ABSTRACT
Functions of exogenous L-ascorbic acid in plant roots are poorly understood. Recent study by Makavitskaya et al. (doi.org/10.1093/jxb/ery056) has demonstrated that exogenous ascorbate can be released from roots in response to salt stress, and can trigger elevation in the cytosolic free Ca2+. Here, we report that exogenous ascorbate significantly modifies root elongation in Arabidopsis thaliana. Using a medium exchange technique, we have shown that 10–100 µM ascorbate induces small but significant increase in root elongation while higher levels cause its dramatic decrease. Root border cells of Pisum sativum have been losing viability twice faster in the presence of ascorbate that under control conditions, as tested by the confocal microscopy and a combined staining with propidium iodide and fluorescein diacetate.
L-ascorbic acid (ascorbate) is the major plant antioxidant and one of the most abundant carbohydrates in plants.Citation1-Citation4 Although ascorbate has been long recognised as a major antioxidant in plants, many evidence were accumulated suggesting that this substance may also play regulatory and signalling roles. Our recent data demonstrate that exogenous L-ascorbic acid induces transient elevation in the cytosolic free Ca2+ in Arabidopsis thaliana roots,Citation5 thus linking ascorbate to Ca2+ signaling; a central signalling phenomenon in plants.Citation6
Calcium signals and a polar increase in the cytosolic free Ca2+ are responsible for a multitude of physiological processes, such as processing of environmental signals, cell elongation, tissue differentiation, gravitropic reactions, adjustment of respiration and photosynthesis.Citation6 Accordingly, we hypothesize that ascorbate, which is ubiquitous and abundant in plants, acts as an extracellular signaling and regulatory molecule having high importance for a number of physiological functions.
Root cell elongation is one of the key processes that are regulated by both ROS and cytosolic calcium.Citation6–Citation9 ROS are produced locally in growing parts of the cell, such as tips of root hairsCitation7 or the frontal part of elongation zone cells.Citation8 This leads to the local ROS-induced activation of Ca2+-permeable ion channels stimulating Ca2+-dependent exocytosis, incorporation of vesicles into the plasma membrane and increase of cell size.Citation7,Citation9,Citation10 Moreover, cell wall needs to be softened locally to allow membrane expansion; this is achieved via cleavage of cell wall polymers by hydroxyl radicals (HO•), which are generated by Haber-Weiss cycle catalysed the cell wall-bound transition metals.Citation11–Citation14 The role of exogenous ascorbate here is obvious: hydroxyl production in Haber-Weiss cycle constantly requires electrons for transition metal reduction from ascorbate. Ascorbate is far more superior reducing agent for Cu2+ and Fe3+ than any other abundant cell metabolites.Citation13 Thus it is anticipated that ascorbate supply should modify the elongation growth.
Here, we report a statistically significant stimulation of root elongation after transferring roots on ascorbate-containing media in 10 to 100 µM concentration range (). For example, 30 µM ascorbate increased root elongation rate from 9.93 ± 0.08 mm d−1 (control: ascorbate-free) to 10.38 ± 0.07 mm d−1 (n = 170; p < 0.0001; ANOVA). Higher levels of ascorbate (over 0.3 mM) inhibited root elongation (), with a 30-fold decrease observed at highest 3 mM ascorbate treatment. Both the root diameter and a length of the elongation root zone also increased slightly in 10–100 µM and then decreased in 0.3–3 mM ascorbate concentration range (A, B).
Figure 1. Effect of ascorbate on Arabidopsis thaliana L. root growth. (A) Typical nine day-old plants cultivated vertically on Murashige and Skoog medium (original concentrations; Duchefa, #M0221) with ascorbate (concentrations are indicated in the figure). During the first four days, plants were growing without ascorbate, then medium below root tips was removed using razor blade and carefully replaced with the warm block of ascorbate-containing medium. This technique allows to avoid disturbance of plants. Root growth rate (B), length of root elongation zone (C) and root diameter (D) at different ascorbate levels in the medium. All parameters were measured at fifth day after exchange of media. Mean values (± SE) were plotted against the tested ascorbate concentrations (0.01 to 3 mM range). Plant cultivation techniques were as described by Makavitskaya et al.Citation5
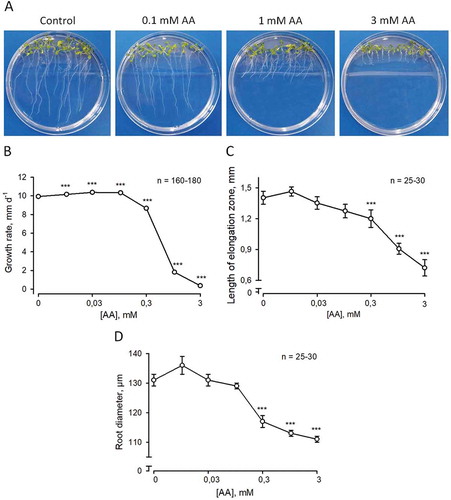
A number of hypotheses can be proposed for interpretation of these data. Ascorbate can potentially be oxidized to dehydroascorbate, which is transported back by cells. Hypothetically, increased dehydroascorbate concentration can affect reduced glutathione pool, which has been shown to be important for maintaining root elongation.Citation15 The obtained results can also be interpreted from a viewpoint where the moderate polar HO• production and a local Ca2+ influx are necessary for root elongation. The physiological levels of ascorbate (10–100 µM) promote these processes (). It can be hypothesized that higher ascorbate concentrations can over-produce HO•. This over-production is non-polar and so it cannot stimulate elongation growth.Citation7 Supporting this Foreman et al.Citation7 reported that addition of HO•-generating mixture containing 1 mM ascorbate and transition metal copper stopped polar expansion of root hairs leading to a formation of bubble-like outgrowings.
