.
- Wani AL, Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8(2):55–64.
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58.
- Binns HJ, Campbell C, Brown MJ. Centers for disease control and prevention advisory committee on childhood lead poisoning prevention. interpreting and managing blood lead levels of less than 10 microg/dl in children and reducing childhood exposure to lead: recommendations of the centers for disease control and prevention advisory committee on childhood lead poisoning prevention. Pediatrics. 2007;120(5):e1285–1298.
- Lanphear BP, Dietrich K, Auinger P, et al. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep Wash DC. 1974;115:2000.521–529.
- Halusková J. Epigenetic studies in human diseases. Folia Biol (Praha). 2010;56(3):83–96.
- Ehrlich M, Lacey M. DNA methylation and differentiation: silencing, upregulation and modulation of gene expression. Epigenomics. 2013;5(5):553–568.
- Yang AS, Estécio MRH, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38.
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97.
- IARC. Inorganic and organic lead compounds, IARC monographs on the evaluation of carcinogenic risks to humans. 2006; Vol. 87. Lyon: IARC
- Devóz PP, Gomes WR, De Araújo ML, et al. Lead (Pb) exposure induces disturbances in epigenetic status in workers exposed to this metal. J Toxicol Environ Health A. 2017;80(19–21):1098–1105.
- Pilsner JR, Hu H, Ettinger A, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect. 2009;117(9):1466–1471.
- Wright RO, Schwartz J, Wright RJ, et al. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect. 2010;118(6):790–795.
- L-B Y, Y-T T, Huang J-W, et al. Hypermethylation of CpG islands is associated with increasing chromosomal damage in Chinese lead-exposed workers. Environ Mol Mutagen. 2018;59(6):549–556.
- Li C, Yang X, Xu M, et al. Association between GSTP1 CpG methylation and the early phase of lead exposure. Toxicol Mech Methods. 2014;24(2):111–115.
- Li Y, Xie C, Murphy SK, et al. Lead exposure during early human development and DNA methylation of imprinted gene regulatory elements in adulthood. Environ Health Perspect. 2016;124(5):666–673.
- Ghanjati F, Beermann A, Hermanns T, et al. Unreserved application of epigenetic methods to define differences of DNA methylation between urinary cellular and cell-free DNA. Cancer Biomark. 2014;14(5):295–302.
- Ponomaryova AA, Rykova EY, Gervas PA, et al. Aberrant methylation of LINE-1 transposable elements: a search for cancer biomarkers. Cells. 2020;9(9):2017.
- Chan QKY, Khoo U-S, Chan KYK, et al. Promoter methylation and differential expression of π-class glutathione S-transferase in endometrial carcinoma. J Mol Diagn. 2005;7(1):8–16.
- Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154–166.
- Nakata H, Nakayama SMM, Yabe J, et al. Assessment of LeadCare® II analysis for testing of a wide range of blood lead levels in comparison with ICP-MS analysis. Chemosphere. 2021;271:129832.
- Harden SV, Guo Z, Epstein JI, et al. Quantitative GSTP1 methylation clearly distinguishes benign prostatic tissue and limited prostate adenocarcinoma. J Urol. 2003;169(3):1138–1142.
- CDC (Centers for Disease Control and Prevention). CDC updates blood lead reference value for children. https://www.cdc.gov/media/releases/2021/p1028-blood-lead.html. Cited 2021 Nov 1. (2021).
- Pelc W, Pawlas N, Dobrakowski M, et al. Environmental and socioeconomic factors contributing to elevated blood lead levels in children from an industrial area of Upper Silesia. Environ Toxicol Chem. 2016;35(10):2597–2603.
- Llop S, Lopez-Espinosa M-J, Rebagliato M, et al. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1–2):3–12.
- Roels HA, Lauwerys RR, Buchet JP, et al. Response of free erythrocyte porphyrin and urinary delta-aminolevulinic acid in men and women moderately exposed to lead. Int Arch Arbeitsmed. 1975;34(2):97–108.
- La Llave León O, Salas Pacheco MJ. Effects of lead on reproductive health [internet]. In: Chooto P, editor. Lead chemistry. IntechOpen; 2020, pp. 4-8. cited 2021 Dec 7. Available from 2021 Dec 7. https://www.intechopen.com/books/lead-chemistry/effects-of-lead-on-reproductive-health
- Zardast M, Khorashadi-Zadeh SS, Nakhaee S, et al. Blood lead concentration and its associated factors in preschool children in eastern Iran: a cross-sectional study. BMC Pediatr. 2020;20(1):435.
- Calabrese EJ, Stanek EJ, James RC, et al. Soil ingestion: a concern for acute toxicity in children. Environ Health Perspect. 1997;105(12):1354–1358.
- US EPA. Child-specific exposure factors handbook (2008) US environmental protection agency. Washington: DC; 2008. EPA/600/R-06/096F, 2008.
- Li C, Yang X, Xu M, et al. Epigenetic marker (LINE-1 promoter) methylation level was associated with occupational lead exposure. Clin Toxicol Phila Pa. 2013;51(4):225–229.
- Wang K, Meng Y, Wang T, et al. Global and gene-specific promoter methylation, and micronuclei induction in lead-exposed workers: a cross-sectional study. Environ Mol Mutagen. 2021;62(7):428–434.
- Sanchez OF, Lee J, Yu King Hing N, et al. Lead (Pb) exposure reduces global DNA methylation level by non-competitive inhibition and alteration of dnmt expression. Met Integr Biometal Sci. 2017;9(2):149–160.
- Senut M-C, Sen A, Cingolani P, et al. Lead exposure disrupts global DNA methylation in human embryonic stem cells and alters their neuronal differentiation. Toxicol Sci Off J Soc Toxicol. 2014;139(1):142–161.
- Barchitta M, Quattrocchi A, Maugeri A, et al. LINE-1 hypomethylation in blood and tissue samples as an epigenetic marker for cancer risk: a systematic review and meta-analysis. PloS One. 2014;9(10):e109478.
- Fang C, Wei X-M, Zeng X-T, et al. Aberrant GSTP1 promoter methylation is associated with increased risk and advanced stage of breast cancer: a meta-analysis of 19 case-control studies. BMC Cancer. 2015;15(1):920.
- Lee JS. GSTP1 promoter hypermethylation is an early event in breast carcinogenesis. Virchows Arch Int J Pathol. 2007;450(6):637–642.
- Cairns P, Esteller M, Herman JG, et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7:2727–2730.
- Kim S-U, Lee K-M, Park S-K, et al. Genetic polymorphism of glutathione S-transferase P1 and breast cancer risk. J Biochem Mol Biol. 2004;37(5):582–585.
- Yohannes YB, Nakayama SM, Yabe J, et al. Blood lead levels and aberrant DNA methylation of the ALAD and p16 gene promoters in children exposed to environmental-lead. Environ Res. 2020;188:109759.
- Yohannes YB, Nakayama SMM, Yabe J, et al. Delta-aminolevulinic acid dehydratase (ALAD) and vitamin D receptor (VDR) genes polymorphisms in children residing in an abandoned lead‑zinc mine area in Kabwe, Zambia. Meta Gene. 2021;27:100838.
- Yohannes YB, Nakayama SMM, Yabe J, et al. Glutathione S-transferase gene polymorphisms in association with susceptibility to lead toxicity in lead- and cadmium-exposed children near an abandoned lead-zinc mining area in Kabwe, Zambia. Environ Sci Pollut Res Int. 2022;29(5):6622–6632.