Our recent work also showed that 1 mM ascorbate increased numbers of cells with symptoms of the programmed cell death (PCD) so it can be toxic.Citation5 Toxicity of 0.5–2 mM ascorbate has also been reported for animal cells where it depended on medium composition.Citation16,Citation17 It was hypothesized that, in the presence of ascorbate, transition metal such as copper and iron (in medium) catalyze hydroxyl radical production, which cause toxicity.Citation16,Citation17 Our previous study demonstrated that hydroxyl radicals are generated in the presence of transition metals and 1 mM ascorbate and that this can induce cell death in Arabidopsis thaliana roots in 1–2 days.Citation18 The medium salines used for Arabidopsis thaliana growth test here were from Murashige and Skoog (original concentrations; #M0221; Duchefa, Netherlands), which includes 0.1 µM Cu2+ (CuSO4•5H2O) and chelated 100 µM Fe3+ (FeNaEDTA). So some moderate catalysis of HO• was hypothetically possible.
Another explanation of the ascorbate-induced inhibition of root elongation is that the elevated ascorbate levels lead to HO•-scavenging effect.Citation13 Ascorbate can also be a scavenger of ‘secondary’ radical production in HO•-induced reactions.Citation13 HO•-producing capacity of ascorbate relies on transition metals, and if ascorbate level grows up without equivalent increase in the transition metal concentration, ascorbate starts scavenge radicals that produced by the ascorbate-dependent Haber-Weiss cycle (hypothetically, at concentrations above 0.1 mM).
The effect of ascorbate on root growth is fundamental to plant physiology. Ascorbate concentration inside root cells can reach millimoles per liter.Citation19–Citation21 According to Smirnoff,Citation4 the apoplastic ascorbate level is lower (about 0.1 mM) that corresponds to stimulatory concentration for root growth found here (). An increase in the apoplastic ascorbate concentration can occur in the case of stress effects that cause cell collapse. Dying cells can release ascorbate causing its local elevation up to 1–10 mM (cytosolic level). This can trigger root growth inhibition. Ascorbate can also be released without cell collapse, in a process mediated by anion channelsCitation5 following membrane depolarization by NaCl.
Root border cells are outer layers of cells and, hence, the first target for stresses.Citation22-Citation24 These cells normally live for a short period of time (only a few days). When dying, they release metabolites to the rhizosphere; those potentially act as signalsCitation22,Citation23 Hypothetically, border cells can be involved in sensing new environment, interacting with neighboring roots and other organisms. Here we used border cells from pea roots to see if their viability will be affected by the presence of ascorbate. In the presence of ascorbate, border cells died twice faster than in control conditions (combined propidium iodide and fluorescein diacetate test; ). This suggests that a release of ascorbate from inner root layers can be a signal for elimination of root border cells. In the viability test with border cells, medium contained only 0.3 mM CaCl2, 0.3 mM KCl, pH 6.0 (2 mM Mes) prepared using deionized water of Type I (18 mOhm). However pea seeds can contain significant quantity of transition metals, such as copper and iron in seeds. This means that the mechanism of HO•-induced toxicity is not excluded for explaining of ascorbate effects on root border cells.
Figure 2. Effect of exogenous L-ascorbic acid on viability of pea root border cells. (A) Typical root border cells of Pisum sativum L. (bright field). (B) Epi-fluorescent images (laser scanning confocal microscopy) of propidium iodide (PI; dead cells) and fluorescein diacetate (FDA; viable cells) staining. (C) Mean numbers (± SE) of viable cells (four independent trials) at different times of treatment by ascorbate. Seeds of pea were germinated under the mist culture as described elsewhere.Citation22–Citation24 Root border cells were harvested and purified as described previously.Citation22–Citation24 Pellets with root border cells were resuspended in a solution containing 0.3 mM CaCl2, 0.3 mM KCl, pH 6.0 (2 mM Mes), and then were treated with 1 mM L-ascorbic acid. At 1 min, 1 h, 2 h and 6 h, 90 μL of solution containing root border cells was mixed with 10 μL of FDA/1 μL PI solution (FDA from Fluka; PI from Sigma), and cell viability was determined as described elsewhere.Citation20
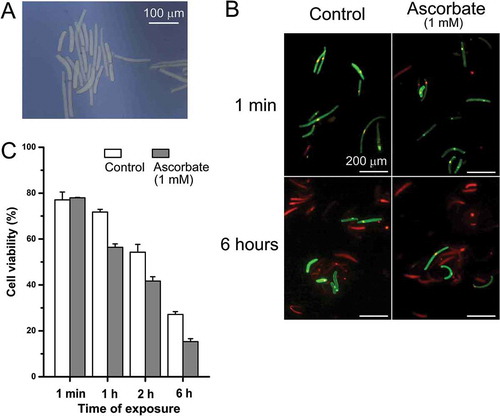
In conclusion, reported data show new insights into ascorbate functions in plants. This includes slight stimulation at physiological levels of 10–100 µM while strong inhibition by 0.3–3 mM. 1 mM ascorbate decreased viability of root border cells. This can potentially be a mechanism of their elimination because ascorbate can be released by inner cell layers via anion channels.Citation5
Additional information
Funding
References
- Gallie DR. L-Ascorbic acid: a multifunctional molecule supporting plant growth and development. Scientifica. 2013;2013:1–24. Article ID 795964. doi:10.1155/2013/795964.
- Gest N, Gautier H, Stevens R. Ascorbate as seen through plant evolution: the rise of a successful molecule? J Exp Bot. 2013;64:33–53. doi:10.1093/jxb/ers297.
- Maruta T, Sawa Y, Shigeoka S, Ishikawa T. Diversity and evolution of ascorbate peroxidase functions in chloroplasts: more than just a classical antioxidant enzyme? Plant Cell Physiol. 2016;57:1377–1386. doi:10.1093/pcp/pcv203.
- Smirnoff N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Rad Biol Med. 2018. Corrected Proofs. doi:10.1016/j.freeradbiomed.2018.03.033.
- Makavitskaya M, Svistunenko D, Navaselsky I, Hryvusevich P, Mackievic V, Rabadanova C, Tyutereva E, Samokhina V, Straltsova D, Sokolik A, et al. Novel roles of ascorbate in plants: induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J Exp Bot. 2018;69:3477–3489. Corrected Proofs. doi:10.1093/jxb/ery056.
- Demidchik V, Shabala S. Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct Plant Biol. 2018;45:9–27. doi:10.1071/FP16420.
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi:10.1038/nature01485.
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci. 2003;116:81–88. doi:10.1242/jcs.00201.
- Mangano S, Juárez SP, Estevez JM. ROS regulation of polar growth in plant cells. Plant Physiol. 2016;171:1593–1605. doi:10.1104/pp.16.00191.
- Demidchik V, Maathuis FJM. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol. 2007;175:387–405. doi:10.1111/j.1469-8137.2007.02128.x.
- Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28:679–688. doi:10.1046/j.1365-313x.2001.01187.x.
- Müller K, Linkies A, Vreeburg RA, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009;150:1855–1865. doi:10.1104/pp.109.139204.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Fifth ed. Oxford: Oxford University Press; 2015. ISBN: 9780198717485.
- Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Env Exp Bot. 2015;109:212–228. doi:10.1016/j.envexpbot.2014.06.021.
- Yu X, Pasternak T, Eiblmeier M, Ditengou F, Kochersperger P, Sun J, Wang H, Rennenberg H, Teale W, Paponov I, et al. Plastid-localized glutathione reductase2-regulated glutathione redox status is essential for Arabidopsis root apical meristem maintenance. Plant Cell. 2013;25:4451–4468. doi:10.1105/tpc.113.117028.
- Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540:3–6.
- Clément MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Red Signal. 2001;3:157–163. doi:10.1089/152308601750100687.
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. Arabidopsis root K+ efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci. 2010;123:1468–1479. doi:10.1242/jcs.064352.
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci. 2000;19:267–290. doi:10.1080/10409230008984166.
- Kawa D, Julkowska MM, Sommerfeld HM, Ter Horst A, Haring MA, Testerink C. Phosphate-dependent root system architecture responses to salt stress. J Plant Physiol. 2016;172:690–706. doi:10.1104/pp.16.00712.
- Alscher RG, Hess JL. Antioxidants in higher plants. Boca Raton, FL: CRC Press; 2017.
- Yu M, Feng YM, Goldbach HE. Mist culture for mass harvesting of root border cells: aluminum effects. J Plant Nutr Soil Sci. 2006;169:670–674. doi:10.1002/jpln.200620604.
- Yu M, Shen R, Liu J, Chen R, Xu M, Yang Y, Xiao H, Wang H, Wang H, Wang C. The role of root border cells in aluminum resistance of pea (Pisum sativum) grown in mist culture. J Plant Nutr Soil Sci. 2009;172:528–534. doi:10.1002/jpln.200800039.
- Ma J, Zhang X, Zhang W, Wang L. Multifunctionality of silicified nanoshells at cell interfaces of Oryza sativa. ACS Sust Chem Engin. 2016;4:6792–6799. doi:10.1021/acssuschemeng.6b01736.

